Abstract
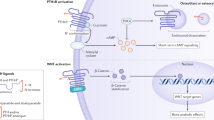
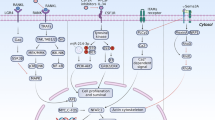
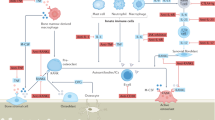
Denosumab, a human monoclonal antibody against receptor activator of nuclear factor-κB ligand (RANKL), is a potent inhibitor of osteoclast differentiation and activity. As the first biologic drug used to treat osteoporosis, denosumab has shown potent anti-resorptive properties and anti-fracture efficacy. The effects of this drug are also unique compared with the effects of bisphosphonates: namely, long-term treatment with this drug results in a continuous gain of bone mineral density, whereas withdrawal of the drug results in a transient overshoot in bone turnover and rapid bone loss. Although the mechanisms for these specific effects remain incompletely understood, emerging experimental and clinical data have started to highlight potential biological and pharmacological mechanisms by which denosumab might affect osteoclasts, as well as osteoblasts, and cause both sustained bone gain and bone loss upon treatment cessation. This Perspective discusses those potential mechanisms and the future studies and clinical implications that might ensue from these findings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kendler, D. L., Cosman, F., Stad, R. K. & Ferrari, S. Denosumab in the treatment of osteoporosis: 10 years later: a narrative review. Adv. Ther. 39, 58–74 (2022).
Baron, R., Ferrari, S. & Russell, R. G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48, 677–692 (2011).
Bone, H. G. et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 5, 513–523 (2017).
Black, D. M. et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 8, 672–682 (2020).
Ferrari, S. et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J. Clin. Endocrinol. Metab. 104, 3450–3461 (2019).
Adachi, J. D. et al. Influence of subject discontinuation on long-term nonvertebral fracture rate in the denosumab FREEDOM Extension study. BMC Musculoskelet. Disord. 18, 174 (2017).
Boonen, S. et al. Postmenopausal osteoporosis treatment with antiresorptives: effects of discontinuation or long-term continuation on bone turnover and fracture risk — a perspective. J. Bone Min. Res. 27, 963–974 (2012).
Brown, J. P. et al. Bone remodeling in postmenopausal women who discontinued denosumab treatment: off-treatment biopsy study. J. Bone Min. Res. 26, 2737–2744 (2011).
Tsourdi, E. et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J. Clin. Endocrinol. Metab. 106, 264–281 (2020).
Reid, I. R., Horne, A. M., Mihov, B. & Gamble, G. D. Bone loss after denosumab: only partial protection with zoledronate. Calcif. Tissue Int. 101, 371–374 (2017).
Eastell, R. et al. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J. Bone Min. Res. 29, 458–466 (2014).
Eisman, J. A. et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J. Bone Min. Res. 26, 242–251 (2011).
Fuller, K. et al. Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone 42, 200–211 (2008).
Gallagher, J. C., Rapuri, P. B., Haynatzki, G. & Detter, J. R. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J. Clin. Endocrinol. Metab. 87, 4914–4923 (2002).
Eghbali-Fatourechi, G. et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Invest. 111, 1221–1230 (2003).
Jiang, L., Cui, X., Ma, H. & Tang, X. Comparison of denosumab and zoledronic acid for the treatment of solid tumors and multiple myeloma with bone metastasis: a systematic review and meta-analysis based on randomized controlled trials. J. Orthop. Surg. Res. 16, 400 (2021).
Anastasilakis, A. D. et al. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J. Bone Min. Res. 32, 1291–1296 (2017).
Cummings, S. R. et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J. Bone Min. Res. 33, 190–198 (2018).
Cosman, F., Huang, S., McDermott, M. & Cummings, S. R. Multiple vertebral fractures after denosumab discontinuation: FREEDOM and FREEDOM Extension trials additional post hoc analyses. J. Bone Min. Res. 37, 2112–2120 (2022).
Burckhardt, P., Faouzi, M., Buclin, T. & Lamy, O., The Swiss Denosumab Study Group. Fractures after denosumab discontinuation: a retrospective study of 797 cases. J. Bone Min. Res. 36, 1717–1728 (2021).
Omiya, T. et al. Sustained anti-osteoporotic action of risedronate compared to anti-RANKL antibody following discontinuation in ovariectomized mice. Bone Rep. 13, 100289 (2020).
Solling, A. S., Harslof, T. & Langdahl, B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J. Bone Min. Res. 35, 1858–1870 (2020).
Solling, A. S., Harslof, T. & Langdahl, B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a 2-year randomized study. J. Bone Min. Res. 36, 1245–1254 (2021).
Makras, P. et al. The duration of denosumab treatment and the efficacy of zoledronate to preserve bone mineral density after its discontinuation. J. Clin. Endocrinol. Metab. 106, e4155–e4162 (2021).
Kearns, A. E., Khosla, S. & Kostenuik, P. J. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 29, 155–192 (2008).
Dempster, D. W. et al. Effects of long-term denosumab on bone histomorphometry and mineralization in women with postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 103, 2498–2509 (2018).
Watanabe, H. et al. Transcription factor hematopoietically expressed homeobox protein (Hhex) negatively regulates osteoclast differentiation by controlling cyclin-dependent kinase inhibitors. JBMR Plus 6, e10608 (2022).
Fontalis A, G. F., Schini, M., Walsh, J. & Eastell, R. The effect of denosumab treatment on osteoclast precursor cells in postmenopausal osteoporosis. Bone Rep. 135, 39 (2020).
