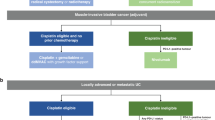
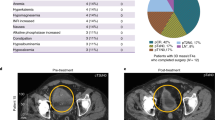
Abstract
Despite recent advances, advanced-stage urothelial carcinoma (aUC) remains incurable, with 5-year survival rates of approximately 10%. Platinum-based chemotherapy has a major role as first-line therapy for most patients with aUC. The approval of the anti-PD-L1 antibody avelumab as maintenance therapy for patients without initial disease progression on platinum-based chemotherapy is an important development that has improved the survival outcomes of patients with this disease. Otherwise, the use of first-line immune-checkpoint inhibitors (ICIs) targeting PD-1 or PD-L1 has been restricted to patients who are ineligible for platinum-containing chemotherapy regimens. Other important developments include the FDA-accelerated approval of first-line enfortumab vedotin plus pembrolizumab for patients ineligible to receive cisplatin and the availability of FGFR inhibitors, enfortumab vedotin and sacituzumab govitecan for subsequent lines of therapy. Several research questions remain unaddressed including the lack of adequate biomarkers, how to assign priority to the different treatment options for individual patients and which agents can be effective as monotherapies. The future is promising with the emergence of modalities such as antibody–drug conjugate-like drugs, next-generation ICIs, bispecific antibodies and cellular therapies. In this Review, we summarize the evolution of systemic therapy for patients with aUC and provide insights into the unmet needs.
Key points
-
With the advent of effective frontline non-chemotherapy-based regimens, the currently adopted approach for selection of frontline therapy based on cisplatin fitness is insufficient.
-
Treatment with immune-checkpoint inhibitors (ICIs) is already consolidated in the treatment of patients with aUC. Currently, the main benefit is seen with the anti-PD-L1 antibody avelumab as switch maintenance therapy following first-line chemotherapy.
-
Antibody–drug conjugates as monotherapies are already part of the therapeutic portfolio for patients with aUC and are indicated in patients with disease progression on platinum-based therapy and/or ICIs.
-
ICI-containing combinations are now positioned as the most promising treatments for patients with aUC, especially the combination of enfortumab vedotin and pembrolizumab.
-
Greater knowledge of the molecular biology of aUC has enabled the identification of several therapeutic targets, including FGFR. Erdafitinib has become the standard-of-care therapy for patients with FGFR2/3 alterations, after previous treatment with platinum-based chemotherapy and ICIs.
-
Designing clinical trials with adequate correlative biomarker studies is essential for broadening our knowledge of tumour biology, identifying new targets and developing more effective treatment combinations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Smith, A. B. et al. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU Int. 114, 719–726 (2014).
Burger, M. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 63, 234–241 (2013).
National Institutes of Health, National Cancer Institute. Cancer stat facts: bladder cancer. seer.cancer.gov, https://seer.cancer.gov/statfacts/html/urinb.html (2022).
Gupta, S. E. A. Defining “platinum-ineligible” patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 40, 4577 (2022).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
FDA. FDA approves avelumab for urothelial carcinoma maintenance treatment. FDA www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment (2020).
Rosenberg, J. E. A. Long-term outcomes in EV-301: 24-month findings from the phase 3 trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. J. Clin. Oncol. 40, 4516 (2022).
O’Donnell, P. H. et al. Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 41, 4107–4117 (2023).
Merck. Merck announces phase 3 KEYNOTE-A39/EV-302 trial met dual primary endpoints of overall survival (OS) and progression-free survival (PFS) in certain patients with previously untreated locally advanced or metastatic urothelial cancer. Merck www.merck.com/news/merck-announces-phase-3-keynote-a39-ev-302-trial-met-dual-primary-endpoints-of-overall-survival-os-and-progression-free-survival-pfs-in-certain-patients-with-previously-untreated-locally-advanced/ (2023).
Dash, A. et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107, 506–513 (2006).
Galsky, M. D. et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 29, 2432–2438 (2011).
Russell, B. M., Boussi, L. & Bellmunt, J. Management of advanced urothelial carcinoma in older and frail patients: have novel treatment approaches improved their care? Drugs Aging 39, 271–284 (2022).
Loehrer, P. J. Sr et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 10, 1066–1073 (1992).
von der Maase, H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 23, 4602–4608 (2005).
von der Maase, H. et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 18, 3068–3077 (2000).
Sternberg, C. N. et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer 42, 50–54 (2006).
Sternberg, C. N. et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin. Oncol. 19, 2638–2646 (2001).
