Abstract
Monogenic autoinflammatory diseases are a group of rheumatologic disorders caused by dysregulation in the innate immune system. The molecular mechanisms of these disorders are linked to defects in inflammasome-mediated, NF-κB-mediated or interferon-mediated inflammatory signalling pathways, cytokine receptors, the actin cytoskeleton, proteasome complexes and various enzymes. As with other human disorders, disease-causing variants in a single gene can present with variable expressivity and incomplete penetrance. In some cases, pathogenic variants in the same gene can be inherited either in a recessive or dominant manner and can cause distinct and seemingly unrelated phenotypes, although they have a unifying biochemical mechanism. With an enhanced understanding of protein structure and functionality of protein domains, genotype–phenotype correlations are beginning to be unravelled. Many of the mutated proteins are primarily expressed in haematopoietic cells, and their malfunction leads to systemic inflammation. Disease presentation is also defined by a specific effect of the mutant protein in a particular cell type and, therefore, the resulting phenotype might be more deleterious in one tissue than in another. Many patients present with the expanded immunological disease continuum that includes autoinflammation, immunodeficiency, autoimmunity and atopy, which necessitate genetic testing.
Key points
-
Mendelian-inherited pathogenic variants in a given gene can have different inheritance patterns and can cause distinct and sometimes opposing clinical phenotypes.
-
Pathogenic variants in the same gene can have variable disease expressivity depending on their effect on protein function.
-
Pathogenic variants in different genes can result in a similar phenotype by virtue of converging on the same signalling pathway (as exemplified by the spectrum of phenotypes denoted as familial cold autoinflammatory syndromes (FCAS).
-
A growing number of autoinflammatory diseases can be explained by non-Mendelian inheritance of somatic variants.
-
Although systemic inflammation and fevers are common features of autoinflammatory diseases, specific organ manifestations are determined by the tissue expression of mutated proteins.
-
Other genetic alleles and risk factors, such as infections and stress, also contribute to the phenotypic variability of autoinflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Manthiram, K., Zhou, Q., Aksentijevich, I. & Kastner, D. L. Corrigendum: the monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 18, 1271 (2017).
Savic, S., Caseley, E. A. & McDermott, M. F. Moving towards a systems-based classification of innate immune-mediated diseases. Nat. Rev. Rheumatol. 16, 222–237 (2020).
Beck, D. B. & Aksentijevich, I. Biochemistry of autoinflammatory diseases: catalyzing monogenic disease. Front. Immunol. 10, 101 (2019).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Feng, S., Fox, D. & Man, S. M. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J. Mol. Biol. 430 (18 Pt B), 3068–3080 (2018).
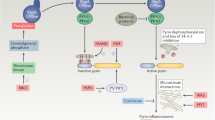
Xu, H. et al. Innate immune sensing of bacterial modifications of rho GTPases by the pyrin inflammasome. Nature 513, 237–241 (2014).
Prudnikova, T. Y., Rawat, S. J. & Chernoff, J. Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin. Cancer Res. 21, 24–29 (2015).
Gao, W., Yang, J., Liu, W., Wang, Y. & Shao, F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl Acad. Sci. USA 113, E4857–E4866 (2016).
Park, Y. H., Wood, G., Kastner, D. L. & Chae, J. J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 17, 914–921 (2016).
Tidow, N. et al. Hematopoietic-specific expression of MEFV, the gene mutated in familial Mediterranean fever, and subcellular localization of its corresponding protein, pyrin. Blood 95, 1451–1455 (2000).
Schnappauf, O., Chae, J. J., Kastner, D. L. & Aksentijevich, I. The pyrin inflammasome in health and disease. Front. Immunol. 10, 1745 (2019).
Ozdogan, H. & Ugurlu, S. Familial Mediterranean fever. Presse Med. 48, e61–e76 (2019).
Maggio, M. C. & Corsello, G. FMF is not always "fever": from clinical presentation to "treat to target". Ital. J. Pediatr. 46, 7 (2020).
Weinert, C., Grutter, C., Roschitzki-Voser, H., Mittl, P. R. & Grutter, M. G. The crystal structure of human pyrin b30.2 domain: implications for mutations associated with familial Mediterranean fever. J. Mol. Biol. 394, 226–236 (2009).
Grossman, C., Kassel, Y., Livneh, A. & Ben-Zvi, I. Familial Mediterranean fever (FMF) phenotype in patients homozygous to the MEFV M694V mutation. Eur. J. Med. Genet. 62, 103532 (2019).
Stoler, I. et al. Gene-dose effect of MEFV gain-of-function mutations determines ex vivo neutrophil activation in familial Mediterranean fever. Front. Immunol. 11, 716 (2020).
Kone-Paut, I. et al. The clinical spectrum of 94 patients carrying a single mutated MEFV allele. Rheumatology 48, 840–842 (2009).
Lachmann, H. J. et al. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology 45, 746–750 (2006).
