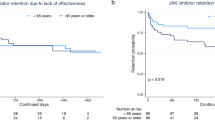
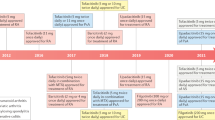
Abstract
Janus kinase (JAK) inhibitors, including tofacitinib, baricitinib, upadacitinib and filgotinib, are increasingly used in the treatment of rheumatoid arthritis (RA). There has been debate about their safety, particularly following the issuance of guidance by regulatory agencies advising caution in their use in certain patients. The registrational clinical trials and registry data of JAK inhibitors did not identify a difference in the risk of major adverse cardiovascular events (MACEs), venous thromboembolism, malignancies or infections (other than herpes zoster) with a JAK inhibitor versus a biologic DMARD. In the ORAL Surveillance trial, which enrolled patients >50 years of age with ≥1 cardiovascular risk factor, tofacitinib was statistically not non-inferior to TNF inhibitors for the occurrence of MACEs and malignancy. Further post hoc analysis of the data revealed that an age of ≥65 years, a high baseline cardiovascular risk, a history of smoking, sustained inflammation, disease activity and suboptimal treatment of cardiovascular comorbidities all increase the risk of these outcomes. The guidance issued by regulatory agencies should be carefully considered to ensure appropriate and safe treatment of patients with RA without undertreatment of patients who might benefit from JAK inhibitor, as well as biologic, treatment. As always, the risks associated with the use of these agents, treatment goals, costs and patient preferences should be discussed with the patient.
Key points
-
Registrational clinical trials and registries of Janus kinase (JAK) inhibitors did not identify a difference in the risk of major adverse cardiovascular events (MACEs), venous thromboembolism, malignancies or infections versus biologic DMARDs.
-
The post-marketing ORAL Surveillance trial enrolled patients with rheumatoid arthritis (RA) >50 years of age with ≥1 cardiovascular risk factor to assess the relative risk of a JAK inhibitor versus a TNF inhibitor.
-
In ORAL Surveillance, tofacitinib was not non-inferior to TNF inhibitor therapy for the occurrence of MACEs and malignancy, although this finding does not mean that tofacitinib is inferior to TNF inhibitor therapy.
-
Post hoc analyses of the trial data revealed that an age of ≥65 years, a history or high risk of cardiovascular disease, smoking, active RA and suboptimal treatment of cardiovascular comorbidities all increased the risk of primarily MACEs and malignancies, but also venous thromboembolism and serious infections.
-
The benefit:risk ratio of JAK inhibitors strongly favours their use in the vast majority of patients.
-
Guidance from regulatory agencies and professional bodies on the use of JAK inhibitors should be considered carefully to ensure appropriate treatment of patients with RA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
01 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41584-024-01085-w
References
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82, 3–18 (2023).
Fraenkel, L. et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 73, 1108–1123 (2021).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 4, 18001 (2018).
O’Shea, J. J. Targeting the Jak/STAT pathway for immunosuppression. Ann. Rheum. Dis. 63, ii67–ii71 (2004).
Fleischmann, R. Novel small-molecular therapeutics for rheumatoid arthritis. Curr. Opin. Rheumatol. 24, 335–341 (2012).
Winthrop, K. L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13, 234–243 (2017).
Szekanecz, Z., Hamar, A. & Soós, B. [Safety issues of JAK inhibitors in rheumatoid arthritis]. Immunol. Q. 13, 5–20 (2021).
van der Heijde, D. et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 65, 559–570 (2013).
Kremer, J. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 159, 253–261 (2013).
van Vollenhoven, R. F. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367, 508–519 (2012).
Burmester, G. R. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381, 451–460 (2013).
Fleischmann, R. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507 (2012).
Fleischmann, R. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69, 506–517 (2017).
Taylor, P. C. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376, 652–662 (2017).
Dougados, M. et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann. Rheum. Dis. 76, 88–95 (2017).
Genovese, M. C. et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374, 1243–1252 (2016).
Burmester, G. R. et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 2503–2512 (2018).
van Vollenhoven, R. et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 72, 1607–1620 (2020).
Fleischmann, R. M. et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann. Rheum. Dis. 78, 1454–1462 (2019).
Genovese, M. C. et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 391, 2513–2524 (2018).
Smolen, J. S. et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 393, 2303–2311 (2019).
Genovese, M. C. et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. J. Am. Med. Assoc. 322, 315–325 (2019).
Combe, B. et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann. Rheum. Dis. 80, 848–858 (2021).
Westhovens, R. et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann. Rheum. Dis. 80, 727–738 (2021).