Bellmunt, J. et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 30, 1107–1113 (2012).
Bamias, A. et al. Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: a Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Ann. Oncol. 29, 361–369 (2018).
De Santis, M. et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 30, 191–199 (2012).
Bellmunt, J., de Wit, R., Albanell, J. & Baselga, J. A feasibility study of carboplatin with fixed dose of gemcitabine in “unfit” patients with advanced bladder cancer. Eur. J. Cancer 37, 2212–2215 (2001).
Galsky, M. D. et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann. Oncol. 23, 406–410 (2012).
Powles, T. DANUBE post-hoc analysis: outcomes for durvalumab with or without tremelimumab by cisplatin eligibility and PD-L1 biomarker status in metastatic urothelial carcinoma. In 36th Annual EAU Congress, https://bladder.uroonco.uroweb.org/webcast/danube-post-hoc-analysis-outcomes-for-durvalumab-with-or-without-tremelimumab-by-cisplatin-eligibility-and-pd-l1-biomarker-status-in-metastatic-urothelial-carcinoma/ (Uroweb, 2021).
Grande, E. E. A. Overall survival (OS) by response to first-line (1L) induction treatment with atezolizumab (atezo) + platinum/gemcitabine (plt/gem) vs placebo + plt/gem in patients (pts) with metastatic urothelial carcinoma (mUC): updated data from the IMvigor130 OS final analysis. J. Clin. Oncol. 41, 4503 (2023).
Sridhar, S. S. et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): long-term follow-up from the JAVELIN Bladder 100 trial in subgroups defined by 1L chemotherapy regimen and analysis of overall survival (OS) from start of 1L chemotherapy. J. Clin. Oncol. 41, 3486–3492 (2023).
von der Maase, H., Andersen, L., Crino, L., Weinknecht, S. & Dogliotti, L. Weekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: a phase II clinical trial. Ann. Oncol. 10, 1461–1465 (1999).
Morales-Barrera, R. et al. Cisplatin and gemcitabine administered every two weeks in patients with locally advanced or metastatic urothelial carcinoma and impaired renal function. Eur. J. Cancer 48, 1816–1821 (2012).
Mourey, L. E. A. Vefora, GETUG-AFU V06 study: randomized multicenter phase II/III trial of fractionated cisplatin (CI)/gemcitabine (G) or carboplatin (CA)/g in patients (pts) with advanced urothelial cancer (UC) with impaired renal function (IRF) — results of a planned interim analysis. J. Clin. Oncol. 38, 461 (2020).
Douillard, J. Y. et al. Combined paclitaxel and gemcitabine as first-line treatment in metastatic non-small cell lung cancer: a multicentre phase II study. Br. J. Cancer 84, 1179–1184 (2001).
Calabro, F. et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer 115, 2652–2659 (2009).
Hussain, M., Vaishampayan, U., Du, W., Redman, B. & Smith, D. C. Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J. Clin. Oncol. 19, 2527–2533 (2001).
Galsky, M. D. et al. Phase II trial of dose-dense doxorubicin plus gemcitabine followed by paclitaxel plus carboplatin in patients with advanced urothelial carcinoma and impaired renal function. Cancer 109, 549–555 (2007).
De Santis, M. et al. Vinflunine-gemcitabine versus vinflunine-carboplatin as first-line chemotherapy in cisplatin-unfit patients with advanced urothelial carcinoma: results of an international randomized phase II trial (JASINT1). Ann. Oncol. 27, 449–454 (2016).
Loriot, Y. Avelumab (Ave) first-line (1L) maintenance plus best supportive care (BSC) versus BSC alone for advanced urothelial carcinoma (UC): JAVELIN Bladder 100 subgroup analysis based on duration and cycles of 1L chemotherapy. J. Clin. Oncol. 39, 438 (2021).
Raggi, D. et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann. Oncol. 27, 49–61 (2016).
Petrylak, D. P. et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial.Lancet 390, 2266–2277 (2017).
Nadal, R. & Bellmun, J. Management of metastatic bladder cancer.Cancer Treat Rev. 76, 10–21 (2019).
Bellmunt, J. et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 27, 4454–4461 (2009).
Bellmunt, J. et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann. Oncol. 24, 1466–1472 (2013).
Papamichael, D., Gallagher, C. J., Oliver, R. T., Johnson, P. W. & Waxman, J. Phase II study of paclitaxel in pretreated patients with locally advanced/metastatic cancer of the bladder and ureter. Br. J. Cancer 75, 606–607 (1997).
