Abstract
Bladder cancer is the tenth most common cancer type worldwide. Urothelial carcinoma is the most common type of bladder cancer and accounts for 90% of bladder cancer cases in the USA and Europe. Novel approaches are needed to improve patient outcomes. Nectin-4 is a tumour-associated antigen found on the surface of most urothelial carcinoma cells. In the antibody–drug conjugate enfortumab vedotin, human anti-nectin-4 antibody is linked to the cytotoxic microtubule-disrupting agent monomethyl auristatin E. In ongoing phase I, II and III clinical trials, enfortumab vedotin has been evaluated as a monotherapy and in combination with a checkpoint inhibitor and/or chemotherapy in locally advanced and metastatic urothelial carcinoma. Encouraging data from the phase II study resulted in the FDA granting accelerated approval for enfortumab vedotin in December 2019 for patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum and a checkpoint inhibitor therapy. Moreover, data from a phase I study led to the FDA granting breakthrough therapy designation to enfortumab vedotin combined with pembrolizumab as a first-line treatment in February 2020 for cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma. Results of ongoing and future combination studies of enfortumab vedotin with immunotherapy and other novel agents are eagerly awaited.
Key points
-
The majority of patients with locally advanced or metastatic urothelial carcinoma treated with an anti-PDL1 checkpoint inhibitor immunotherapy given in the post-platinum or cisplatin-ineligible setting will fail to achieve complete remission; novel treatment approaches are needed to improve clinical outcomes for these patients.
-
An emerging target for systemic treatment of locally advanced or metastatic urothelial carcinoma is the tumour-associated antigen nectin-4, which is overexpressed in various cancer types, including 97% of urothelial carcinomas.
-
In the nectin-4 targeting antibody–drug conjugate enfortumab vedotin, human anti-nectin-4 antibody is linked to the cytotoxic microtubule-disrupting agent monomethyl auristatin E, and its preclinical activity has been successfully demonstrated in several solid tumours, including bladder cancer.
-
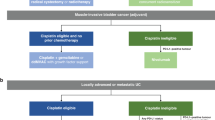
Enfortumab vedotin is being evaluated in ongoing phase I, II and III clinical trials either as a monotherapy or in combination with the checkpoint inhibitor pembrolizumab and/or chemotherapy in patients with locally advanced or metastatic urothelial carcinoma.
-
On the basis of data from the phase II EV-201 study, the FDA granted accelerated approval to enfortumab vedotin in December 2019 for patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed on platinum and checkpoint inhibitor therapy.
-
Data from the phase Ib/II EV-103 study led to the FDA granting breakthrough therapy designation to enfortumab vedotin combined with pembrolizumab in February 2020 as a first-line treatment for cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Cancer.Net Editorial Board. Bladder cancer: statistics. Cancer.Net https://www.cancer.net/cancer-types/bladder-cancer/statistics (2020).
American Cancer Society. Survival rates for bladder cancer. American Cancer Society https://www.cancer.org/cancer/bladder-cancer/detection-diagnosis-staging/survival-rates.html (2020).
Flaig, T. W. et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 18, 329–354 (2020).
Fuge, O., Vasdev, N., Allchorne, P. & Green, J. S. Immunotherapy for bladder cancer. Res. Rep. Urol. 7, 65–79 (2015).
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 196, 1021–1029 (2016).
Rayn, K. N., Hale, G. R., Grave, G. P. & Agarwal, P. K. New therapies in nonmuscle invasive bladder cancer treatment. Indian J. Urol. 34, 11–19 (2018).
FDA. FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer (2020).
Aragon-Ching, J. B., Werntz, R. P., Zietman, A. L. & Steinberg, G. D. Multidisciplinary management of muscle-invasive bladder cancer: current challenges and future directions. Am. Soc. Clin. Oncol. Educ. Book 38, 307–318 (2018).
Oing, C. et al. Second line chemotherapy for advanced and metastatic urothelial carcinoma: vinflunine and beyond — a comprehensive review of the current literature. J. Urol. 195, 254–263 (2016).
Rosenberg, J. E. et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 37, 2592–2600 (2019).
Dietrich, B., Siefker-Radtke, A. O., Srinivas, S. & Yu, E. Y. Systemic therapy for advanced urothelial carcinoma: current standards and treatment considerations. Am. Soc. Clin. Oncol. Educ. Book 38, 342–353 (2018).
Challita-Eid, P. M. et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
Beck, A., Goetsch, L., Dumontet, C. & Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Garcia-Alonso, S., Ocana, A. & Pandiella, A. Resistance to antibody-drug conjugates. Cancer Res. 78, 2159–2165 (2018).
Birrer, M. J., Moore, K. N., Betella, I. & Bates, R. C. Antibody-drug conjugate-based therapeutics: state of the science. J. Natl Cancer Inst. 111, 538–549 (2019).