Ytterberg, S. R. et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 386, 316–326 (2022).
Lauper, K. & Hyrich, K. L. How effective are JAK-inhibitors? Perspectives from clinical trials and real-world studies. Expert Rev. Clin. Immunol. 18, 207–220 (2022).
Nash, P. et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann. Rheum. Dis. 80, 71–87 (2021).
Baillet, A. et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann. Rheum. Dis. 75, 965–973 (2016).
Agca, R. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76, 17–28 (2017).
Choy, E., Ganeshalingam, K., Semb, A. G., Szekanecz, Z. & Nurmohamed, M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 53, 2143–2154 (2014).
Choi, H. K. et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann. Rheum. Dis. 72, 1182–1187 (2013).
Szekanecz, Z. et al. Eight pillars of oncorheumatology: crossroads between malignancies and musculoskeletal diseases. Autoimmun. Rev. 19, 102658 (2020).
Bosco, E., Hsueh, L., McConeghy, K. W., Gravenstein, S. & Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med. Res. Methodol. 21, 241 (2021).
Kerschbaumer, A. et al. Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 82, 95–106 (2023).
Fleischmann, R. et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390, 457–468 (2017).
Rubbert-Roth, A. et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N. Engl. J. Med. 383, 1511–1521 (2020).
Lee, E. B. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370, 2377–2386 (2014).
Sholter, D. et al. Maintenance of patient-reported outcomes in baricitinib-treated patients with moderate-to-severe active rheumatoid arthritis: post hoc analyses from two phase 3 trials. Rheumatol. Ther. 9, 541–553 (2022).
Conaghan, P. et al. Benefit-risk analysis of upadacitinib compared with adalimumab in the treatment of patients with moderate-to-severe rheumatoid arthritis. Rheumatol. Ther. 9, 191–206 (2022).
Atsumi, T. et al. Number needed to treat and cost per responder of Janus kinase inhibitors approved for the treatment of moderate-to-severe rheumatoid arthritis in Japan. Mod. Rheumatol. 33, 54–63 (2023).
Atzeni, F. et al. Cardiovascular effects of approved drugs for rheumatoid arthritis. Nat. Rev. Rheumatol. 17, 270–290 (2021).
Salinas, C. A. et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol. Ther. 10, 201–223 (2023).
Smolen, J. S. et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J. Rheumatol. 46, 7–18 (2019).
Taylor, P. C., Abdul Azeez, M. & Kiriakidis, S. Filgotinib for the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs 26, 1181–1187 (2017).
Curtis, J. R. et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann. Rheum. Dis. 75, 831–841 (2016).
Harigai, M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology 58, i34–i42 (2019).
Fleischmann, R. et al. Safety profile of upadacitinib in patients at risk of cardiovascular disease: integrated post hoc analysis of the SELECT phase III rheumatoid arthritis clinical programme. Ann. Rheum. Dis. 82, 1130–1141 (2023).
Khosrow-Khavar, F., Desai, R. J., Lee, H., Lee, S. B. & Kim, S. C. Tofacitinib and risk of malignancy: results from the safety of tofacitinib in routine care patients with rheumatoid arthritis (STAR-RA) study. Arthritis Rheumatol. 74, 1648–1659 (2022).
Khosrow-Khavar, F., Kim, S. C., Lee, H., Lee, S. B. & Desai, R. J. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann. Rheum. Dis. 81, 798–804 (2022).
Russell, M. D. et al. JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications. Ann. Rheum. Dis. 82, 1059–1067 (2023).
Burmester, G. R. et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 9, e002735 (2023).
Winthrop, K. L. et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 69, 1960–1968 (2017).
Myasoedova, E. et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 70, 482–487 (2011).
McInnes, I. B. et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann. Rheum. Dis. 73, 124–131 (2014).
Winthrop, K. L. et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1133–1138 (2015).
Queeney, K., Housley, W., Sokolove, J. & Long, A. Elucidating the mechanism underlying creatine phosphokinase upregulation with upadacitinib [abstract]. Ann. Rheum. Dis. 78, 734–735 (2019).
Choy, E. H. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology 58, 953–962 (2019).
Cohen, S. B. et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 6, e001395 (2020).
Taylor, P. C. et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann. Rheum. Dis. 81, 335–343 (2022).
Cohen, S. B. et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann. Rheum. Dis. 30, 304–311 (2020).
Winthrop, K. L. et al. Integrated safety analysis of filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 1.6 years. Ann. Rheum. Dis. 81, 184–192 (2022).
