Abstract
Joint distraction, the prolonged mechanical separation of the bones at a joint, has emerged as a joint-preserving treatment for end-stage osteoarthritis, with the gradually growing promise of implementation in regular clinical practice. Joint distraction of the knee has been most extensively studied, with these studies showing prolonged symptomatic improvement in combination with repair of cartilage tissue in degenerated knee joints, supporting the concept that cartilage repair can translate into real clinical benefit. The reversal of tissue degeneration observed with joint distraction could be the result of one or a combination of various proposed mechanisms, including partial unloading, synovial fluid pressure oscillation, mechanical and biochemical changes in subchondral bone, adhesion and chondrogenic commitment of joint-derived mesenchymal stem cells or a change in the molecular milieu of the joint. The overall picture that emerges from the combined evidence is relevant for future research and treatment-related improvements of joint distraction and for translation of the insights gained about tissue repair to other joint-preserving techniques. It remains to be elucidated whether optimizing the biomechanical conditions during joint distraction can actually cure osteoarthritis rather than only providing temporary symptomatic relief, but even temporary relief might be relevant for society and patients, as it will delay joint replacement with a prosthesis at an early age and thereby avert revision surgery later in life. Most importantly, improved insights into the underlying mechanisms of joint repair might provide new leads for more targeted treatment options.
Key points
-
Joint distraction can induce tissue structure modifications in degenerated knee joints, accompanied by prolonged symptomatic improvement that supports the concept that cartilage repair can translate into real clinical benefit.
-
It remains to be elucidated whether achieving optimal biomechanical and molecular conditions during distraction can lead to cure in osteoarthritis rather than providing only temporary symptomatic relief.
-
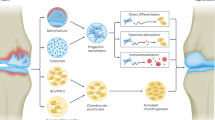
Partial unloading, synovial fluid pressure oscillation, subchondral bone changes, adhesion and chondrogenic commitment of joint-derived mesenchymal stem cells, and an altered molecular milieu in the joint are the proposed mechanisms underlying the therapeutic effects of joint distraction.
-
Greater insight into the molecular mechanisms of cartilage repair and their interplay in joint distraction might provide new leads to more targeted treatment options.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Mastbergen, S. C., Saris, D. B. F. & Lafeber, F. P. J. G. Functional articular cartilage repair: Here, near, or is the best approach not yet clear? Nat. Rev. Rheumatol. 9, 277–290 (2013).
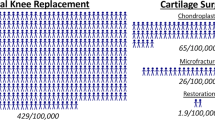
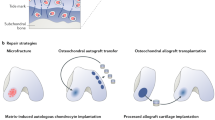
Palmer, J. S. et al. Surgical interventions for symptomatic mild to moderate knee osteoarthritis. Cochrane Database Syst. Rev. 7, CD012128 (2019).
Jatinder Singh, L., Salim AL, H. & Suwailim AL, G. Surgical options for treating knee osteoarthritis — a concise review. J. Musculoskelet. Disord. Treat. 6, 084 (2020).
Lafeber, F. P., Intema, F., Van Roermund, P. M. & Marijnissen, A. C. Unloading joints to treat osteoarthritis, including joint distraction. Curr. Opin. Rheumatol. 18, 519–525 (2006).
Aldegheri, R., Trivella, G. & Saleh, M. Articulated distraction of the hip: conservative surgery for arthritis in young patients. Clin. Orthop. Relat. Res. 301, 94–101 (1994).
Van Valburg, A. A. et al. Can Ilizarov joint distraction delay the need for an arthrodesis of the ankle? A preliminary report. J. Bone Jt. Surg. B 77, 720–725 (1995).
Van Valburg, A. A. et al. Joint distraction in treatment of osteoarthritis: a two-year follow-up of the ankle. Osteoarthritis Cartilage 7, 474–479 (1999).
Bain, G. I., Mehta, J. A., Heptinstall, R. J. & Bria, M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J. Bone Jt. Surg. B 80, 1014–1019 (1998).
DeVries, J. G., Amiot, R. A., Cummings, P. & Sockrider, N. Freiberg’s infraction of the second metatarsal treated with autologous osteochondral transplantation and external fixation. J. Foot Ankle Surg. 47, 565–570 (2008).
Spaans, A. J., van Minnen, L. P., Braakenburg, A. & Mink van der Molen, A. B. Joint distraction for thumb carpometacarpal osteoarthritis: a feasibility study with 1-year follow-up. J. Plast. Surg. Hand Surg. 51, 254–258 (2017).
