Abstract
In real-world scenarios, Janus Kinase (JAK) inhibitors are often offered to "difficult-to-treat" rheumatoid arthritis patients, quite different from those included in randomized controlled trials. Our study aimed to evaluate the influence of patient-related factors on the effectiveness and safety of JAK inhibitors in real-world clinical practice. This observational retrospective study involved rheumatoid arthritis patients who received treatment with either tofacitinib, baricitinib, upadacitinib, or filgotinib. At 12 months of treatment, reasons for and rates of JAK inhibitor treatment discontinuation were examined. Treatment retentions were analyzed through Cox proportional hazard regression models and Kaplan–Meier estimates. Patient-related factors that could influence treatment retention were evaluated for the discontinuation reasons of lack of effectiveness and adverse events. At 12 months of treatment, discontinuation rates for 189 JAK inhibitor treatments were: lack of effectiveness (24.3%), adverse events (20.6%), and other reasons (3.7%). The remaining 51.4% represents the treatment continuation rate. No patient-related factors evaluated had an influence on treatment discontinuation due to lack of effectiveness. Ae significantly increased the risk of treatment discontinuation due to adverse events (p = 0.030). In terms of age, at 12 month of treatment, discontinuation rates due to adverse events were: < 65 years, 14.4% vs. 65 years or older, 26.3% (p = 0.019). Rheumatoid arthritis patients aged 65 years or older showed an increased risk of JAK inhibitor treatment discontinuation due to adverse events. Factors not related to treatment discontinuation were: sex, rheumatoid arthritis disease duration, rheumatoid arthritis disease activity, seropositivity for rheumatoid factor, seropositivity for anti-cyclic citrullinated peptides, number of prior biologic treatments, number of prior JAK inhibitor treatments, concomitant use of glucocorticoids, and concomitant use of conventional synthetic disease-modifying antirheumatic drugs.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disorder that primarily affects women and typically presents during the sixth decade of life1. The pathophysiology of RA is characterized by chronic inflammation of the synovial membrane, leading to the progressive destruction of articular cartilage and marginal bone2. The primary goal for the treatment of patients with RA is to control the inflammation, aiming to prevent irreversible damage2. The recommendations from the European League Against Rheumatism (EULAR), in accordance with the treatment guidelines of the American College of Rheumatology (ACR), emphasize the importance of initiating treatment at the time of diagnosis3,4. First-line treatment typically consists of administering conventional synthetic (cs) Disease-Modifying Antirheumatic Drugs (DMARDs), mainly methotrexate (MTX), either as monotherapy or in combination with short-term and low dose glucocorticoids (GC)3,4. If this treatment fails, and remission or low disease activity is not achieved within a 6-month period, a second-line treatment approach should be pursued. This approach involves add-on therapy with a biologic DMARD (bDMARD) or, assuming risk assessment, a Janus Kinase (JAK) inhibitor3. If a JAK inhibitor fails, it turns out to consider again other JAK inhibitor or a bDMARD to end the loop3.
Several orally available JAK inhibitors have been developed for the management of RA. Tofacitinib (TOF), baricitinib (BAR), upadacitinib (UPA), and filgotinib (FIL) have demonstrated significant long-term efficacy and safety across diverse randomized controlled trials (RCTs)5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24, and currently, they constitute the four approved small molecules for RA treatment in Europe. However, RCTs commonly involve a smaller, well-selected study population that is closely monitored under predefined conditions and time intervals25,26. Furthermore, patients commonly enrolled in RCTs differ from those typically encountered in real-world settings26.
In real-world clinical practice, JAK inhibitors are often offered to patients who experienced multiple failures to bDMARDs and, increasingly, to other JAK inhibitors; who exhibit active/progressive disease activity; and whose RA management is perceived as problematic. Those patients are commonly referred to as "difficult-to-treat" RA patients27. Therefore, real-world evidence (RWE) studies, whether prospective or retrospective, can significantly complement the information obtained from RCTs, providing valuable insights that enhance healthcare decision-making25,26.
The main aim of the present study was to evaluate the influence of patient-related factors on the retention of JAK inhibitor treatment in RA patients within real-world scenarios. Within clinical practice, treatment retention serves as a composite metric that indirectly indicates the effectiveness and safety of a given treatment.
Methods
Study design and patient population
This is an observational, retrospective, single-center study that involved real-world patients who fulfilled the 2010 ACR-EULAR classification criteria for RA28.