Banerjee, S. Spatial data analysis. Annu. Rev. Public. Health 37, 47–60 (2016).
Mease, P. et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann. Rheum. Dis. 79, 1400–1413 (2020).
Kremer, J. M. et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a united states-based rheumatoid arthritis registry. ACR Open Rheumatol. 3, 173–184 (2021).
Maneiro, J. R., Souto, A. & Gomez-Reino, J. J. Risks of malignancies related to tofacitinib and biological drugs in rheumatoid arthritis: systematic review, meta-analysis, and network meta-analysis. Semin. Arthritis Rheum. 47, 149–156 (2017).
Robertson, J., Peters, M. J., McInnes, I. B. & Sattar, N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat. Rev. Rheumatol. 9, 513–523 (2013).
Charles-Schoeman, C. et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 67, 616–625 (2015).
Charles-Schoeman, C. et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin. Arthritis Rheum. 46, 71–80 (2016).
Isaacs, J. D. et al. Changes in serum creatinine in patients with active rheumatoid arthritis treated with tofacitinib: results from clinical trials. Arthritis Res. Ther. 16, R158 (2014).
Panaccione, R. et al. Characterization of creatine kinase levels in tofacitinib-treated patients with ulcerative colitis: results from clinical trials. Dig. Dis. Sci. 66, 2732–2743 (2021).
Curtis, J. R. et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 35, 390–400 (2017).
Cohen, S. B. et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann. Rheum. Dis. 76, 1253–1262 (2017).
Choy, E. H. S. et al. The effect of JAK1/JAK2 inhibition in rheumatoid arthritis: efficacy and safety of baricitinib. Clin. Exp. Rheumatol. 37, 694–704 (2019).
Harigai, M. et al. Safety profile of baricitinib in Japanese patients with active rheumatoid arthritis with over 1.6 years median time in treatment: an integrated analysis of Phases 2 and 3 trials. Mod. Rheumatol. 30, 36–43 (2020).
Taylor, P. C. et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 71, 1042–1055 (2019).
Kremer, J. M. et al. Effects of baricitinib on lipid, apolipoprotein, and lipoprotein particle profiles in a phase IIb study of patients with active rheumatoid arthritis. Arthritis Rheumatol. 69, 943–952 (2017).
Taylor, P. C. et al. Lipid profile and effect of statin treatment in pooled phase II and phase III baricitinib studies. Ann. Rheum. Dis. 77, 988–995 (2018).
Fleischmann, R. et al. Safety and efficacy of baricitinib in elderly patients with rheumatoid arthritis. RMD Open 3, e000546 (2017).
Serhal, L. & Edwards, C. J. Upadacitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Immunol. 15, 13–25 (2018).
Hellstrom, W. J. G. et al. MANTA and MANTA-ray: rationale and design of trials evaluating effects of filgotinib on semen parameters in patients with inflammatory diseases. Adv. Ther. 39, 3403–3422 (2022).
Reinisch, W. et al. Effects of filgotinib on semen parameters and sex hormones in male patients with inflammatory diseases: results from the phase 2, randomised, double-blind, placebo-controlled MANTA and MANTA-RAy studies. Ann. Rheum. Dis. 82, 1049–1058 (2023).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Giles, J. T. et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 72, 31–40 (2020).
Charles-Schoeman, C. et al. Risk of venous thromboembolism with tofacitinib versus tumor necrosis factor inhibitors in cardiovascular risk-enriched rheumatoid arthritis patients. Arthritis Rheumatol. 82, 901–910 (2023).
Charles-Schoeman, C. et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann. Rheum. Dis. 82, 119–129 (2023).
Balanescu, A. R. et al. Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL Surveillance trial. Ann. Rheum. Dis. 81, 1491–1503 (2022).
Curtis, J. R. et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann. Rheum. Dis. 82, 331–343 (2023).
Dougados, M. et al. Impact of cardiovascular risk enrichment on incidence of major adverse cardiovascular events in the tofacitinib rheumatoid arthritis clinical programme. Ann. Rheum. Dis. 82, 575–577 (2023).
Giles, J. et al. Association between baseline statin treatment and major adverse cardiovascular events in patients with rheumatoid arthritis: a post hoc analysis of ORAL Surveillance [abstract]. Ann. Rheum. Dis. 81, 518–519 (2022).
Karpouzas, G. et al. Relationship between disease activity and major adverse events in patients with rheumatoid arthritis on tofacitinib or TNF inhibitors: a post hoc analysis of ORAL Surveillance [abstract]. Ann. Rheum. Dis. 81, 517–518 (2022).