Jamilloux, Y. et al. Familial Mediterranean fever mutations are hypermorphic mutations that specifically decrease the activation threshold of the Pyrin inflammasome. Rheumatology 57, 100–111 (2018).
Koshy, R., Sivadas, A. & Scaria, V. Genetic epidemiology of familial Mediterranean fever through integrative analysis of whole genome and exome sequences from Middle East and North Africa. Clin. Genet. 93, 92–102 (2018).
Park, Y. H. et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat. Immunol. 21, 857–867 (2020).
Booth, D. R. et al. The genetic basis of autosomal dominant familial Mediterranean fever. QJM 93, 217–221 (2000).
Rowczenio, D. M. et al. Autosomal dominant familial Mediterranean fever in Northern European Caucasians associated with deletion of p.M694 residue — a case series and genetic exploration. Rheumatology 56, 209–213 (2017).
Papa, R. et al. A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry. Orphanet J. Rare Dis. 12, 167 (2017).
Masters, S. L. et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci. Transl. Med. 8, 332ra345 (2016).
Moghaddas, F. et al. A novel Pyrin-associated autoinflammation with neutrophilic dermatosis mutation further defines 14-3-3 binding of pyrin and distinction to familial Mediterranean fever. Ann. Rheum. Dis. 76, 2085–2094 (2017).
Jeru, I. et al. Interaction of pyrin with 14.3.3 in an isoform-specific and phosphorylation-dependent manner regulates its translocation to the nucleus. Arthritis Rheum. 52, 1848–1857 (2005).
Hong, Y. et al. Autoinflammation due to homozygous S208 MEFV mutation. Ann. Rheum. Dis. 78, 571–573 (2019).
Aldea, A. et al. A severe autosomal-dominant periodic inflammatory disorder with renal AA amyloidosis and colchicine resistance associated to the MEFV H478Y variant in a Spanish kindred: an unusual familial Mediterranean fever phenotype or another MEFV-associated periodic inflammatory disorder? Am. J. Med. Genet. A 124, 67–73 (2004).
Stoffels, M. et al. MEFV mutations affecting pyrin amino acid 577 cause autosomal dominant autoinflammatory disease. Ann. Rheum. Dis. 73, 455–461 (2014).
Rowczenio, D. M. et al. British kindred with dominant FMF associated with high incidence of AA amyloidosis caused by novel MEFV variant, and a review of the literature. Rheumatology 59, 554–558 (2020).
Honda, Y. et al. Rapid flow cytometry-based assay for the functional classification of MEFV variants. J. Clin. Immunol. https://doi.org/10.1007/s10875-021-01021-7 (2021).
Van Gorp, H. et al. Blood-based test for diagnosis and functional subtyping of familial Mediterranean fever. Ann. Rheum. Dis. 79, 960–968 (2020).
Gattorno, M. et al. Differentiating PFAPA syndrome from monogenic periodic fevers. Pediatrics 124, e721–e728 (2009).
Taniuchi, S. et al. MEFV variants in patients with PFAPA syndrome in Japan. Open Rheumatol. J. 7, 22–25 (2013).
De Benedetti, F. et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 378, 1908–1919 (2018).
Badour, K. et al. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199, 99–112 (2004).
Thrasher, A. J. & Burns, S. O. WASP: a key immunological multitasker. Nat. Rev. Immunol. 10, 182–192 (2010).
Yu, J. W. et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol. Cell 28, 214–227 (2007).
Demidowich, A. P. et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum. 64, 2022–2027 (2012).
Wise, C. A. et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 11, 961–969 (2002).
Toplak, N. et al. An International registry on Autoinflammatory diseases: the Eurofever experience. Ann. Rheum. Dis. 71, 1177–1182 (2012).
Fathalla, B. M., Al-Wahadneh, A. M., Al-Mutawa, M., Kambouris, M. & El-Shanti, H. A novel de novo PSTPIP1 mutation in a boy with pyogenic arthritis, pyoderma gangrenosum, acne (PAPA) syndrome. Clin. Exp. Rheumatol. 32, 956–958 (2014).
Holzinger, D. et al. Single amino acid charge switch defines clinically distinct proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1)-associated inflammatory diseases. J. Allergy Clin. Immunol. 136, 1337–1345 (2015).
Holzinger, D. & Roth, J. Alarming consequences – autoinflammatory disease spectrum due to mutations in proline-serine-threonine phosphatase-interacting protein 1. Curr. Opin. Rheumatol. 28, 550–559 (2016).
Dai, P. et al. Autoinflammation masquerading as autoimmunity in an adult with heterozygous p.E250K PSTPIP1 mutation. J. Clin. Immunol. 39, 519–522 (2019).
Belelli, E. et al. Haematological involvement associated with a mild autoinflammatory phenotype, in two patients carrying the E250K mutation of PSTPIP1. Clin. Exp. Rheumatol. 35, 113–115 (2017).
Mistry, P. et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann. Rheum. Dis. 77, 1825–1833 (2018).
Shoham, N. G. et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl Acad. Sci. USA 100, 13501–13506 (2003).