In a tertiary-care university hospital from Spain, three rheumatologists, following clinical guidelines based on the EULAR recommendations3, attended to patients with RA. Patients who received treatment with either TOF, BAR, UPA, or FIL between September 2017 and May 2023, and who had comprehensive data regarding treatment initiation, potential discontinuation, and reasons for discontinuation, were eligible for inclusion in this study. All patients included were individually informed about the study and were given the option to decline the extraction of data from their electronic medical records. Data were retrospectively collected from patients’ records between March 2022 and May 2023.
Assessments
The retention of treatment was defined as the time interval between treatment initiation and definitive treatment discontinuation. The reasons for discontinuation were classified into three primary categories, as outlined below: (1) lack of effectiveness (including primary and secondary failure), (2) adverse events, and (3) other reasons. Physicians were restricted to select a single reason for discontinuation.
The potential predictive factors for JAK inhibitor retention included socio-demographic information (age, sex), RA anamnesis (RA disease duration), RA disease activity (measured using the Clinical Disease Activity Index (CDAI)), RA seropositivity (Rheumatoid Factor (RF), anti-Cyclic Citrullinated Peptides (anti-CCP)), number of prior RA treatments (previous bDMARDs, previous JAK inhibitors), and the presence or absence of concomitant RA treatments (GC, csDMARDs).
While RA can present at any age, its prevalence notably escalates with advanced age, with a substantial portion of patients experiencing initial symptoms after 60 years of old1. Accordingly, patients were categorized by age: young (< 65 years) and old (65 years or older). The CDAI scale was deemed appropriate for measuring disease activity. The CDAI does not incorporate acute phase reactants, making it more applicable for assessing disease activity, particularly when drugs have significantly influenced these inflammation biomarkers.
Statistical analyses
Differences in baseline patient characteristics among the JAK inhibitor groups (TOF, BAR, UPA, FIL) were evaluated using the Kruskal–Wallis test or the analysis of variance (for ordinal or quantitative variables) and the Fisher's exact test (for categorical variables).
Treatment retention was examined through Cox proportional hazard regression models and Kaplan–Meier estimates. Cox proportional hazard regression models (bivariate and multivariate) were applied to analyze the potential predictive patient-related factors described previously, which were present at the initiation of JAK inhibitor treatment. These potential predictive factors could influence the treatment retention for the discontinuation reasons of (1) lack of effectiveness and (2) adverse events, while excluding (3) other reasons. Covariates with a P value < 0.1 from the bivariate analysis were included in the multivariate analysis. Kaplan–Meier estimates, for the specified discontinuation reasons, were employed to evaluate the survival curves of treatment retention based on the potential predictive factors, with the log-rank test used for comparison. At the 12-month mark of JAK inhibitor treatment, reasons for and rates of treatment discontinuation were examined.
The statistical analyses were performed using STATA software version 15. A P value of < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the Research Ethics Committee of Hospital de la Santa Creu i Sant Pau who determined that our study did not need ethical approval. An official waiver of ethical approval was granted from the Research Ethics Committee of Hospital de la Santa Creu i Sant Pau.
All procedures involved in the present study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from patient included in the study.
Results
Study population
Between September 2017 and May 2023, a total of 189 JAK inhibitor treatments were identified, corresponding to 123 RA patients. Demographic and clinical characteristics of the included patients at the initiation of treatment are summarized in Table 1.
Reasons and rates of treatment discontinuation
After 12 months of treatment, JAK inhibitor discontinuation rates due to the corresponding reasons were as follows: lack of effectiveness (24.3%), adverse events (20.6%), and other reasons (3.7%). The remaining 51.4% represents the treatment continuation rate.
With regard to JAK inhibitor treatment discontinuation due to lack of effectiveness (Table 2), bivariate Cox regression analyses suggested that a greater number of previous bDMARDs treatments, a higher disease activity according to the CDAI scale, the concomitant use of GC, and an increased number of previous JAK inhibitors treatments, could represent potential patient-related factors associated with the prognostic risk of treatment discontinuation. However, following multiple imputation, no independent risk factors were found to significantly impact the JAK inhibitors' effectiveness to lead to treatment discontinuation.