Vaughn, D. J., Broome, C. M., Hussain, M., Gutheil, J. C. & Markowitz, A. B. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J. Clin. Oncol. 20, 937–940 (2002).
McCaffrey, J. A. et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J. Clin. Oncol. 15, 1853–1857 (1997).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study.Lancet Oncol. 18, 1483–1492 (2017).
Rosenberg, J. E. et al. Atezolizumab monotherapy in cisplatin-ineligible patients with previously untreated metastatic urothelial carcinoma: 5-year response and survival analysis from the phase II IMvigor210 study (cohort 1). Ann. Oncol. 32, S678–S724 (2021).
O’Donnell, P. H. et al. First-line pembrolizumab (pembro) in cisplatin-ineligible patients with advanced urothelial cancer (UC): response and survival results up to five years from the KEYNOTE-052 phase 2 study. J. Clin. Oncol. 39, 4508 (2021).
Galsky, M. D. et al. Atezolizumab (atezo) + platinum/gemcitabine (plt/gem) vs placebo + plt/gem for first-line (1L) treatment (tx) of locally advanced or metastatic urothelial carcinoma (mUC): final OS from the randomized phase 3 IMvigor130 study. J. Clin. Oncol. 41, LBA440 (2023).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
FDA. FDA approves new, targeted treatment for bladder cancer. FDA www.fda.gov/news-events/press-announcements/fda-approves-new-targeted-treatment-bladder-cancer (2016).
FDA. Pembrolizumab (Keytruda): advanced or metastatic urothelial carcinoma. FDA www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-advanced-or-metastatic-urothelial-carcinoma (2017).
FDA. Nivolumab for treatment of urothelial carcinoma. FDA www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-treatment-urothelial-carcinoma (2017).
FDA. FDA grants accelerated approval to avelumab for urothelial carcinoma. FDA www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-avelumab-urothelial-carcinoma (2017).
Powles, T. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391, 748–757 (2018).
Bellmunt, J. et al. Pembrolizumab versus investigator’s choice of paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: 5-year follow-up from the phase 3 KEYNOTE-045 trial. J. Clin. Oncol. 39, 4532 (2021).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
Vaughn, D. J. et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J. Clin. Oncol. 36, 1579–1587 (2018).
Massard, C. et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 34, 3119–3125 (2016).
Powles, T. et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 3, e172411 (2017).
Powles, T. et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 21, 1574–1588 (2020).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial.Lancet Oncol. 18, 312–322 (2017).
Sharma, P. et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 17, 1590–1598 (2016).
Sharma, P. et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J. Clin. Oncol. 37, 1608–1616 (2019).
Sharma, P. et al. Nivolumab (N) alone or in combination with ipilimumab (I) in patients (pts) with platinum-pretreated metastatic urothelial carcinoma (mUC): extended follow-up from CheckMate 032. Ann. Oncol. 31, S550 (2020).
Apolo, A. B. et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J. Clin. Oncol. 35, 2117–2124 (2017).
Patel, M. R. et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 19, 51–64 (2018).
Grimm, M. O. et al. Safe use of immune checkpoint inhibitors in the multidisciplinary management of urological cancer: the European Association of Urology Position in 2019. Eur. Urol. 76, 368–380 (2019).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021).
Galsky, M. D. et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395, 1547–1557 (2020).
Powles, T. et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 931–945 (2021).
Galsky, M. D. et al. A phase 3, open-label, randomized study of nivolumab plus ipilimumab or standard of care (SOC) versus SOC alone in patients (pts) with previously untreated unresectable or metastatic urothelial carcinoma (mUC; CheckMate 901). J. Clin. Oncol. 36, TPS539 (2018).
van der Heijden, M. S. et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2309863 (2023).
Galsky, M. D. et al. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J. Clin. Oncol. 39, TPS504 (2021).
Perez Valderrama, B. et al. Phase II multicenter, randomized study to evaluate efficacy and safety of avelumab with gemcitabine/carboplatin (CG) vs CG alone in patients with unresectable or metastatic urothelial carcinoma (mUC) who are ineligible to receive cisplatin-based therapy. Ann. Oncol. 4, S1158–S1159 (2020).
Yu, E. M., Mudireddy, M., Biswas, R. & Aragon-Ching, J. B. The role of switch maintenance therapy in urothelial cancers. Ther. Adv. Urol. 15, 17562872221147760 (2023).
Powles, T. et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383, 1218–1230 (2020).
Powles, T. et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up.J. Clin. Oncol. 41, 3486–3492 (2023).