Janssen, W. J. M. et al. Proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) controls immune synapse stability in human T cells. J. Allergy Clin. Immunol. 142, 1947–1955 (2018).
Starnes, T. W. et al. The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood 123, 2703–2714 (2014).
Zeeli, T. et al. Pyoderma gangrenosum, acne and ulcerative colitis in a patient with a novel mutation in the PSTPIP1 gene. Clin. Exp. Dermatol. 40, 367–372 (2015).
Marzano, A. V. et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br. J. Dermatol. 176, 1588–1598 (2017).
Calderon-Castrat, X. et al. PSTPIP1 gene mutation in a pyoderma gangrenosum, acne and suppurative hidradenitis (PASH) syndrome. Br. J. Dermatol. 175, 194–198 (2016).
Cugno, M., Borghi, A. & Marzano, A. V. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am. J. Clin. Dermatol. 18, 555–562 (2017).
Saito, N. et al. Novel PSTPIP1 gene mutation in pyoderma gangrenosum, acne and suppurative hidradenitis syndrome. J. Dermatol. 45, e213–e214 (2018).
Cote, J. F. et al. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J. Biol. Chem. 277, 2973–2986 (2002).
Omenetti, A. et al. Disease activity accounts for long-term efficacy of IL-1 blockers in pyogenic sterile arthritis pyoderma gangrenosum and severe acne syndrome. Rheumatology 55, 1325–1335 (2016).
Laberko, A. et al. HSCT is effective in patients with PSTPIP1-associated myeloid-related proteinemia inflammatory (PAMI) syndrome. J. Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2020.11.043 (2020).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Akula, M. K. et al. Control of the innate immune response by the mevalonate pathway. Nat. Immunol. 17, 922–929 (2016).
Skinner, O. P. et al. Lack of protein prenylation promotes NLRP3 inflammasome assembly in human monocytes. J. Allergy Clin. Immunol. 143, 2315–2317.e3 (2019).
Ter Haar, N. M. et al. The phenotype and genotype of mevalonate kinase deficiency: a series of 114 cases from the Eurofever registry. Arthritis Rheumatol. 68, 2795–2805 (2016).
Frenkel, J. et al. Lack of isoprenoid products raises ex vivo interleukin-1beta secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum. 46, 2794–2803 (2002).
Houten, S. M. et al. Temperature dependence of mutant mevalonate kinase activity as a pathogenic factor in hyper-IgD and periodic fever syndrome. Hum. Mol. Genet. 11, 3115–3124 (2002).
Houten, S. M., van Woerden, C. S., Wijburg, F. A., Wanders, R. J. & Waterham, H. R. Carrier frequency of the V377I (1129G>A) MVK mutation, associated with Hyper-IgD and periodic fever syndrome, in the Netherlands. Eur. J. Hum. Genet. 11, 196–200 (2003).
Mandey, S. H., Schneiders, M. S., Koster, J. & Waterham, H. R. Mutational spectrum and genotype-phenotype correlations in mevalonate kinase deficiency. Hum. Mutat. 27, 796–802 (2006).
Zhang, S. Q. et al. Exome sequencing identifies MVK mutations in disseminated superficial actinic porokeratosis. Nat. Genet. 44, 1156–1160 (2012).
Kubo, A. et al. Clonal expansion of second-hit cells with somatic recombinations or C>T transitions form Porokeratosis in MVD or MVK mutant heterozygotes. J. Invest. Dermatol. 139, 2458–2466.e9 (2019).
Neven, B. et al. Allogeneic bone marrow transplantation in mevalonic aciduria. N. Engl. J. Med. 356, 2700–2703 (2007).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Mathur, A., Hayward, J. A. & Man, S. M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Biol. 103, 233–257 (2018).
Sandall, C. F., Ziehr, B. K. & MacDonald, J. A. ATP-binding and hydrolysis in inflammasome activation. Molecules 25, 4572 (2020).
Alehashemi, S. & Goldbach-Mansky, R. Human autoinflammatory diseases mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-inflammasome dysregulation updates on diagnosis, treatment, and the respective roles of IL-1 and IL-18. Front. Immunol. 11, 1840 (2020).
Chavarria-Smith, J., Mitchell, P. S., Ho, A. M., Daugherty, M. D. & Vance, R. E. Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 inflammasome activation. PLoS Pathog. 12, e1006052 (2016).
Masters, S. L. et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37, 1009–1023 (2012).
Yu, C. H., Moecking, J., Geyer, M. & Masters, S. L. Mechanisms of NLRP1-mediated autoinflammatory disease in humans and mice. J. Mol. Biol. 430, 142–152 (2018).
Bauernfried, S., Scherr, M. J., Pichlmair, A., Duderstadt, K. E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 371, eabd0811 (2021).
Kummer, J. A. et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 55, 443–452 (2007).
Finger, J. N. et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 287, 25030–25037 (2012).
Hollingsworth, L. R. et al. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature https://doi.org/10.1038/s41586-021-03350-4 (2021).