Jansen, M. P. et al. Knee joint distraction as treatment for osteoarthritis results in clinical and structural benefit: a systematic review and meta-analysis of the limited number of studies and patients available. Cartilage https://doi.org/10.1177/1947603520942945 (2020).
Jansen, M. P. et al. Knee joint distraction in regular care for treatment of knee osteoarthritis: a comparison with clinical trial data. PLoS ONE 15, e0227975 (2020).
Deie, M., Ochi, M., Adachi, N., Kajiwara, R. & Kanaya, A. A new articulated distraction arthroplasty device for treatment of the osteoarthritic knee joint: a preliminary report. Arthroscopy 23, 833–838 (2007).
Deie, M. et al. Knee articulated distraction arthroplasty for the middle-aged osteoarthritic knee joint. Tech. Knee Surg. 9, 80–84 (2010).
Abouheif, M. M. et al. Repair of a large osteochondral defect in the knee joint using autologous and artificial bone graft combined with motion preserving distraction arthroplasty: a case report. Arch. Orthop. Trauma. Surg. 130, 231–236 (2010).
Intema, F. et al. Tissue structure modification in knee osteoarthritis by use of joint distraction: an open 1-year pilot study. Ann. Rheum. Dis. 70, 1441–1446 (2011).
Aly, T. A., Hafez, K. & Amin, O. Arthrodiatasis for management of knee osteoarthritis. Orthopedics 34, e338–e343 (2011).
van der Woude, J. A. D. et al. Knee joint distraction compared with high tibial osteotomy: a randomized controlled trial. Knee Surg. Sport. Traumatol. Arthrosc. 25, 876–886 (2017).
Van Der Woude, J. A. D. et al. Knee joint distraction compared with total knee arthroplasty a randomised controlled trial. Bone Jt. J. 99-B, 51–58 (2017).
Jansen, M. P. Knee joint distraction: moving forward. (Utrecht University, 2021).
ISRCTN. A comparison of knee replacement surgery and knee joint distraction for treating osteoarthritis of the knee (2020).
van der Woude, J. A. D. et al. Six weeks of continuous joint distraction appears sufficient for clinical benefit and cartilaginous tissue repair in the treatment of knee osteoarthritis. Knee 23, 785–791 (2016).
Struik, T. et al. Technical feasibility of personalized articulating knee joint distraction for treatment of tibiofemoral osteoarthritis. Clin. Biomech. 49, 40–47 (2017).
Wiegant, K. et al. Knee joint distraction as an alternative surgical treatment for osteoarthritis: rationale and design of two randomized controlled trials (vs high tibial osteotomy and total knee prosthesis). Int. J. Orthop. 2, 353–360 (2015).
Wiegant, K. et al. Sustained clinical and structural benefit after joint distraction in the treatment of severe knee osteoarthritis. Osteoarthritis Cartilage 21, 1660–1667 (2013).
van der Woude, J. T. A. D. et al. Five-year follow-up of knee joint distraction: clinical benefit and cartilaginous tissue repair in an open uncontrolled prospective study. Cartilage 8, 263–271 (2017).
Jansen, M. P. et al. Initial tissue repair predicts long-term clinical success of knee joint distraction as treatment for knee osteoarthritis. Osteoarthritis Cartilage 26, 1604–1608 (2018).
Jansen, M. P. et al. Knee joint distraction compared with high tibial osteotomy and total knee arthroplasty: two-year clinical, radiographic, and biochemical marker outcomes of two randomized controlled trials. Cartilage 12, 181–191 (2019).
Intema, F. et al. Subchondral bone remodeling is related to clinical improvement after joint distraction in the treatment of ankle osteoarthritis. Osteoarthritis Cartilage 19, 668–675 (2011).
Escobar, A. et al. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage 15, 273–280 (2007).
Hoorntje, A. et al. Return to sport and work after randomization for knee distraction versus high tibial osteotomy: is there a difference? J. Knee Surg. https://doi.org/10.1055/s-0040-1721027 (2020).
Bayliss, L. E. et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 389, 1424–1430 (2017).
Jansen, M. P. et al. Changes in cartilage thickness and denuded bone area after knee joint distraction and high tibial osteotomy — post-hoc analyses of two randomized controlled trials. J. Clin. Med. 10, 368 (2021).
Jansen, M. P., Mastbergen, S. C., MacKay, J. W., Turmezei, T. D. & Lafeber, F. Knee joint distraction results in MRI cartilage thickness increase up to ten years after treatment. Rheumatology https://doi.org/10.1093/rheumatology/keab456 (2021).
van Spil, W. E., DeGroot, J., Lems, W. F., Oostveen, J. C. M. & Lafeber, F. P. J. G. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthrits Cartilage 18, 605–612 (2010).