Garcia-Donas, J. et al. Maintenance therapy with vinflunine plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after first-line chemotherapy (MAJA; SOGUG 2011/02): a multicentre, randomised, controlled, open-label, phase 2 trial. Lancet Oncol. 18, 672–681 (2017).
Bellmunt Molins, J. et al. Final overall survival analysis of the SOGUG phase 2 MAJA study: maintenance vinflunine versus best supportive care after first-line chemotherapy in advanced urothelial carcinoma. Clin. Genitourin. Cancer 18, 452–460 (2020).
Galsky, M. D. et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J. Clin. Oncol. 38, 1797–1806 (2020).
Simon, C. et al. A randomized, double blind, biomarker selected, phase II clinical trial of maintenance PARP inhibition following chemotherapy for metastatic urothelial carcinoma (mUC): final analysis of the ATLANTIS rucaparib arm. J. Clin. Oncol. 40, 436 (2022).
Jones, R. et al. A randomised, double blind, phase II clinical trial of maintenance cabozantinib following chemotherapy for metastatic urothelial carcinoma (mUC): final analysis of the ATLANTIS cabozantinib comparison. J. Clin. Oncol. 40, LBA4505 (2022).
Vignani, F. et al. Randomized phase II study of niraparib plus best supportive care (BSC) versus BSC alone as maintenance treatment in patients with advanced urothelial carcinoma (UC) whose disease did not progress after first-line platinum-based chemotherapy (PBCT): the Meet-URO12 trial. J. Clin. Oncol. 40, 442 (2022).
Sonpavde, G. P. et al. Phase II trial of lurbinectedin combined with avelumab as maintenance therapy for metastatic urothelial carcinoma with stable or responding disease following platinum-based chemotherapy. J. Clin. Oncol. 41, TPS590 (2023).
Coquan, E. et al. TALASUR trial: a single arm phase II trial assessing efficacy and safety of talazoparib and avelumab as maintenance therapy in platinum-sensitive metastatic or locally advanced urothelial carcinoma. BMC Cancer 22, 1213 (2022).
Kertesz, N. et al. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood 107, 2330–2338 (2006).
Sadeghi, S. et al. EphrinB2 inhibition and pembrolizumab in metastatic urothelial carcinoma. J. Clin. Oncol. 41, 640–650 (2023).
Aggen, D. et al. Initial results from a phase 1a/b study of IK-175, an oral AHR inhibitor, as single agent and in combination with nivolumab in patients with advanced solid tumors and urothelial cancer. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-SITC2022.0661 (2022).
Luke, J. et al. BMS-986205, an indoleamine 2, 3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (nivo): updated safety across all tumor cohorts and efficacy in advanced bladder cancer (advBC). J. Clin. Oncol. 37, 358 (2019).
Siefker-Radtke, A. O. et al. Bempegaldesleukin plus nivolumab in first-line metastatic urothelial carcinoma: results from PIVOT-02. Eur. Urol. 82, 365–373 (2022).
Rosa, K. Bempegaldesleukin/nivolumab combo misses mark in RCC and urothelial cancer. OncLive www.onclive.com/view/bempegaldesleukin-nivolumab-combo-misses-mark-in-rcc-and-urothelial-cancer (2022).
Meeks, J. et al. A pilot study of tazemetostat and MK-3475 (pembrolizumab) in advanced urothelial carcinoma (ETCTN 10183). J. Clin. Oncol. 38, TPS607 (2020).
Tomlinson, D. C., Baldo, O., Harnden, P. & Knowles, M. A. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 213, 91–98 (2007).
Helsten, T. et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 22, 259–267 (2016).
Robertson, A. G. et al. Comprehensive molecular characterization of muscle-invasive bladder cancer.Cell 171, 540–556.e25 (2017).
Marandino, L., Raggi, D., Giannatempo, P., Fare, E. & Necchi, A. Erdafitinib for the treatment of urothelial cancer. Expert Rev. Anticancer Ther. 19, 835–846 (2019).
Loriot, Y. et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 381, 338–348 (2019).
Siefker-Radtke, A. O. et al. Management of fibroblast growth factor inhibitor treatment-emergent adverse events of interest in patients with locally advanced or metastatic urothelial carcinoma. Eur. Urol. Open Sci. 50, 1–9 (2023).
Loriot, Y. et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2308849 (2023).