Concerning JAK inhibitor cessation due to adverse events (Table 3), bivariate Cox regression analyses showed that age greater than 65 years and female sex could be potential patient-related factors associated with an increased prognostic risk of treatment discontinuation. In contrast, anti-CCP positivity and RF positivity could be associated with a potential protective prognostic effect against treatment discontinuation due to safety concerns. The results of the multivariate Cox regression analysis indicated that an age greater than 65 years seems to significantly increase the risk of JAK inhibitor treatment discontinuation due to adverse events (HR: 1.98; p = 0.030).
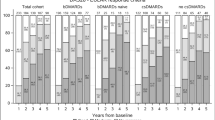
In terms of age, at 12 months of treatment, no differences were observed in the discontinuation rates due to lack of effectiveness (p = 0.436; Fig. 1a), while there were significant differences noted for those related to adverse events, which were: young, 14.4% vs. old, 26.3% (p = 0.019; Fig. 1b).
Discussion
This study assessed patient-related factors associated with the retention of JAK inhibitor treatment, thereby investigating how patient characteristics impact the effectiveness and safety of these small molecules. Based on the available literature, there is limited evidence addressing these issues within real-world conditions. Ebina et al. explored Asian RA patients treated in accordance with Japanese guidelines using either TOF or BAR29. To the best of our knowledge, this present study is the first to include treatments with TOF, BAR, UPA, and FIL, the four JAK inhibitors currently approved for RA management in Europe.
Regarding the impact of age, both young and old RA patients displayed similar efficacy and effectiveness when treated with JAK inhibitors29,30,31. However, in patients aged 65 years or older, an association was observed between TOF treatment and an increased risk of cardiovascular events and malignancies when compared to Tumor Necrosis Factor inhibitor (TNFi) treatment32. Consequently, due to potential shared effects within the drug class, in accordance with the European Medicines Agency (EMA)33 and the EULAR3, careful consideration should be given to individuals aged over 65 years when considering the prescription of a JAK inhibitor. Consistently with this approach, in our current study, age was not found to be related to treatment discontinuation due to lack of effectiveness, but it was significantly associated with treatment discontinuation due to adverse events (HR: 1.98; p = 0.030). Regarding adverse events, the discontinuation rates at 12 months of treatment were as follows: young, 14.4% vs. old, 26.3% (p = 0.019; Fig. 1b).
In terms of sex, there is currently a lack of substantial evidence concerning its potential impact on the retention of JAK inhibitor treatment29,34. Our study findings suggest that being female or male does not significantly influence the effectiveness and safety of a JAK inhibitor treatment.
With regard to RA seropositivity, a post-hoc analysis of TOF treatment indicated that the treatment outcome is not significantly affected by the positivity or negativity of anti-CCP or RF34. In a recent study, seropositivity (anti-CCP or RF) was found to have no influence on JAK inhibitor treatment retention34. In line with both, the existing literature34,35 and our study' results, neither anti-CCP nor RF were found to have significant effects on treatment retention.
Concerning RA disease activity, when poor prognostic factors are present and patients experience moderate to severe RA activity despite the initial csDMARDs strategy, treatment with a JAK inhibitor may be considered. At 6 months of treatment, dose reduction or interval adjustment can be safety implemented with any JAK inhibitor if clinical remission, or at least low disease activity, is achieved3. At baseline, the patients of our study exhibited severe or, at least moderate RA activity, according with the guideline recommendations. These baseline RA disease activity values did not significantly impact the effectiveness and safety of the JAK inhibitor treatment in our study.
With respect to RA disease duration, published literature suggests that it should not be considered a factor influencing treatment retention29,34. Similarly, our present study did not identify any significant association between RA disease duration and discontinuation of treatment due to lack of effectiveness or adverse events.
Regarding the number of prior bDMARDs treatments, recent evidence indicates that it does not have a significant impact on JAK inhibitor treatment retention29,34. Similarly, in our study, comparable JAK inhibitor treatment retentions were observed regardless of the number of previous bDMARDs used. It is worth nothing that specific mechanisms of action of prior bDMARDs might suggest an increased risk of JAK inhibitor treatment discontinuation due to lack of effectiveness, such as, interleukin (IL)-6 inhibition29.
Another consideration relates to the number of prior JAK inhibitor treatments. The strategy of subsequent JAK inhibitor treatments, referred to as the JAK inhibitor cycling strategy, has shown to be effective and safe as an eligible option following the failure of a prior JAK inhibitor in terms of lack of effectiveness or adverse events36. The number of prior JAK inhibitor treatments does not seem to impact subsequent JAK inhibitor treatment retentions, as supported by both the existing literature29 and the findings of our study.