Besselink, N. J. et al. Cartilage quality (dGEMRIC Index) following knee joint distraction or high tibial osteotomy. Cartilage 11, 19–31 (2018).
Jansen, M. P. et al. Knee joint distraction is more efficient in rebuilding cartilage thickness in the more affected compartment than high tibial osteotomy in patients with knee osteoarthritis. Osteoarthritis Cartilage 27, S330–S331 (2019).
Wiegant, K. et al. Total knee prosthesis after knee joint distraction treatment. J. Surg. Surg. Res. 1, 066–071 (2015).
Jansen, M. P., Struijk, T., Mastbergen, S. C., Custers, R. J. & Lafeber, F. P. User-friendliness of a novel dedicated knee joint distraction device: experiences from clinical practice. Osteoarthritis Cartilage 28, S474 (2020).
Jansen, M. P. et al. Reduction of pin tract infections during external fixation using cadexomer iodine. J. Exp. Orthop. 7, 88 (2020).
Baboolal, T. G. et al. Synovial fluid hyaluronan mediates MSC attachment to cartilage, a potential novel mechanism contributing to cartilage repair in osteoarthritis using knee joint distraction. Ann. Rheum. Dis. 75, 908–915 (2016).
Sanjurjo-Rodriguez, C. et al. Gene expression signatures of synovial fluid multipotent stromal cells in advanced knee osteoarthritis and following knee joint distraction. Front. Bioeng. Biotechnol. 8, 1178 (2020).
Watt, F. E. et al. The molecular profile of synovial fluid changes upon joint distraction and is associated with clinical response in knee osteoarthritis. Osteoarthritis Cartilage 28, 324–333 (2020).
Caldwell, K. L. & Wang, J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthritis Cartilage 23, 351–362 (2015).
Funck-Brentano, T. & Cohen-Solal, M. Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor. Rev. 22, 91–97 (2011).
Yuan, X. L. et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage 22, 1077–1089 (2014).
Griffin, T. M. & Guilak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport. Sci. Rev. 33, 195–200 (2005).
Widmyer, M. R. et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 65, 2615–2622 (2013).
Coleman, J. L. et al. Diurnal variations in articular cartilage thickness and strain in the human knee. J. Biomech. 46, 541–547 (2013).
Eckstein, F. et al. Effect of physical exercise on cartilage volume and thickness in vivo: MR imaging study. Radiology 207, 243–248 (1998).
Eckstein, F., Hudelmaier, M. & Putz, R. The effects of exercise on human articular cartilage. J. Anat. 208, 491–512 (2006).
Eckstein, F., Tieschky, M., Faber, S., Englmeier, K. H. & Reiser, M. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat. Embryol. 200, 419–424 (1999).
Sutter, E. G. et al. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am. J. Sports Med. 43, 370–376 (2015).
Liu, F. et al. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J. Biomech. 43, 658–665 (2010).
Van De Velde, S. K. et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 60, 3693–3702 (2009).
Kurz, B. et al. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J. Orthop. Res. 19, 1140–1146 (2001).
Patwari, P., Cheng, D. M., Cole, A. A., Kuettner, K. E. & Grodzinsky, A. J. Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human post-mortem knee and ankle cartilage. Biomech. Model. Mechanobiol. 6, 83–89 (2007).
Patwari, P. et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 48, 1292–1301 (2003).
Stolberg-Stolberg, J. A. et al. Effects of cartilage impact with and without fracture on chondrocyte viability and the release of inflammatory markers. J. Orthop. Res. 31, 1283–1292 (2013).
Natoli, R. M., Scott, C. C. & Athanasiou, K. A. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann. Biomed. Eng. 36, 780–792 (2008).
Guilak, F., Meyer, B. C., Ratcliffe, A. & Mow, V. C. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage 2, 91–101 (1994).
Ng, K. W. et al. Duty cycle of deformational loading influences the growth of engineered articular cartilage. Cell. Mol. Bioeng. 2, 386–394 (2009).
Nam, J., Aguda, B. D., Rath, B. & Agarwal, S. Biomechanical thresholds regulate inflammation through the NF-κB pathway: experiments and modeling. PLoS ONE 4, e5262 (2009).
Mauck, R. L., Nicoll, S. B., Seyhan, S. L., Ateshian, G. A. & Hung, C. T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 9, 597–611 (2003).
Torzilli, P. A., Bhargava, M. & Chen, C. T. Mechanical loading of articular cartilage reduces IL-1-induced enzyme expression. Cartilage 2, 364–373 (2011).