Shim, H. Bispecific antibodies and antibody-drug conjugates for cancer therapy: technological considerations. Biomolecules 10, 360 (2020).
Reichert, J. FDA approves sacituzumab govitecan (Trodelvy®) for triple-negative breast cancer. Antibody Society https://www.antibodysociety.org/adc/ (2020).
Reichert, J. FDA grants first approval to belantamab mafodotin-blmf. Antibody Society https://www.antibodysociety.org/food-and-drug-administration/fda-grants-first-approval-to-belantamab-mafodotin-blmf/ (2020).
FDA. FDA grants accelerated approval to enfortumab vedotin-ejfv for metastatic urothelial cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-enfortumab-vedotin-ejfv-metastatic-urothelial-cancer (2019).
Astellas. Astellas and Seattle Genetics receive FDA breakthrough therapy designation for PADCEV™ (enfortumab vedotin-ejfv) in combination with pembrolizumab in first-line advanced bladder cancer. Astellas https://newsroom.astellas.us/2020-02-19-Astellas-and-Seattle-Genetics-Receive-FDA-Breakthrough-Therapy-Designation-for-PADCEV-TM-enfortumab-vedotin-ejfv-in-Combination-with-Pembrolizumab-in-First-Line-Advanced-Bladder-Cancer (2020).
Reymond, N. et al. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276, 43205–43215 (2001).
Fabre, S. et al. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C’-C”-D beta-strands of the nectin1 V domain. J. Biol. Chem. 277, 27006–27013 (2002).
Brancati, F. et al. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am. J. Hum. Genet. 87, 265–273 (2010).
Fabre-Lafay, S. et al. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 280, 19543–19550 (2005).
Buchanan, P. C. et al. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J. Biol. Chem. 292, 6339–6351 (2017).
Maruoka, M., Kedashiro, S., Ueda, Y., Mizutani, K. & Takai, Y. Nectin-4 co-stimulates the prolactin receptor by interacting with SOCS1 and inhibiting its activity on the JAK2-STAT5a signaling pathway. J. Biol. Chem. 292, 6895–6909 (2017).
Muhlebach, M. D. et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480, 530–533 (2011).
Noyce, R. S. & Richardson, C. D. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 20, 429–439 (2012).
Jelani, M., Chishti, M. S. & Ahmad, W. Mutation in PVRL4 gene encoding nectin-4 underlies ectodermal-dysplasia-syndactyly syndrome (EDSS1). J. Hum. Genet. 56, 352–357 (2011).
Fortugno, P. et al. Nectin-4 mutations causing ectodermal dysplasia with syndactyly perturb the Rac1 pathway and the kinetics of adherens junction formation. J. Invest. Dermatol. 134, 2146–2153 (2014).
Raza, S. I., Nasser Dar, R., Shah, A. A. & Ahmad, W. A novel homozygous nonsense mutation in the PVRL4 gene and expansion of clinical spectrum of EDSS1. Ann. Hum. Genet. 79, 92–98 (2015).
Dardour, L., Cosyns, K. & Devriendt, K. A novel missense variant in the PVRL4 gene underlying ectodermal dysplasia-syndactyly syndrome in a Turkish child. Mol. Syndromol. 9, 22–24 (2018).
Takano, A. et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 69, 6694–6703 (2009).
Zhang, Y. et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett. 375, 179–189 (2016).
Pavlova, N. N. et al. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife. 2, e00358 (2013).
Siddharth, S. et al. Nectin-4 is a breast cancer stem cell marker that induces WNT/beta-catenin signaling via Pi3k/Akt axis. Int. J. Biochem. Cell Biol. 89, 85–94 (2017).
Zhang, Y. et al. Nectin-4 promotes gastric cancer progression via the PI3K/AKT signaling pathway. Hum. Pathol. 72, 107–116 (2018).
Sithanandam, G. & Anderson, L. M. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 15, 413–448 (2008).
Kedashiro, S., Sugiura, A., Mizutani, K. & Takai, Y. Nectin-4 cis-interacts with ErbB2 and its trastuzumab-resistant splice variants, enhancing their activation and DNA synthesis. Sci. Rep. 9, 18997 (2019).
M Rabet, M. et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. 28, 769–776 (2017).
Nishiwada, S. et al. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 34, 30 (2015).
Ma, J. et al. Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. Onco Targets Ther. 9, 183–190 (2016).
Deng, H., Shi, H., Chen, L., Zhou, Y. & Jiang, J. Over-expression of Nectin-4 promotes progression of esophageal cancer and correlates with poor prognosis of the patients. Cancer Cell Int. 19, 106 (2019).
Teutsch, S. M. et al. The evaluation of genomic applications in practice and prevention (EGAPP) initiative: methods of the EGAPP Working Group. Genet. Med. 11, 3–14 (2009).