Siefker-Radtke, A. O. et al. Erdafitinib versus pembrolizumab in pretreated patients with advanced or metastatic urothelial cancer with select FGFR alterations: cohort 2 of the randomized phase III THOR trial. Ann. Oncol. https://doi.org/10.1016/j.annonc.2023.10.003 (2023).
Lyou, Y. et al. Infigratinib and treatment response in advanced/unresectable or metastatic urothelial carcinoma in first-line and later-line treatment settings. J. Clin. Oncol. 38, 5038 (2020).
Flaherty, C. FIDES-02 data dail to support the development of derazantinib in metastatic urothelial cancer. OncLive www.onclive.com/view/fides-02-data-fail-to-support-the-development-of-derazantinib-in-metastatic-urothelial-cancer (2023).
Chaudhry, A. et al. FIDES-02, a phase Ib/II study of derazantinib (DZB) as monotherapy and combination therapy with atezolizumab (A) in patients with surgically unresectable or metastaticurothelial cancer (UC) and FGFR genetic aberrations. J. Clin. Oncol. 38, TPS590 (2020).
Sternberg, C. N. et al. FORT-1: phase II/III study of rogaratinib versus chemotherapy in patients with locally advanced or metastatic urothelial carcinoma selected based on FGFR1/3 mRNA expression. J. Clin. Oncol. 41, 629–639 (2023).
Powles, T. et al. Erdafitinib (ERDA) or ERDA plus cetrelimab (CET) for patients with metastatic or locally advanced urothelial carcinoma (mUC) and fibroblast growth factor receptor alterations (FGFRa): first phase (Ph) II results from the NORSE study. Ann. Oncol. 32, S1283–S1346 (2021).
URO Today. ESMO 2021: the phase 2 NORSE trial discussion: erdafitinib or erdafitinib plus cetrelimab for patients with metastatic or locally advanced urothelial carcinoma and FGFR alterations. URO Today www.urotoday.com/conference-highlights/esmo-2021/esmo-2021-bladder-cancer/132175-esmo-2021-invited-discussant-the-phase-2-norse-trial.html (2021).
Rosenberg, J. et al. Safety and efficacy of rogaratinib in combination with atezolizumab in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression in the phase Ib/II FORT-2 study. J. Clin. Oncol. 39, 4521 (2021).
Hoffman-Censits, J. H. et al. Expression of nectin-4 in bladder urothelial carcinoma, in morphologic variants, and nonurothelial histotypes. Appl. Immunohistochem. Mol. Morphol. 29, 619–625 (2021).
Challita-Eid, P. M. et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
Doronina, S. O. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 21, 778–784 (2003).
Rosenberg, J. E. et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 37, 2592–2600 (2019).
Powles, T. et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384, 1125–1135 (2021).
Balar, A. et al. EV-201 cohort 2: enfortumab vedotin in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer who received prior PD-1/PD-L1 inhibitors. J. Clin. Oncol. 39, 394 (2021).
Seagen. U.S. FDA grants regular approval and expands indication for PADCEV® (enfortumab vedotin-ejfv) for patients with locally advanced or metastatic urothelial cancer. Seagen investor.seagen.com/press-releases/news-details/2021/U.S.-FDA-Grants-Regular-Approval-and-Expands-Indication-for-PADCEV-enfortumab-vedotin-ejfv-for-Patients-with-Locally-Advanced-or-Metastatic-Urothelial-Cancer/default.aspx (2021).
Hoimes, C. J. et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J. Clin. Oncol. 41, 22–31 (2023).
Rosenberg, J. E. et al. Study EV-103 cohort K: antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann. Oncol. 33, S808–S869 (2022).
Gupta, A. et al. Study EV-103 dose escalation/cohort A: long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1L) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. J. Clin. Oncol. 41, 4505 (2023).
Friedlander, T. W. et al. Enfortumab vedotin (EV) with or without pembrolizumab (P) in patients (pts) who are cisplatin-ineligible with previously untreated locally advanced or metastatic urothelial cancer (la/mUC): additional 3-month follow-up on cohort K data. J. Clin. Oncol. 41, 4568 (2023).
O’Donnell, P. H. et al. Enfortumab vedotin (EV) alone or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer (la/mUC): subgroup analyses of confirmed objective response rate (cORR) from EV-103 cohort K. J. Clin. Oncol. 41, 4107–4117 (2023).
FDA. FDA grants regular approval to enfortumab vedotin-ejfv for locally advanced or metastatic urothelial cancer. FDA www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer (2021).
Powles, T. B. et al. LBA6 EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann. Oncol. 34, S1340 (2023).