In reference to the presence or absence of concomitant GC, when initiating a JAK inhibitor treatment or making changes in concomitant csDMARDs, it is recommended by both the ACR guidelines4 and EULAR recommendations3 to consider short-term GC in different dose regimens and routes of administration. However, it is crucial to gradually taper and discontinue GC as soon as it is clinically feasible due to the potential risk of adverse events3,4. Doses exceeding 7.5 mg/day of oral prednisone (PDN) equivalent were identified as a risk factor for serious infections in TOF treatment37. In our present study, 56.6% of the patients were receiving GC at the initiation of JAK inhibitor treatment, with a median (IQR) PDN dose equivalent of 5 (5–8) mg. At baseline, in JAK inhibitor treatment, the presence or absence of concomitant GC at low doses (≤ 7.5 mg/day PDN equivalent3) is not related to the lack of effectiveness or the occurrence of adverse events, as evidenced by both the published literature34 and the findings of our study.
No compelling evidence exists regarding the monotherapy of JAK inhibitor compared to the combination therapy with csDMARDs27,34. According to the EULAR recommendations3, it is advocated to continue MTX (or other csDMARDs) when planning treatment with a JAK inhibitor, after assessing the associated risks. The MTX dose can be reduced to convey the added benefit of combination vs. monotherapy while, mitigating the risk of adverse events3. In our study, 15.9% of patients continued MTX upon JAK inhibitor initiation (Table 1). Based on our findings, no significant differences were observed between the presence or absence of concomitant csDMARDs regarding the risk of JAK inhibitor treatment discontinuation due to lack of effectiveness nor adverse events. These results are consistent with recent literature29.
There are limitations to the present study that inevitably influence the interpretation of the results obtained. Firstly, when extending the findings of this study to the broader population, it is crucial to take into account both the population size and the fact that the study was performed exclusively at a single healthcare center. However, it is worth mentioning that the results obtained in that study align with the previous existing evidence. The other limitation stems from the uneven distribution of JAK inhibitor treatment groups, which reflects real-world clinical practice and represents a common limitation in observational studies. Given that UPA and FIL are the most recently approved JAK inhibitors for RA, the majority of patients were treated with TOF or BAR. Due to limited statistical power, the assessment of factors influencing treatment retention based on the type of JAK inhibitor was not feasible. There is a lack of head-to-head randomized clinical trials comparing these small molecules38. Future research should aim to determine potential differences among various types of JAK inhibitors.
The main strength of our study resides in the inclusion of RA patients being treated on real life, examining factors that could impact the effectiveness and safety of the JAK inhibitor treatment. This is particularly significant for "difficult-to-treat" RA patients who might not be included in RCTs.
In summary, age (65 years or older) was significantly linked to an increased risk of the treatment discontinuation of JAK inhibitors due to adverse events. Patient-related factors not associated with treatment discontinuation were as follows: sex, RA disease duration, RA disease activity, seropositivity for RF, seropositivity for anti-CCP, number of prior bDMARD treatments, number of prior JAK inhibitor treatments, concomitant use of GC, and concomitant use of csDMARDs. These findings can significantly complement the information obtained from randomized controlled trials, providing valuable insights that enhance healthcare decision-making.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACR:
-
American college of rheumatology
- anti-CCP:
-
Anti-cyclic citrullinated peptides
- BAR:
-
Baricitinib
- bDMARD:
-
Biologic disease-modifying antirheumatic drug
- BMI:
-
Body mass index
- CD20i:
-
Cluster of differentiation 20 inhibitor
- CD80/86i:
-
Cluster of differentiation 80/86 inhibitor
- CDAI:
-
Clinical disease activity index
- CI:
-
Confidence interval
- csDMARD:
-
Conventional synthetic disease-modifying antirheumatic drug
- DAS28-CRP:
-
Disease activity score 28‐joint count using C‐reactive protein
- DAS28-ESR:
-
Disease activity score 28‐joint count using erythrocyte sedimentation rate
- DMARD:
-
Disease-modifying antirheumatic drug
- EMA:
-
European medicines agency
- EULAR:
-
European league against rheumatism
- FIL:
-
Filgotinib
- GC:
-
Glucocorticoids
- HR:
-
Hazard ratio
- IL:
-
Interleukin
- IL6i:
-
Interleukin 6 inhibitor
- JAK:
-
Janus Kinase
- LEF:
-
Leflunomide
- MTX:
-
Methotrexate
- PDN:
-
Prednisone
- RA:
-
Rheumatoid arthritis
- RCTs:
-
Randomized controlled trials
- RF:
-
Rheumatoid factor
- SDAI:
-
Simplified disease activity index
- SSZ:
-
Sulfasalazine
- TNFi:
-
Tumor necrosis factor inhibitor
- TOF:
-
Tofacitinib
- UPA:
-
Upadacitinib
References
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis: A review. JAMA. 320(13), 1360–1372. https://doi.org/10.1001/jama.2018.13103 (2018).