Febbo, P. G. et al. NCCN task force report: evaluating the clinical utility of tumor markers in oncology. J. Natl Compr. Canc. Netw. https://doi.org/10.6004/jnccn.2011.0137 (2011).
Riester, M. et al. Integrative analysis of 1q23.3 copy-number gain in metastatic urothelial carcinoma. Clin. Cancer Res. 20, 1873–1883 (2014).
Bambury, R. M. et al. DNA copy number analysis of metastatic urothelial carcinoma with comparison to primary tumors. BMC Cancer 15, 242 (2015).
Riester, M. et al. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin. Cancer Res. 18, 1323–1333 (2012).
Kalim, M. et al. Intracellular trafficking of new anticancer therapeutics: antibody-drug conjugates. Drug Des. Devel. Ther. 11, 2265–2276 (2017).
Lambert, J. M. & Berkenblit, A. Antibody-drug conjugates for cancer treatment. Annu. Rev. Med. 69, 191–207 (2018).
Masters, J. C., Nickens, D. J., Xuan, D., Shazer, R. L. & Amantea, M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest. N. Drugs 36, 121–135 (2018).
Li, F. et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 76, 2710–2719 (2016).
Green, L. L. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J. Immunol. Methods 231, 11–23 (1999).
Doronina, S. O. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 21, 778–784 (2003).
Sussman, D. et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol. Cancer Ther. 13, 2991–3000 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01409135 (2015).
Galsky, M. D. et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 29, 2432–2438 (2011).
Saxman, S. B. et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 15, 2564–2569 (1997).
Milowsky, M. I. et al. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol. 34, 1945–1952 (2016).
Bukhari, N., Al-Shamsi, H. O. & Azam, F. Update on the treatment of metastatic urothelial carcinoma. ScientificWorldJournal. 2018, 5682078 (2018).
Siefker-Radtke, A. & Curti, B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat. Rev. Urol. 15, 112–124 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02091999 (2020).
Rosenberg, J. et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J. Clin. Oncol. 38, 1041–1049 (2020).
Komlodi-Pasztor, E., Sackett, D., Wilkerson, J. & Fojo, T. Mitosis is not a key target of microtubule agents in patient tumors. Nat. Rev. Clin. Oncol. 8, 244–250 (2011).
US Food & Drug Administration. Breakthrough therapy. FDA https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03070990 (2020).
Takahashi, S. et al. A phase I study of enfortumab vedotin in Japanese patients with locally advanced or metastatic urothelial carcinoma. Invest. New Drugs 38, 1056–1066 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03219333 (2020).
Rudmann, D. G. On-target and off-target-based toxicologic effects. Toxicol. Pathol. 41, 310–314 (2013).
Goldberg, H. ESMO virtual congress 2020: long-term results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer in the EV-201 study in patients previously treated with platinum and PD-1/PD-L1 inhibitors. UroToday https://www.urotoday.com/conference-highlights/esmo-2020/bladder-cancer/124501-esmo-virtual-congress-2020-long-term-results-of-enfortumab-vedotin-monotherapy-for-locally-advanced-or-metastatic-urothelial-cancer-in-the-ev-201-study-in-patients-previously-treated-with-platinum-and-pd-1-pd-l1-inhibitors.html (2020).
Astellas Pharma Inc. Astellas and Seagen announce positive topline results from second cohort of patients in phase 2 pivotal trial of PADCEV® (enfortumab vedotin-ejfv) in advanced urothelial cancer. PR Newswire https://www.prnewswire.com/news-releases/astellas-and-seagen-announce-positive-topline-results-from-second-cohort-of-patients-in-phase-2-pivotal-trial-of-padcev-enfortumab-vedotin-ejfv-in-advanced-urothelial-cancer-301149817.html (2020).
National Cancer Institute. Enfortumab vedotin approved for recurrent bladder cancer. NCI https://www.cancer.gov/news-events/cancer-currents-blog/2020/enfortumab-vedotin-bladder-cancer-fda-approval (2020).
Cao, A., Heiser, R., Law, C.-L. & Gardai, S. J. Auristatin-based antibody drug conjugates activate multiple ER stress response pathways resulting in immunogenic cell death and amplified T-cell responses [abstract 4914]. Cancer Res. 76, 4914 (2016).
Muller, P., Rios-Doria, J., Harper, J. & Cao, A. in Innovations for Next-Generation Antibody-Drug Conjugates (ed. Damelin, M.) 11–44 (Humana Press, 2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03288545 (2020).
Hoimes, C. J. et al. EV-103: Initial results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma [abstract 901O]. Ann. Oncol. 30, v356–v402 (2019).