Soler, V. J. et al. Whole exome sequencing identifies a mutation for a novel form of corneal intraepithelial dyskeratosis. J. Med. Genet. 50, 246–254 (2013).
Zhong, F. L. et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell 167, 187–202.e17 (2016).
Herlin, T. et al. Autoinflammatory disease with corneal and mucosal dyskeratosis caused by a novel NLRP1 variant. Rheumatology 59, 2334–2339 (2020).
Grandemange, S. et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann. Rheum. Dis. 76, 1191–1198 (2017).
Drutman, S. B. et al. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc. Natl Acad. Sci. USA 116, 19055–19063 (2019).
Magitta, N. F. et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 10, 120–124 (2009).
Jin, Y. et al. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 356, 1216–1225 (2007).
Levandowski, C. B. et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc. Natl Acad. Sci. USA. 110, 2952–2956 (2013).
Fenini, G., Karakaya, T., Hennig, P., Di Filippo, M. & Beer, H. D. The NLRP1 inflammasome in human skin and beyond. Int. J. Mol. Sci. 21, 4788 (2020).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008).
de Torre-Minguela, C., Mesa Del Castillo, P. & Pelegrin, P. The NLRP3 and pyrin inflammasomes: implications in the pathophysiology of autoinflammatory diseases. Front. Immunol. 8, 43 (2017).
Guarda, G. et al. Differential expression of NLRP3 among hematopoietic cells. J. Immunol. 186, 2529–2534 (2011).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Booshehri, L. M. & Hoffman, H. M. CAPS and NLRP3. J. Clin. Immunol. 39, 277–286 (2019).
Jéru, I., Hayrapetyan, H., Duquesnoy, P., Sarkisian, T. & Amselem, S. PYPAF1 nonsense mutation in a patient with an unusual autoinflammatory syndrome: role of PYPAF1 in inflammation. Arthritis Rheum. 54, 508–514 (2006).
Frenkel, J., van Kempen, M. J., Kuis, W. & van Amstel, H. K. Variant chronic infantile neurologic, cutaneous, articular syndrome due to a mutation within the leucine-rich repeat domain of CIAS1. Arthritis Rheum. 50, 2719–2720 (2004).
Matsubayashi, T., Sugiura, H., Arai, T., Oh-Ishi, T. & Inamo, Y. Anakinra therapy for CINCA syndrome with a novel mutation in exon 4 of the CIAS1 gene. Acta Paediatr. 95, 246–249 (2006).
Jeru, I. et al. Functional consequences of a germline mutation in the leucine-rich repeat domain of NLRP3 identified in an atypical autoinflammatory disorder. Arthritis Rheum. 62, 1176–1185 (2010).
Nakanishi, H. et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl Acad. Sci. USA 114, E7766–E7775 (2017).
Hu, J., Zhu, Y., Zhang, J. Z., Zhang, R. G. & Li, H. M. A novel mutation in the pyrin domain of the NOD-like receptor family pyrin domain containing protein 3 in Muckle-Wells syndrome. Chin. Med. J. 130, 586–593 (2017).
Turunen, J. A. et al. Keratoendotheliitis fugax hereditaria: a novel cryopyrin-associated periodic syndrome caused by a mutation in the nucleotide-binding domain, leucine-rich repeat family, pyrin domain-containing 3 (NLRP3) gene. Am. J. Ophthalmol. 188, 41–50 (2018).
Saito, M. et al. Somatic mosaicism of CIAS1 in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 52, 3579–3585 (2005).
Tanaka, N. et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 63, 3625–3632 (2011).
Zhou, Q. et al. Brief report: cryopyrin-associated periodic syndrome caused by a myeloid-restricted somatic NLRP3 mutation. Arthritis Rheumatol. 67, 2482–2486 (2015).
Lasiglie, D. et al. Cryopyrin-associated periodic syndromes in Italian patients: evaluation of the rate of somatic NLRP3 mosaicism and phenotypic characterization. J. Rheumatol. 44, 1667–1673 (2017).
Rowczenio, D. M. et al. Late-onset cryopyrin-associated periodic syndromes caused by somatic NLRP3 mosaicism-UK single center experience. Front. Immunol. 8, 1410 (2017).
Assrawi, E. et al. Somatic mosaic NLRP3 mutations and inflammasome activation in late-onset chronic urticaria. J. Invest. Dermatol. 140, 791–798.e2 (2020).
Louvrier, C. et al. NLRP3-associated autoinflammatory diseases: phenotypic and molecular characteristics of germline versus somatic mutations. J. Allergy Clin. Immunol. 145, 1254–1261 (2020).
Gusdorf, L. & Lipsker, D. Schnitzler syndrome: a review. Curr. Rheumatol. Rep. 19, 46 (2017).
de Koning, H. D. et al. Myeloid lineage-restricted somatic mosaicism of NLRP3 mutations in patients with variant Schnitzler syndrome. J. Allergy Clin. Immunol. 135, 561–564 (2015).