McInnes, IB. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365(23), 2205–2219. https://doi.org/10.1056/NEJMra1004965 (2011).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82(1), 3–18. https://doi.org/10.1136/ard-2022-223356 (2023).
Fraenkel, L. et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 73(7), 924–939. https://doi.org/10.1002/acr.24596 (2021).
Fleischmann, R. et al. ORAL-Solo Investigators. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367(6), 495–507. https://doi.org/10.1056/NEJMoa1109071 (2012).
Kremer, J. et al. ORAL-Sync Investigators. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 159(4), 253–261. https://doi.org/10.7326/0003-4819-159-4-201308200-00006 (2013).
Van Vollenhoven, R. F. et al. ORAL-Standard Investigators. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367(6), 508–519. https://doi.org/10.1056/NEJMoa1112072 (2012).
Van der Heijde, D. et al. ORAL-Scan Investigators. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: Twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 65(3), 559–570. https://doi.org/10.1002/art.37816 (2013).
Burmester, G. R. et al. ORAL-Step Investigators. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: A randomised phase 3 trial. Lancet. 381(9865), 451–460. https://doi.org/10.1016/S0140-6736(12)61424-X (2013).
Lee, EB. et al. ORAL-Start Investigators. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370(25), 2377–2386. https://doi.org/10.1056/NEJMoa1310476 (2014).
Fleischmann, R. et al. ORAL-Strategy Investigators. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): A phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 390(10093), 457–468. https://doi.org/10.1016/S0140-6736(17)31618-5 (2017).
Fleischmann, R. et al. RA-BEGIN Investigators. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69(3), 506–517. https://doi.org/10.1002/art.39953 (2017).
Taylor, P. C. et al. RA-BEAM Investigators. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376(7), 652–662. https://doi.org/10.1056/NEJMoa1608345 (2017).
Dougados, M. et al. RA-BUILD Investigators. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: Results from the RA-BUILD study. Ann. Rheum. Dis. 76(1), 88–95. https://doi.org/10.1136/annrheumdis-2016-210094 (2017).
Genovese, M. C. et al; RA-BEACON Investigators. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374(13), 1243–1252. https://doi.org/10.1056/NEJMoa1507247 (2016).
Van Vollenhoven, R. et al. SELECT-EARLY Investigators. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): A multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 72(10), 1607–1620. https://doi.org/10.1002/art.41384 (2020).
Smolen, J. S. et al. SELECT-MONOTHERAPY Investigators. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): A randomised, placebo-controlled, double-blind phase 3 study. Lancet. 393(10188), 2303–2311. https://doi.org/10.1016/S0140-6736(19)30419-2 (2019).
Burmester, G. R. et al. SELECT-NEXT Investigators. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 391(10139), 2503–2512. https://doi.org/10.1016/S0140-6736(18)31115-2 (2018).
Fleischmann, R. et al. SELECT-COMPARE Investigators. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 71(11), 1788–1800. https://doi.org/10.1002/art.41032 (2019).
Genovese, M. C. et al. SELECT-BEYOND Investigators. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): A double-blind, randomised controlled phase 3 trial. Lancet. 391(10139), 2513–2524. https://doi.org/10.1016/S0140-6736(18)31116-4 (2018).
Rubbert-Roth, A. et al. SELECT-CHOICE Investigators. Trial of upadacitinib or abatacept in rheumatoid arthritis. N. Engl. J. Med. 383(16), 1511–1521. https://doi.org/10.1056/NEJMoa2008250 (2020).