Chan, P. S. et al. Gene expression profile of mechanically impacted bovine articular cartilage explants. J. Orthop. Res. 23, 1146–1151 (2005).
Ashwell, M. S. et al. Changes in chondrocyte gene expression following in vitro impaction of porcine articular cartilage in an impact injury model. J. Orthop. Res. 31, 385–391 (2013).
Atkinson, T. S., Haut, R. C. & Altiero, N. J. An investigation of biphasic failure criteria for impact-induced fissuring of articular cartilage. J. Biomech. Eng. 120, 536–537 (1998).
Fragomen, A. T., McCoy, T. H., Meyers, K. N. & Rozbruch, S. R. Minimum distraction gap: how much ankle joint space is enough in ankle distraction arthroplasty? HSS J. 10, 6–12 (2014).
Sanchez-Adams, J., Leddy, H. A., McNulty, A. L., O’Conor, C. J. & Guilak, F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 16, 451 (2014).
Lafeber, F., Veldhuijzen, J. P., Vanroy, J. L. A. M., Huber-Bruning, O. & Bijlsma, J. W. J. Intermittent hydrostatic compressive force stimulates exclusively the proteoglycan synthesis of osteoarthritic human cartilage. Br. J. Rheumatol. 31, 437–442 (1992).
Van Valburg, A. A., Van Roy, H. L. A. M., Lafeber, F. P. J. G. & Bijlsma, J. W. J. Beneficial effects of intermittent fluid pressure of low physiological magnitude on cartilage and inflammation in osteoarthritis. An in vitro study. J. Rheumatol. 25, 515–520 (1998).
Slynarski, K., Walawski, J., Smigielski, R. & van der Merwe, W. Two-year results of the PHANTOM high flex trial: a single-arm study on the atlas unicompartmental knee system load absorber in patients with medial compartment osteoarthritis of the knee. Clin. Med. Insights Arthritis Musculoskelet. Disord. 12, 1–7 (2019).
Jung, W. H. et al. Second-look arthroscopic assessment of cartilage regeneration after medial opening-wedge high tibial osteotomy. Arthrosc. J. Arthrosc. Relat. Surg. 30, 72–79 (2014).
Slynarski, K. & Lipinski, L. Treating early knee osteoarthritis with the Atlas® Unicompartmental Knee System in a 26-year-old ex-professional basketball player: a case study. Case Rep. Orthop. 2017, 1–5 (2017).
Spahn, G., Klinger, H. M., Harth, P. & Hofmann, G. O. Cartilage regeneration after high tibial osteotomy results of an arthroscopic study. Z. Orthop. Unfall. 150, 272–279 (2012).
Bode, G. et al. Comparison of the efficiency of an extra-articular absorber system and high tibial osteotomy for unloading the medial knee compartment: an in vitro study. Knee Surg. Sport. Traumatol. Arthrosc. 25, 3695–3703 (2017).
Slynarski, K., Walawski, J., Smigielski, R. & van der Merwe, W. Feasibility of the Atlas Unicompartmental Knee System Load Absorber in improving pain relief and function in patients needing unloading of the medial compartment of the knee: 1-year follow-up of a prospective, multicenter, single-arm pilot study (PHANTOM). Clin. Med. Insights Arthritis Musculoskelet. Disord. 10, 1–9 (2017).
Bikle, D. D. & Halloran, B. P. The response of bone to unloading. J. Bone Miner. Metab. 17, 233–244 (1999).
Marijnissen, A. C. A. et al. Ankle images digital analysis (AIDA): Digital measurement of joint space width and subchondral sclerosis on standard radiographs. Osteoarthritis Cartilage 9, 264–272 (2001).
Pouders, C. et al. Prevalence and MRI-anatomic correlation of bone cysts in osteoarthritic knees. Am. J. Roentgenol. 190, 17–21 (2008).
Tanamas, S. K. et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis Res. Ther. 12, R58 (2010).
van Dijk, C. N., Reilingh, M. L., Zengerink, M. & van Bergen, C. J. A. The natural history of osteochondral lesions in the ankle. Instr. Course Lect. 59, 375–386 (2010).
Hwang, J. et al. Increased hydraulic conductance of human articular cartilage and subchondral bone plate with progression of osteoarthritis. Arthritis Rheum. 58, 3831–3842 (2008).
Carrino, J. A., Blum, J., Parellada, J. A., Schweitzer, M. E. & Morrison, W. B. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage 14, 1081–1085 (2006).