Drakaki, A. et al. Phase Ib/II umbrella trial to evaluate the safety and efficacy of multiple 2L cancer immunotherapy (CIT) combinations in advanced/metastatic urothelial carcinoma (mUC): MORPHEUS-mUC. J. Clin. Oncol. 38, TPS591 (2020).
Tagawa, S. T. et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 39, 2474–2485 (2021).
Tagawa, S. et al. Updated outcomes in TROPHY-U-01 cohort 1, a phase 2 study of sacituzumab govitecan (SG) in patients (pts) with metastatic urothelial cancer (mUC) that progressed after platinum (PT)-based chemotherapy and a checkpoint inhibitor (CPI). J. Clin. Oncol. 41, 526 (2023).
Grivas, P. et al. TROPiCS-04: study of sacituzumab govitecan in metastatic or locally advanced unresectable urothelial cancer that has progressed after platinum and checkpoint inhibitor therapy. J. Clin. Oncol. 39, TPS498 (2021).
Lattanzi, M. et al. Incidence and clinical outcomes of HER2-altered bladder cancer (BC) patients (pts). J. Clin. Oncol. 40, 556 (2022).
Choudhury, N. J. et al. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J. Clin. Oncol. 34, 2165–2171 (2016).
Culine, S. et al. Combining paclitaxel and lapatinib as second-line treatment for patients with metastatic transitional cell carcinoma: a case series. Anticancer Res. 32, 3949–3952 (2012).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Li, H. et al. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer. Cancer Biol. Ther. 17, 346–354 (2016).
Staudacher, A. H. & Brown, M. P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer 117, 1736–1742 (2017).
Xu, H. et al. A phase II study of RC48-ADC in HER2-negative patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 40, 4519 (2022).
Sheng, X. et al. C48-ADC for metastatic urothelial carcinoma with HER2-positive: combined analysis of RC48-C005 and RC48-C009 trials. J. Clin. Oncol. 40, 4520 (2022).
Sheng, X. et al. Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 40, 4518 (2022).
Galsky, M. D. et al. Primary analysis from DS8201-A-U105: a phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J. Clin. Oncol. 40, 438 (2022).
Mudd, G. E. et al. Discovery of BT8009: a nectin-4 targeting bicycle toxin conjugate for the treatment of cancer. J. Med. Chem. 65, 14337–14347 (2022).
Baldini, C. et al. BT8009-100: a phase I/II study of novel bicyclic peptide and MMAE conjugate BT8009 in patients (pts) with advanced malignancies associated with nectin-4 expression, including urothelial cancer (UC). J. Clin. Oncol. 41, 498 (2023).
Gerullis, H. et al. Combined treatment with pazopanib and vinflunine in patients with advanced urothelial carcinoma refractory after first-line therapy. Anticancer Drugs 24, 422–425 (2013).
Krege, S. et al. Prospective randomized double-blind multicentre phase II study comparing gemcitabine and cisplatin plus sorafenib chemotherapy with gemcitabine and cisplatin plus placebo in locally advanced and/or metastasized urothelial cancer: SUSE (AUO-AB 31/05). BJU Int. 113, 429–436 (2014).
Apolo, A. B. et al. Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: an open-label, single-centre, phase 2 trial. Lancet Oncol. 21, 1099–1109 (2020).
Apolo, A. B. et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J. Clin. Oncol. 38, 3672–3684 (2020).
Pal, S. et al. Cabozantinib in combination with atezolizumab in urothelial carcinoma previously treated with platinum-containing chemotherapy: results from cohort 2 of the COSMIC-021 study. J. Clin. Oncol. 38, 5013 (2020).
Pal, S. K. et al. Cabozantinib (C) in combination with atezolizumab (A) in urothelial carcinoma (UC): results from cohorts 3, 4, 5 of the COSMIC-021 study. J. Clin. Oncol. 40, 4504 (2022).
Loriot, Y. et al. First-line pembrolizumab (pembro) with or without lenvatinib (lenva) in patients with advanced urothelial carcinoma (LEAP-011): a phase 3, randomized, double-blind study. J. Clin. Oncol. 40, 432 (2022).
Msaouel, P. et al. Sitravatinib (sitra) in combination with nivolumab (nivo) demonstrates clinical activity in checkpoint inhibitor (CPI) naïve, platinum-experienced patients (pts) with advanced or metastatic urothelial carcinoma (UC). Ann. Oncol. 31, S556 (2020).
Van Allen, E. M. et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 4, 1140–1153 (2014).