Combe, B. et al. FINCH-1 Investigators. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: A phase III randomised clinical trial. Ann. Rheum Dis. 80(7), 848–858. https://doi.org/10.1136/annrheumdis-2020-219214 (2021).
Genovese, M. C. et al; FINCH-2 Investigators. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: The FINCH 2 randomized clinical trial. JAMA. 322(4), 315–325. https://doi.org/10.1001/jama.2019.9055 (2019).
Westhovens, R. et al. FINCH-3 Investigators. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: The phase 3, randomised controlled FINCH 3 trial. Ann. Rheum. Dis. 80(6), 727–738. https://doi.org/10.1136/annrheumdis-2020-219213 (2021).
Mahajan, R. Real world data: Additional source for making clinical decisions. Int. J. Appl. Basic Med. Res. 5(2), 82. https://doi.org/10.4103/2229-516X.157148 (2015).
Sherman, RE. et al. Real-world evidence - What is it and what can it tell us? N Engl J Med. 375(23), 2293–2297. https://doi.org/10.1056/NEJMsb1609216 (2016).
Nagy, G. et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 80(1), 31–35. https://doi.org/10.1136/annrheumdis-2020-217344 (2021).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against rheumatism collaborative initiative. Ann. Rheum Dis. 69, 1580–1588. https://doi.org/10.1136/ard.2010.138461 (2010).
Ebina, K. et al. Factors affecting drug retention of Janus kinase inhibitors in patients with rheumatoid arthritis: The ANSWER cohort study. Sci. Rep. 12(1), 134. https://doi.org/10.1038/s41598-021-04075-0 (2022).
Curtis, J. R. et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 35(3), 390–400 (2017).
Fleischmann, R. et al. Safety and efficacy of baricitinib in elderly patients with rheumatoid arthritis. RMD Open. 3(2), e000546. https://doi.org/10.1136/rmdopen-2017-000546 (2017).
Kristensen, L. E. et al. Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: An analysis of the open label, randomised controlled study ORAL Surveillance. Ann Rheum Dis. 82(7), 901–910. https://doi.org/10.1136/ard-2022-223715 (2023).
European Medicines Agency – EMA (2023). EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. EMA/142279/2023. https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki. Accessed 22 May 2023.
Pombo-Suarez, M. et al. After JAK inhibitor failure: To cycle or to switch, that is the question—data from the JAK-pot collaboration of registries. Ann. Rheum. Dis. 82(2), 175–181. https://doi.org/10.1136/ard-2022-222835 (2023).
Bird, P. et al. Treatment outcomes in patients with seropositive versus seronegative rheumatoid arthritis in Phase III randomised clinical trials of tofacitinib. RMD Open. 5(1), e000742. https://doi.org/10.1136/rmdopen-2018-000742 (2019).
Retuerto, M. et al. Efficacy and safety of switching Jak inhibitors in rheumatoid arthritis: An observational study. Clin. Exp. Rheumatol. 39(3), 453–455. https://doi.org/10.55563/clinexprheumatol/cbanza (2021).
Cohen, S. et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 66(11), 2924–2937. https://doi.org/10.1002/art.38779 (2014).
Sanmartí, R. & Corominas, H. Upadacitinib for patients with rheumatoid arthritis: A comprehensive review. J. Clin. Med. 12(5), 1734. https://doi.org/10.3390/jcm12051734 (2023).
Acknowledgements
The authors express their sincere gratitude to the patients who were involved in the present study.
Author information
Authors and Affiliations
Contributions
H.C. and S.V. were responsible for the conception and design of the study. C.M. conducted the data extraction, and, together with I.G., performed the statistical analyses. C.M. drafted the initial manuscript. H.C. and S.V. supervised the final version, critically revising it for important intellectual content. All authors (A.F., C.D., C.M., H.C., H.P., I.G., S.V.) read and contributed to the final approval of the published version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. HC has received speaker honoraria from Grünenthal, MSD, Biogen, Galapagos, Abbvie, Roche, and Bristol Myers Squibb and consultancy/lectures fees from Galapagos, Gebro, Abbvie, Sanofi, and UCB. Other authors have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinez-Molina, C., Gich, I., Diaz-Torné, C. et al. Patient-related factors influencing the effectiveness and safety of Janus Kinase inhibitors in rheumatoid arthritis: a real-world study. Sci Rep 14, 172 (2024). https://doi.org/10.1038/s41598-023-50379-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50379-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.