Rowczenio, D. M. et al. Molecular genetic investigation, clinical features, and response to treatment in 21 patients with Schnitzler syndrome. Blood 131, 974–981 (2018).
Kuemmerle-Deschner, J. B. et al. Clinical and molecular phenotypes of low-penetrance variants of NLRP3: diagnostic and therapeutic challenges. Arthritis Rheumatol. 69, 2233–2240 (2017).
Goldbach-Mansky, R. et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N. Engl. J. Med. 355, 581–592 (2006).
Lachmann, H. J. et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 (2009).
Vance, R. E. The NAIP/NLRC4 inflammasomes. Curr. Opin. Immunol. 32C, 84–89 (2015).
Rauch, I. et al. NAIP-NLRC4 Inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46, 649–659 (2017).
Duncan, J. A. & Canna, S. W. The NLRC4 inflammasome. Immunol. Rev. 281, 115–123 (2018).
Poyet, J. L. et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276, 28309–28313 (2001).
Hu, Z. et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341, 172–175 (2013).
Qu, Y. et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490, 539–542 (2012).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014).
Romberg, N. et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46, 1135–1139 (2014).
Liang, J. et al. Novel NLRC4 mutation causes a syndrome of perinatal autoinflammation with hemophagocytic lymphohistiocytosis, hepatosplenomegaly, fetal thrombotic vasculopathy, and congenital anemia and ascites. Pediatr. Dev. Pathol. 20, 498–505 (2017).
Kawasaki, Y. et al. Identification of a high-frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell-based phenotype dissection. Arthritis Rheumatol. 69, 447–459 (2017).
Moghaddas, F. et al. Autoinflammatory mutation in NLRC4 reveals a leucine-rich repeat (LRR)–LRR oligomerization interface. J. Allergy Clin. Immunol. 142, 1956–1967.e6 (2018).
Chear, C. T. et al. A novel de novo NLRC4 mutation reinforces the likely pathogenicity of specific LRR domain mutation. Clin. Immunol. 211, 108328 (2020).
Kitamura, A., Sasaki, Y., Abe, T., Kano, H. & Yasutomo, K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 211, 2385–2396 (2014).
Raghawan, A. K. et al. A disease-associated mutant of NLRC4 shows enhanced interaction with SUG1 leading to constitutive FADD-dependent caspase-8 activation and cell death. J. Biol. Chem. 292, 1218–1230 (2017).
Volker-Touw, C. M. et al. Erythematous nodes, urticarial rash and arthralgias in a large pedigree with NLRC4-related autoinflammatory disease, expansion of the phenotype. Br. J. Dermatol. 176, 244–248 (2017).
Canna, S. W. et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 139, 1698–1701 (2017).
Trindade, B. C. & Chen, G. Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol. Rev. 297, 139–161 (2020).
Gutierrez, O. et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 277, 41701–41705 (2002).
Philpott, D. J., Sorbara, M. T., Robertson, S. J., Croitoru, K. & Girardin, S. E. NOD proteins: regulators of inflammation in health and disease. Nat. Rev. Immunol. 14, 9–23 (2014).
Travassos, L. H., Carneiro, L. A., Girardin, S. & Philpott, D. J. Nod proteins link bacterial sensing and autophagy. Autophagy 6, 409–411 (2010).
Strober, W., Asano, N., Fuss, I., Kitani, A. & Watanabe, T. Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn’s disease. Immunol. Rev. 260, 249–260 (2014).
Okafuji, I. et al. Role of the NOD2 genotype in the clinical phenotype of Blau syndrome and early-onset sarcoidosis. Arthritis Rheum. 60, 242–250 (2009).
Rose, C. D. et al. Blau syndrome: cross-sectional data from a multicentre study of clinical, radiological and functional outcomes. Rheumatology 54, 1008–1016 (2015).
de Inocencio, J. et al. Somatic NOD2 mosaicism in Blau syndrome. J. Allergy Clin. Immunol. 136, 484–487 e482 (2015).
Mensa-Vilaro, A. et al. Brief report: first identification of intrafamilial recurrence of Blau syndrome due to gonosomal NOD2 mosaicism. Arthritis Rheumatol. 68, 1039–1044 (2016).
Kanazawa, N. et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105, 1195–1197 (2005).
Maekawa, S., Ohto, U., Shibata, T., Miyake, K. & Shimizu, T. Crystal structure of NOD2 and its implications in human disease. Nat. Commun. 7, 11813 (2016).
Takada, S. et al. Pluripotent stem cell models of Blau syndrome reveal an IFN-gamma-dependent inflammatory response in macrophages. J. Allergy Clin. Immunol. 141, 339–349.e11 (2018).
Yao, Q. et al. NOD2-associated autoinflammatory disease: a large cohort study. Rheumatology 54, 1904–1912 (2015).
McDonald, C., Shen, M., Johnson, E. E., Kabi, A. & Yao, Q. Alterations in nucleotide-binding oligomerization domain-2 expression, pathway activation, and cytokine production in Yao syndrome. Autoimmunity 51, 53–61 (2018).