Seagen. Seattle Genetics and Astellas announce updated results from phase 1b/2 Trial of PADCEV™ (enfortumab vedotin-ejfv) in combination with immune therapy pembrolizumab as investigational first-line treatment for advanced bladder cancer. Seagen https://investor.seattlegenetics.com/press-releases/news-details/2020/Seattle-Genetics-and-Astellas-Announce-Updated-Results-from-Phase-1b2-Trial-of-PADCEV-enfortumab-vedotin-ejfv-in-Combination-with-Immune-Therapy-Pembrolizumab-as-Investigational-First-Line-Treatment-for-Advanced-Bladder-Cancer/default.aspx (2020).
Rosenberg, J. E. et al. Study EV-103: preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma [abstract 441]. J. Clin. Oncol. 38, 441 (2020).
Goldberg, H. ASCO 2020: study EV-103: new randomized cohort testing enfortumab vedotin as monotherapy or in combination with pembrolizumab in locally advanced or metastatic urothelial cancer (Trial in Progress). UroToday https://www.urotoday.com/conference-highlights/asco-2020/asco-2020-bladder-cancer/121869-asco-2020-study-ev-103-new-randomized-cohort-testing-edfortumab-vedotin-as-monotherapy-or-in-combination-with-pembrolizumab-in-locally-advanced-or-metastatic-urothelial-cancer-trial-in-progress.html (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03474107 (2020).
Petrylak, D. P. et al. EV-301: phase III study to evaluate enfortumab vedotin (EV) versus chemotherapy in patients with previously treated locally advanced or metastatic urothelial cancer (la/mUC) [abstract TPS497]. J. Clin. Oncol. https://doi.org/10.1200/JCO.2019.37.7_suppl.TPS497 (2019).
Seagen. Seattle Genetics and Astellas announce PADCEV® (enfortumab vedotin-ejfv) significantly improved overall survival in phase 3 trial in previously treated locally advanced or metastatic urothelial cancer. Seagen https://investor.seattlegenetics.com/press-releases/news-details/2020/Seattle-Genetics-and-Astellas-Announce-PADCEV-enfortumab-vedotin-ejfv-Significantly-Improved-Overall-Survival-in-Phase-3-Trial-in-Previously-Treated-Locally-Advanced-or-Metastatic-Urothelial-Cancer/default.aspx (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT04223856 (2020).
Tewari, A. ESMO virtual congress 2020: EV-302: enfortumab vedotin plus pembrolizumab and/or chemotherapy, vs chemotherapy alone, in untreated locally advanced or metastatic urothelial cancer. UroToday https://www.urotoday.com/conference-highlights/esmo-2020/bladder-cancer/124718-esmo-virtual-congress-2020-ev-302-enfortumab-vedotin-plus-pembrolizumab-and-or-chemotherapy-vs-chemotherapy-alone-in-untreated-locally-advanced-or-metastatic-urothelial-cancer.html (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04136808 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03869190 (2020).
Drakaki, A. et al. Phase Ib/II umbrella trial to evaluate the safety and efficacy of multiple 2L cancer immunotherapy (CIT) combinations in advanced/metastatic urothelial carcinoma (mUC): MORPHEUS-mUC [abstract TPS591]. J. Clin. Oncol. https://doi.org/10.1200/JCO.2020.38.6_suppl.TPS591 (2020).
Collins, D. M., Bossenmaier, B., Kollmorgen, G. & Niederfellner, G. Acquired resistance to antibody-drug conjugates. Cancers 11, 394 (2019).
Chen, R. et al. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol. Cancer Ther. 14, 1376–1384 (2015).
De Santis, M. et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 30, 191–199 (2012).
Author information
Authors and Affiliations
Contributions
E.I.H. researched data for the article, made a substantial contribution to discussion of content, wrote and reviewed/edited the manuscript before submission. J.E.R. wrote and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E.I.H. and J.E.R. have received honoraria from Astellas Pharma Global Development and Seagen.
Additional information
Peer review information
Nature Reviews Urology thanks Bradley McGregor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Heath, E.I., Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol 18, 93–103 (2021). https://doi.org/10.1038/s41585-020-00394-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-00394-5
This article is cited by
-
A review of the clinical efficacy of FDA-approved antibody‒drug conjugates in human cancers
Molecular Cancer (2024)
-
Turning up the heat: CTLA4 blockade in urothelial cancer
Nature Reviews Urology (2024)
-
Durable Response to Enfortumab Vedotin Compared to Re-challenging Chemotherapy in Metastatic Urothelial Carcinoma After Checkpoint Inhibitors
Targeted Oncology (2024)
-
Trop-2 and Nectin-4 immunohistochemical expression in metastatic colorectal cancer: searching for the right population for drugs’ development
British Journal of Cancer (2023)
-
Antibody–Drug Conjugates in the Treatment of Urothelial Cancer
BioDrugs (2023)