Hunter, D. J. et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res. Ther. 11, R11 (2009).
Yazawa, M., Kishi, K., Nakajima, H. & Nakajima, T. Expression of bone morphogenetic proteins during mandibular distraction osteogenesis in rabbits. J. Oral. Maxillofac. Surg. 61, 587–592 (2003).
Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133–143 (2012).
Song, J. et al. Fak-Mapk, Hippo and Wnt signalling pathway expression and regulation in distraction osteogenesis. Cell Prolif. 51, e12453 (2018).
Wiegant, K. et al. Evidence of cartilage repair by joint distraction in a canine model of osteoarthritis. Arthritis Rheumatol. 67, 465–474 (2015).
McGonagle, D., Baboolal, T. G. & Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 13, 719–730 (2017).
Jones, B. A. & Pei, M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng. Part B Rev. 18, 301–311 (2012).
Jones, E. A. et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 58, 1731–1740 (2008).
Chen, Y., Sun, Y., Pan, X., Ho, K. & Li, G. Joint distraction attenuates osteoarthritis by reducing secondary inflammation, cartilage degeneration and subchondral bone aberrant change. Osteoarthritis Cartilage 23, 1728–1735 (2015).
Sánchez, M. et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: a pilot study. Biomed. Res. Int. 2016, 4868613 (2016).
Kouroupis, D., Sanjurjo-Rodriguez, C., Jones, E. & Correa, D. Mesenchymal stem cell functionalization for enhanced therapeutic applications. Tissue Eng. Part B Rev. 25, 55–77 (2019).
Kania, K. et al. Expression of growth and differentiation factor 5 during joint repair and osteoarthritis. Osteoarthritis Cartilage 28, S34 (2020).
Kania, K. et al. Regulation of Gdf5 expression in joint remodelling, repair and osteoarthritis. Sci. Rep. 10, 157 (2020).
Leijten, J. C. H. et al. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res. Ther. 15, R126 (2013).
Raghu, H. et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 76, 914–922 (2017).
Miotla Zarebska, J. et al. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthritis Cartilage 25, 406–412 (2017).
Teunissen, M. et al. Knee joint distraction-induced shift from catabolic to anabolic state occurs after the distraction period. Osteoarthritis Cartilage 28, S76–S77 (2020).
Jeon, O. H. et al. Senescence cell–associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 4, e125019 (2019).
ArthroSave. KneeReviver. https://www.arthrosave.com/ (2020).
Acknowledgements
The authors thank F.P.J.G. Lafeber for a thorough review of the manuscript and discussion on the subjects described. This work is partially supported by grants from the Dutch Arthritis Foundation (LLP9) and Nederlandse Oganizatie voor Wetenschappelijk Onderzoek (NWO) Domain Applied and Engineering Sciences (P15-23) to S.C.M.
Review criteria
To find studies involving KJD treatment in OA (summarized in Table 1), MEDLINE, EMBASE and Web of Science libraries were searched with the following terms: (distraction OR arthrodiatasis OR arthrodiastasis) AND (knee OR tibiofemoral OR tibiofibular), and were applied on title and abstract and, in Web of Science, Keywords+. Only full-text publications about clinical studies in which KJD with external fixation was applied and the primary outcomes were patient-reported outcomes and/or cartilage tissue restoration were included in Table 1. Studies that did not fully meet the criteria are discussed in the text where relevant. Ongoing unpublished studies were included based on personal communication with principal investigators.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks E. Jones and T. Matsumoto for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Stress
-
Subject to pressure or tension.
- Strain
-
Relative deformation or change in shape and size of elastic, plastic and fluid materials under applied forces.
- Shear
-
A force tending to cause deformation of a material by slippage along a plane or planes parallel to the imposed stress.
Rights and permissions
About this article
Cite this article
Jansen, M.P., Mastbergen, S.C. Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat Rev Rheumatol 18, 35–46 (2022). https://doi.org/10.1038/s41584-021-00695-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00695-y
This article is cited by
-
Contribution of Soft Tissue Passive Forces in Thumb Carpometacarpal Joint Distraction
Annals of Biomedical Engineering (2024)
-
Atorvastatin loaded lecithin-coated zein nanoparticles based thermogel for the intra-articular management of osteoarthritis: in-silico, in-vitro, and in-vivo studies
Journal of Pharmaceutical Investigation (2024)
-
Patients with advanced lateral osteoarthritis can return to sports and work after distraction arthroplasty plus lateral meniscal allograft transplantation combined with cartilage repair
Knee Surgery, Sports Traumatology, Arthroscopy (2022)