Netea, M. G. et al. NOD2 3020insC mutation and the pathogenesis of Crohn’s disease: impaired IL-1beta production points to a loss-of-function phenotype. Neth. J. Med. 63, 305–308 (2005).
Ma, C. et al. Anti-TNF therapy within 2 years of Crohn’s disease diagnosis improves patient outcomes: a retrospective cohort study. Inflamm. Bowel Dis. 22, 870–879 (2016).
Harden, J. L. et al. CARD14 expression in dermal endothelial cells in psoriasis. PLoS ONE 9, e111255 (2014).
Van Nuffel, E. et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J. Invest. Dermatol. 137, 569–575 (2017).
Howes, A. et al. Psoriasis mutations disrupt CARD14 autoinhibition promoting BCL10-MALT1-dependent NF-κB activation. Biochem. J. 473, 1759–1768 (2016).
Jordan, C. T. et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-κB, in psoriasis. Am. J. Hum. Genet. 90, 796–808 (2012).
Jordan, C. T. et al. PSORS2 is due to mutations in CARD14. Am. J. Hum. Genet. 90, 784–795 (2012).
Fuchs-Telem, D. et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am. J. Hum. Genet. 91, 163–170 (2012).
Ammar, M. et al. CARD14 alterations in Tunisian patients with psoriasis and further characterization in European cohorts. Br. J. Dermatol. 174, 330–337 (2016).
Li, Q. et al. Analysis of CARD14 polymorphisms in pityriasis rubra pilaris: activation of NF-κB. J. Invest. Dermatol. 135, 1905–1908 (2015).
Israel, L. & Mellett, M. Clinical and genetic heterogeneity of CARD14 mutations in psoriatic skin disease. Front. Immunol. 9, 2239 (2018).
Sugiura, K., Muto, M. & Akiyama, M. CARD14 c.526G>C (p.Asp176His) is a significant risk factor for generalized pustular psoriasis with psoriasis vulgaris in the Japanese cohort. J. Invest. Dermatol. 134, 1755–1757 (2014).
Peled, A. et al. Loss-of-function mutations in caspase recruitment domain-containing protein 14 (CARD14) are associated with a severe variant of atopic dermatitis. J. Allergy Clin. Immunol. 143, 173–181.e10 (2019).
Eytan, O., Sarig, O., Sprecher, E. & van Steensel, M. A. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br. J. Dermatol. 171, 420–422 (2014).
Mostowy, S. & Shenoy, A. R. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence. Nat. Rev. Immunol. 15, 559–573 (2015).
Tangye, S. G. et al. Human inborn errors of the actin cytoskeleton affecting immunity: way beyond WAS and WIP. Immunol. Cell Biol. 97, 389–402 (2019).
Takenawa, T. & Suetsugu, S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48 (2007).
Park, H., Chan, M. M. & Iritani, B. M. Hem-1: putting the "WAVE" into actin polymerization during an immune response. FEBS Lett. 584, 4923–4932 (2010).
Martinelli, S. et al. Functional dysregulation of CDC42 causes diverse developmental phenotypes. Am. J. Hum. Genet. 102, 309–320 (2018).
Lam, M. T. et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J. Exp. Med. 216, 2778–2799 (2019).
Gernez, Y. et al. Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1beta inhibition. J. Allergy Clin. Immunol. 144, 1122–1125.e6 (2019).
Aicart-Ramos, C., Valero, R. A. & Rodriguez-Crespo, I. Protein palmitoylation and subcellular trafficking. Biochim. Biophys. Acta 1808, 2981–2994 (2011).
Castro, C. N. et al. NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. J. Exp. Med. 217, e20192275 (2020).
Cook, S. A. et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science 369, 202–207 (2020).
Koronakis, V. et al. WAVE regulatory complex activation by cooperating GTPases Arf and Rac1. Proc. Natl Acad. Sci. USA 108, 14449–14454 (2011).
Kahr, W. H. et al. Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nat. Commun. 8, 14816 (2017).
Brigida, I. et al. T-cell defects in patients with ARPC1B germline mutations account for combined immunodeficiency. Blood 132, 2362–2374 (2018).
Volpi, S. et al. A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. J. Allergy Clin. Immunol. 143, 2296–2299 (2019).
Randzavola, L. O. et al. Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity. J. Clin. Invest. 129, 5600–5614 (2019).
Standing, A. S. et al. Autoinflammatory periodic fever, immunodeficiency, and thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene WDR1. J. Exp. Med. 214, 59–71 (2017).
Kuhns, D. B. et al. Cytoskeletal abnormalities and neutrophil dysfunction in WDR1 deficiency. Blood 128, 2135–2143 (2016).
Rodero, M. P. & Crow, Y. J. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J. Exp. Med. 213, 2527–2538 (2016).
Gruber, C. et al. Homozygous STAT2 gain-of-function mutation by loss of USP18 activity in a patient with type I interferonopathy. J. Exp. Med. 217, e20192319 (2020).