Teo, M. Y. et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 36, 1685–1694 (2018).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Grivas, P. et al. Efficacy and safety of rucaparib in previously treated, locally advanced or metastatic urothelial carcinoma from a phase 2, open-label trial (ATLAS). BMC Cancer 21, 593 (2021).
Sweis, R. F. et al. Clinical activity of olaparib in urothelial bladder cancer with DNA damage response gene mutations. JCO Precis. Oncol. 2, 1–7 (2018).
Powles, T. et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat. Med. 27, 793–801 (2021).
Rosenberg, J. E. et al. Durvalumab plus olaparib in previously untreated, platinum-ineligible patients with metastatic urothelial carcinoma: a multicenter, randomized, phase II trial (BAYOU). J. Clin. Oncol. 41, 43–53 (2023).
Joerger, M. et al. Rogaratinib in patients with advanced urothelial carcinomas prescreened for tumor FGFR mRNA expression and effects of mutations in the FGFR signaling pathway. J. Clin. Oncol. 36, 4513 (2018).
Rezazadeh, A. et al. An observational study of outcomes of patients (pts) with advanced urothelial carcinoma (UC) after anti-programmed death-(ligand) 1 (PD-[L]1) therapy by fibroblast growth factor receptor gene alteration (FGFRa) status. Ann. Oncol. 31, S586–S587 (2020).
Wang, L. et al. Fibroblast growth factor receptor 3 alterations and response to PD-1/PD-L1 blockade in patients with metastatic urothelial cancer. Eur. Urol. 76, 599–603 (2019).
Rose, T. L. et al. Fibroblast growth factor receptor 3 alterations and response to immune checkpoint inhibition in metastatic urothelial cancer: a real world experience. Br. J. Cancer 125, 1251–1260 (2021).
McGregor, B. A. et al. Sacituzumab govitecan (SG) plus enfortumab vedotin (EV) for metastatic urothelial carcinoma (UC) progressing on platinum-based chemotherapy and PD1/L1 inhibitors (ICB): double antibody drug conjugate (DAD) phase I trial. J. Clin. Oncol. 40, TPS588 (2022).
McGregor, B. A. et al. The Double Antibody Drug conjugate (DAD) phase I trial: sacituzumab govitecan plus enfortumab vedotin for metastatic urothelial carcinoma. Ann. Oncol. https://doi.org/10.1016/j.annonc.2023.09.3114 (2023).
Koshkin, V. S. et al. Enfortumab vedotin (EV) outcomes with and without immediate prior immune checkpoint inhibitor (ICI) in patients (pts) with advanced urothelial carcinoma (aUC). J. Clin. Oncol. 41, 514 (2023).
Molina, G. E., Schwartz, B., Srinivas, S., Shah, S. & Zaba, L. C. In patients with advanced urothelial carcinoma, immune checkpoint inhibition prior to enfortumab vedotin is associated with high-grade skin toxicity. Eur. Urol. 83, 377–378 (2023).
Vlachou, E. et al. Enfortumab vedotin-related cutaneous toxicity and radiographic response in patients with urothelial cancer: a single-center experience and review of the literature. Eur. Urol. Open Sci. 49, 100–103 (2023).
Grimm, M. O. et al. Tailored immunotherapy approach with nivolumab with or without ipilimumab in patients with advanced transitional cell carcinoma after platinum-based chemotherapy (TITAN-TCC): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 24, 347–359 (2023).
Girardi, D. M. et al. Cabozantinib plus nivolumab phase I expansion study in patients with metastatic urothelial carcinoma refractory to immune checkpoint inhibitor therapy. Clin. Cancer Res. 28, 1353–1362 (2022).
Hack, J. & Crabb, S. J. Platinum-based chemotherapy ‘rechallenge’ in advanced non-ovarian solid malignancies. Clin. Oncol. 34, e329–e344 (2022).
Wong, R. L. et al. Efficacy of platinum rechallenge in metastatic urothelial carcinoma after previous platinum-based chemotherapy for metastatic disease. Oncologist 26, 1026–1034 (2021).
Bellmunt, J. et al. Long-term outcomes in patients with advanced urothelial carcinoma (UC) who received avelumab first-line (1L) maintenance with or without second-line (2L) treatment: exploratory analyses from JAVELIN Bladder 100. J. Clin. Oncol. 40, 4560 (2022).
Ogihara, K. et al. Can urologists introduce the concept of “oligometastasis” for metastatic bladder cancer after total cystectomy? Oncotarget 8, 111819–111835 (2017).