Anastasilakis, A. D. et al. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur. J. Endocrinol. 176, 677–683 (2017).
Solling, A. S., Harslof, T., Joergensen, N. R. & Langdahl, B. Changes in RANKL and TRAcP 5b after discontinuation of denosumab suggest RANKL mediated recruitment of osteoclasts. Bone Rep. 16, 12 (2022).
McDonald, M. M. et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 184, 1940 (2021).
Cawley, K. M. et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep. 32, 108052 (2020).
Rinotas, V. et al. Novel genetic models of osteoporosis by overexpression of human RANKL in transgenic mice. J. Bone Min. Res. 29, 1158–1169 (2014).
Fu, Q. et al. Low osteoblast number and OPG levels may contribute to rebound resorption after discontinuation of denosumab administration. J. Bone Min. Res 37, 338 (2021).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Martinez-Reina, J., Calvo-Gallego, J. L. & Pivonka, P. Combined effects of exercise and denosumab treatment on local failure in post-menopausal osteoporosis-insights from bone remodelling simulations accounting for mineralisation and damage. Front. Bioeng. Biotechnol. 9, 635056 (2021).
Bonnet, N. et al. Influence of fatigue loading and bone turnover on bone strength and pattern of experimental fractures of the tibia in mice. Calcif. Tissue Int. 99, 99–109 (2016).
Frost, H. M. Bone “mass” and the “mechanostat”: a proposal. Anat. Rec. 219, 1–9 (1987).
Hart, N. H. et al. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 17, 114–139 (2017).
Gerbaix, M., Vico, L., Ferrari, S. L. & Bonnet, N. Periostin expression contributes to cortical bone loss during unloading. Bone 71, 94–100 (2015).
Bonnet, N., Garnero, P. & Ferrari, S. Periostin action in bone. Mol. Cell Endocrinol. 432, 75–82 (2016).
Bonnet, N., Bourgoin, L., Biver, E., Douni, E. & Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Invest. 133, e169317 (2019).
Kistler-Fischbacher, M., Yong, J. S., Weeks, B. K. & Beck, B. R. A comparison of bone-targeted exercise with and without antiresorptive bone medication to reduce indices of fracture risk in postmenopausal women with low bone mass: the MEDEX-OP randomized controlled trial. J. Bone Min. Res. 36, 1680–1693 (2021).
Eastell, R., Walsh, J. S., Watts, N. B. & Siris, E. Bisphosphonates for postmenopausal osteoporosis. Bone 49, 82–88 (2011).
Farlay, D. et al. Bone mineral and organic properties in postmenopausal women treated with denosumab for up to 10 years. J. Bone Min. Res. 37, 856–864 (2022).
Ominsky, M. S. et al. Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J. Bone Min. Res. 30, 1280–1289 (2015).
Dempster, D. W. et al. Modeling-based bone formation in the human femoral neck in subjects treated with denosumab. J. Bone Min. Res. 35, 1282–1288 (2020).
Kim, B. J. Effects of muscles on bone metabolism-with a focus on myokines. Ann. Geriatr. Med. Res. 26, 63–71 (2022).
Pennypacker, B. L. et al. Inhibition of cathepsin K increases modeling-based bone formation, and improves cortical dimension and strength in adult ovariectomized monkeys. J. Bone Min. Res. 29, 1847–1858 (2014).
Bonnet, N., Brun, J., Rousseau, J. C., Duong, L. T. & Ferrari, S. L. Cathepsin K controls cortical bone formation by degrading periostin. J. Bone Min. Res. 32, 1432–1441 (2017).
Bonnet, N. et al. RANKL-induced increase in cathepsin K levels restricts cortical expansion in a periostin-dependent fashion: a potential new mechanism of bone fragility. J. Bone Min. Res. 36, 1636–1645 (2021).
Ikebuchi, Y. et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 561, 195–200 (2018).
Dennison, E. M. et al. Fracture risk following intermission of osteoporosis therapy. Osteoporos. Int. 30, 1733–1743 (2019).
Everts-Graber, J. et al. Risk factors for vertebral fractures and bone loss after denosumab discontinuation: a real-world observational study. Bone 144, 115830 (2021).
Author information
Authors and Affiliations
Contributions
S.F. researched data for the article and wrote the article. B.L. contributed to the writing of the article. Both authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.L. has received research grants from Amgen and Novo Nordisk, and is on the advisory board and performs lectures for Amgen, Astellas, AstraZeneca, Eli Lilly, Gedeon-Richter, Gilead and UCB. S.F. has received research support from Agnovos, Alexion, Amgen, and UCB, and is on the scientific advisory board of Agnovos, Alexion, Amgen, Boehringer, Flowbone, Fresenius, Labatec, Myovant, Parexel, Radius and UCB.
Peer review
Peer review information
Nature Reviews Rheumatology thanks E. Seeman, R. Eastell and R. Baron for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferrari, S., Langdahl, B. Mechanisms underlying the long-term and withdrawal effects of denosumab therapy on bone. Nat Rev Rheumatol 19, 307–317 (2023). https://doi.org/10.1038/s41584-023-00935-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-023-00935-3
This article is cited by
-
The relationship between length of denosumab treatment for postmenopausal osteoporosis and serum TRAcP5b measured six months after the last injection
Osteoporosis International (2024)
-
The Five-Year Effect of a Single Zoledronate Infusion on Bone Mineral Density Following Denosumab Discontinuation in Women with Postmenopausal Osteoporosis
Calcified Tissue International (2023)