Hu, M. et al. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J. Clin. Invest. 131, e139333 (2021).
Burdette, D. L. & Vance, R. E. STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 14, 19–26 (2013).
Kumar, V. A STING to inflammation and autoimmunity. J. Leukoc. Biol. 106, 171–185 (2019).
Ergun, S. L., Fernandez, D., Weiss, T. M. & Li, L. STING polymer structure reveals mechanisms for activation, hyperactivation, and inhibition. Cell 178, 290–301.e10 (2019).
Tanaka, Y. & Chen, Z. J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 5, ra20 (2012).
Fremond, M. L. & Crow, Y. J. STING-mediated lung inflammation and beyond. J. Clin. Immunol. 41, 501–514 (2021).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Fremond, M. L. et al. Overview of STING-associated vasculopathy with onset in infancy (SAVI) among 21 patients. J. Allergy Clin. Immunol. Pract. 9, 803–818.e11 (2021).
Jeremiah, N. et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 124, 5516–5520 (2014).
Picard, C. et al. Severe pulmonary fibrosis as the first manifestation of interferonopathy (TMEM173 mutation). Chest 150, e65–e71 (2016).
Konig, N. et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 76, 468–472 (2017).
Konno, H. et al. Pro-inflammation associated with a gain-of-function mutation (R284S) in the innate immune sensor STING. Cell Rep. 23, 1112–1123 (2018).
Saldanha, R. G. et al. A mutation outside the dimerization domain causing atypical STING-associated vasculopathy with onset in infancy. Front. Immunol. 9, 1535 (2018).
Keskitalo, S. et al. Novel TMEM173 mutation and the role of disease modifying alleles. Front. Immunol. 10, 2770 (2019).
Seo, J. et al. Tofacitinib relieves symptoms of stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy caused by 2 de novo variants in TMEM173. J. Allergy Clin. Immunol. 139, 1396–1399.e12 (2017).
Lin, B. et al. A novel STING1 variant causes a recessive form of STING-associated vasculopathy with onset in infancy (SAVI). J. Allergy Clin. Immunol. 146, 1204–1208.e6 (2020).
Melki, I. et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J. Allergy Clin. Immunol. 140, 543–552.e5 (2017).
Sanchez, G. A. M. et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J. Clin. Invest. 128, 3041–3052 (2018).
Murata, S., Takahama, Y., Kasahara, M. & Tanaka, K. The immunoproteasome and thymoproteasome: functions, evolution and human disease. Nat. Immunol. 19, 923–931 (2018).
Ebstein, F., Poli Harlowe, M. C., Studencka-Turski, M. & Kruger, E. Contribution of the unfolded protein response (UPR) to the pathogenesis of proteasome-associated autoinflammatory syndromes (PRAAS). Front. Immunol. 10, 2756 (2019).
Agarwal, A. K. et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am. J. Hum. Genet. 87, 866–872 (2010).
Arima, K. et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc. Natl Acad. Sci. USA 108, 14914–14919 (2011).
Kitamura, A. et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J. Clin. Invest. 121, 4150–4160 (2011).
Liu, Y. et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 64, 895–907 (2012).
Torrelo, A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front. Immunol. 8, 927 (2017).
Brehm, A. et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Invest. 126, 795 (2016).
Sarrabay, G. et al. PSMB10, the last immunoproteasome gene missing for PRAAS. J. Allergy Clin. Immunol. 145, 1015–1017.e6 (2020).
Kataoka, S. et al. Successful treatment of a novel type I interferonopathy due to a de novo PSMB9 gene mutation with a Janus kinase inhibitor. J Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2021.03.010 (2021).
Poli, M. C. et al. Heterozygous truncating variants in POMP escape nonsense-mediated decay and cause a unique immune dysregulatory syndrome. Am. J. Hum. Genet. 102, 1126–1142 (2018).
Dahlqvist, J. et al. A single-nucleotide deletion in the POMP 5′ UTR causes a transcriptional switch and altered epidermal proteasome distribution in KLICK genodermatosis. Am. J. Hum. Genet. 86, 596–603 (2010).
de Jesus, A. A. et al. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol. 143, 1939–1943.e8 (2019).
Packard, T. A. & Cambier, J. C. B lymphocyte antigen receptor signaling: initiation, amplification, and regulation. F1000Prime Rep. 5, 40 (2013).
Rosen, L. B., Ginty, D. D., Weber, M. J. & Greenberg, M. E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12, 1207–1221 (1994).
Ombrello, M. J. et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 366, 330–338 (2012).
Zhou, Q. et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 91, 713–720 (2012).
Neves, J. F. et al. Novel PLCG2 mutation in a patient with APLAID and Cutis Laxa. Front. Immunol. 9, 2863 (2018).
Moran-Villasenor, E. et al. Expanding the clinical features of autoinflammation and phospholipase Cgamma2-associated antibody deficiency and immune dysregulation by description of a novel patient. J. Eur. Acad. Dermatol. Venereol. 33, 2334–2339 (2019).