Decaestecker, K., Fonteyne, V. & Oosterlinck, W. Perspective on cytoreduction and metastasis-directed therapy in node positive and metastatic urothelial carcinoma of the bladder. Transl. Androl. Urol. 6, 1117–1122 (2017).
Shah, S. et al. Consolidative radiotherapy in metastatic urothelial cancer. Clin. Genitourin. Cancer 15, 685–688 (2017).
Manig, L., Kasmann, L., Janssen, S. & Rades, D. Predicting survival after irradiation of metastases from transitional carcinoma of the bladder. Anticancer Res. 36, 6663–6665 (2016).
Leonetti, A. et al. Radiotherapy for the treatment of distant nodes metastases from oligometastatic urothelial cancer: a retrospective case series. Int. J. Urol. 25, 879–886 (2018).
Miranda, A. F. et al. Metastasis-directed radiation therapy after radical cystectomy for bladder cancer. Urol. Oncol. 39, 790.e1–790.e7 (2021).
Flaig, T. W. et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 18, 329–354 (2020).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Powles, T., Walker, J., Andrew Williams, J. & Bellmunt, J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat. Rev. 82, 101925 (2020).
Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614.e14 (2021).
Bellmunt, J. et al. Association of TMB with efficacy of pembrolizumab (pembro) in patients (pts) with advanced urothelial cancer (UC): results from KEYNOTE-045 and KEYNOTE-052. Ann. Oncol. 31, S580–S581 (2020).
Vuky, J. et al. Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 38, 2658–2666 (2020).
Kamoun, A. et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 77, 420–433 (2020).
Rebouissou, S. et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl. Med. 6, 244ra291 (2014).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017).
Powles, T., Petrylak, D. P. & Rosenberg, J. E. Beyond chemotherapy and checkpoint inhibitors: weighing the risks and benefits of the novel therapies for metastatic urothelial carcinoma. J. Clin. Oncol. 39, 3411–3412 (2021).
Bellmunt, J. et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann. Oncol. 18, 522–528 (2007).
Urun, Y. et al. ERCC1 as a prognostic factor for survival in patients with advanced urothelial cancer treated with platinum based chemotherapy: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 120, 120–126 (2017).
Taber, A. et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat. Commun. 11, 4858 (2020).
Kim, J. et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat. Genet. 48, 600–606 (2016).
Mullane, S. A. et al. Expression levels of DNA damage repair proteins are associated with overall survival in platinum-treated advanced urothelial carcinoma. Clin. Genitourin. Cancer 14, 352–359 (2016).
EMA. EMA restricts use of Keytruda and Tecentriq in bladder cancer. EMA www.ema.europa.eu/en/news/ema-restricts-use-keytruda-tecentriq-bladder-cancer (2018).
Rui, X., Gu, T. T., Pan, H. F. & Zhang, H. Z. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: a meta-analysis. Int. Immunopharmacol. 67, 378–385 (2019).
Powles, T. et al. Avelumab first-line (1L) maintenance + best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma (UC): association between clinical outcomes and exploratory biomarkers. Ann. Oncol. 31, S552–S553 (2020).
Quinn, D. et al. FORT-1: phase II/III study of rogaratinib versus chemotherapy (CT) in patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) selected based on FGFR1/3 mRNA expression. J. Clin. Oncol. 38, 489 (2020).
Grivas, P. D. et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer 120, 692–701 (2014).
Powles, T. et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J. Clin. Oncol. 35, 48–55 (2017).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
B.P.V. has acted as an adviser of AAA, Astellas Pharma, Bayer, Bristol-Myers Squibb, EUSA Pharma, Ipsen, Merck, MSD, Novartis and Pfizer; has been an invited speaker for Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb, EUSA Pharma, Janssen, Merck, MSD, Pfizer and Roche; and has received travel support from Merck and Pfizer. J.B. has acted as a consultant and/or adviser of Astellas Pharma, AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech, Merck, Pierre Fabre, Pfizer and Takeda; has received research funding from Pfizer/EMD Serono, Millennium and Sanofi and trial sponsorship from Associació per a la Recerca Oncológia (APRO); has received royalties from UpToDate; owns stocks in Rainier Therapeutics; and declares a non-financial interest as President of APRO. R.N. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks P. S. Hoon, Y. Loriot, N. Naoun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nadal, R., Valderrama, B.P. & Bellmunt, J. Progress in systemic therapy for advanced-stage urothelial carcinoma. Nat Rev Clin Oncol 21, 8–27 (2024). https://doi.org/10.1038/s41571-023-00826-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00826-2