Szekanecz, Z. et al. Incidence of major adverse cardiovascular events stratified by geographic region and baseline cardiovascular risk: a post hoc analysis of ORAL Surveillance [abstract]. Ann. Rheum. Dis. 81, 278–279 (2022).
Weitz, J. I. et al. Biomarkers to predict risk of venous thromboembolism in patients with rheumatoid arthritis receiving tofacitinib or tumour necrosis factor inhibitors. RMD Open 8, e002571 (2022).
Kristensen, L. E. et al. Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance. Ann. Rheum. Dis. 82, 901–910 (2023).
Karpouzas, G. A. et al. Rheumatoid arthritis disease activity and adverse events in patients receiving tofacitinib or tumor necrosis factor inhibitors: a post hoc analysis of ORAL Surveillance. Ther. Adv. Musculoskelet. Dis. 15, 1759720X231201047 (2023).
Lau, E. S. et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol 3, 48–58 (2021).
Fleischmann, R. Recent issues in JAK inhibitor safety: perspective for the clinician. Expert Rev. Clin. Immunol. 18, 295–307 (2022).
Singh, J. A. Risks and benefits of Janus kinase inhibitors in rheumatoid arthritis — past, present, and future. N. Engl. J. Med. 386, 387–389 (2022).
Szekanecz, Z., Kerekes, G. & Soltesz, P. Vascular effects of biologic agents in RA and spondyloarthropathies. Nat. Rev. Rheumatol. 5, 677–684 (2009).
Meissner, Y. et al. Risk of cardiovascular events under Janus kinase inhibitors in patients with rheumatoid arthritis: observational data from the German RABBIT register [abstract]. Ann. Rheum. Dis. 82, 86–87 (2023).
European Medicines Agency. Xeljanz. https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz (2017).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79, 685–699 (2020).
European Medicines Agency. PRAC recommendations on signals. https://go.nature.com/3vxL0Sj (5 July 2021).
U.S. Food & Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. https://go.nature.com/3NR9FYA (7 December 2021).
European Medicines Agency. EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. https://go.nature.com/48tdDis (11 November 2022).
European Medicines Agency Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 9–12 January 2023. https://go.nature.com/3vihPCI (13 January 2023).
Greenberg, J. D., Furer, V. & Farkouh, M. E. Cardiovascular safety of biologic therapies for the treatment of RA. Nat. Rev. Rheumatol. 8, 13–21 (2011).
Hamar, A. et al. Prospective, simultaneous assessment of joint and vascular inflammation by PET/CT in tofacitinib-treated patients with rheumatoid arthritis: associations with vascular and bone status. RMD Open 7, e001804 (2021).
Soos, B. et al. Effects of tofacitinib therapy on arginine and methionine metabolites in association with vascular pathophysiology in rheumatoid arthritis: a metabolomic approach. Front. Med. 9, 1011734 (2022).
Kume, K. et al. Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatol. Int. 37, 2079–2085 (2017).
Askling, J. et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis Rheum. 60, 3180–3189 (2009).
Ramiro, S. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 76, 1101–1136 (2017).
Author information
Authors and Affiliations
Contributions
Z.S., A.H. and R.F. researched data for the article. All authors contributed substantially to discussion of the content. Z.S., A.H. and R.F. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
Z.S. declares that he has acted as a consultant for AbbVie, Lilly and Pfizer and has received research grants from Pfizer. M.H.B. declares that she has been a consultant for AbbVie, Galapagos and Pfizer. C.C.-S. declares that she has acted as a consultant for AbbVie, Galapagos and Pfizer and has received grants from AbbVie and Pfizer. J.G. declares that he has acted as a consultant for AbbVie, Galapagos, Lilly and Pfizer. G.A.K. declares that he has received research grants from Pfizer. L.E.K. declares that he has acted as a consultant for AbbVie, Galapagos, Lilly and Pfizer. S.R.Y. declares that he has acted as a consultant for Pfizer. R.F. declares that he has acted as a consultant for AbbVie, Galapagos, Lilly and Pfizer. A.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Eduardo Mysler, who co-reviewed with Ana Lizarraga, Andrea Rubbert-Roth and Yoshiya Tanaka for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Szekanecz, Z., Buch, M.H., Charles-Schoeman, C. et al. Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician. Nat Rev Rheumatol 20, 101–115 (2024). https://doi.org/10.1038/s41584-023-01062-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-01062-9
This article is cited by
-
Proposals for the rheumatological use of JAK inhibitors
Nature Reviews Rheumatology (2024)