Martin-Nalda, A. et al. Severe autoinflammatory manifestations and antibody deficiency due to novel hypermorphic PLCG2 mutations. J. Clin. Immunol. 40, 987–1000 (2020).
Milner, J. D. PLAID: a syndrome of complex patterns of disease and unique phenotypes. J. Clin. Immunol. 35, 527–530 (2015).
Walliser, C. et al. Functional characterization of phospholipase C-gamma2 mutant protein causing both somatic ibrutinib resistance and a germline monogenic autoinflammatory disorder. Oncotarget 9, 34357–34378 (2018).
Bastarache, L. et al. Phenotype risk scores identify patients with unrecognized Mendelian disease patterns. Science 359, 1233–1239 (2018).
Liu, T. M. et al. Hypermorphic mutation of phospholipase C, gamma2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood 126, 61–68 (2015).
He, S. & Wang, X. RIP kinases as modulators of inflammation and immunity. Nat. Immunol. 19, 912–922 (2018).
Meng, Y., Sandow, J. J., Czabotar, P. E. & Murphy, J. M. The regulation of necroptosis by post-translational modifications. Cell Death Differ. 28, 861–883 (2021).
Cuchet-Lourenço, D. et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 361, 810–813 (2018).
Li, Y. et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 116, 970–975 (2019).
Tao, P. et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114 (2020).
Lalaoui, N. et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 577, 103–108 (2020).
Navon Elkan, P. et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N. Engl. J. Med. 370, 921–931 (2014).
Zhou, Q. et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N. Engl. J. Med. 370, 911–920 (2014).
Zavialov, A. V. & Engstrom, A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem. J. 391, 51–57 (2005).
Iwaki-Egawa, S., Yamamoto, T. & Watanabe, Y. Human plasma adenosine deaminase 2 is secreted by activated monocytes. Biol. Chem. 387, 319–321 (2006).
Meyts, I. & Aksentijevich, I. Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J. Clin. Immunol. 38, 569–578 (2018).
Hashem, H., Kelly, S. J., Ganson, N. J. & Hershfield, M. S. Deficiency of adenosine deaminase 2 (DADA2), an inherited cause of polyarteritis nodosa and a mimic of other systemic rheumatologic disorders. Curr. Rheumatol. Rep. 19, 70 (2017).
Lee, P. Y. et al. Disrupted N-linked glycosylation as a disease mechanism in deficiency of ADA2. J. Allergy Clin. Immunol. 142, 1363–1365.e8 (2018).
Lee, P. Y. et al. Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2). J. Allergy Clin. Immunol. 145, 1664–1672.e10 (2020).
Ombrello, A. K. et al. Treatment strategies for deficiency of adenosine deaminase 2. N. Engl. J. Med. 380, 1582–1584 (2019).
Hashem, H. et al. Hematopoietic stem cell transplantation rescues the hematological, immunological, and vascular phenotype in DADA2. Blood 130, 2682–2688 (2017).
Nagaike, T. et al. Identification and characterization of mammalian mitochondrial tRNA nucleotidyltransferases. J. Biol. Chem. 276, 40041–40049 (2001).
Wilusz, J. E., Whipple, J. M., Phizicky, E. M. & Sharp, P. A. tRNAs marked with CCACCA are targeted for degradation. Science 334, 817–821 (2011).
Wiseman, D. H. et al. A novel syndrome of congenital sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmental delay (SIFD). Blood 122, 112–123 (2013).
Chakraborty, P. K. et al. Mutations in TRNT1 cause congenital sideroblastic anemia with immunodeficiency, fevers, and developmental delay (SIFD). Blood 124, 2867–2871 (2014).
Leibovitch, M., Hanic-Joyce, P. J. & Joyce, P. B. M. In vitro studies of disease-linked variants of human tRNA nucleotidyltransferase reveal decreased thermal stability and altered catalytic activity. Biochim. Biophys. Acta Proteins Proteom. 1866, 527–540 (2018).
Slade, A., Kattini, R., Campbell, C. & Holcik, M. Diseases associated with defects in tRNA CCA addition. Int. J. Mol. Sci. 21, 3780 (2020).
Frans, G. et al. Homozygous N-terminal missense mutation in TRNT1 leads to progressive B-cell immunodeficiency in adulthood. J. Allergy Clin. Immunol. 139, 360–363.e6 (2017).
Wedatilake, Y. et al. TRNT1 deficiency: clinical, biochemical and molecular genetic features. Orphanet J. Rare Dis. 11, 90 (2016).
Giannelou, A. et al. Aberrant tRNA processing causes an autoinflammatory syndrome responsive to TNF inhibitors. Ann. Rheum. Dis. 77, 612–619 (2018).
Barton, C., Kausar, S., Kerr, D., Bitetti, S. & Wynn, R. SIFD as a novel cause of severe fetal hydrops and neonatal anaemia with iron loading and marked extramedullary haemopoiesis. J. Clin. Pathol. 71, 275–278 (2018).
Lougaris, V. et al. Novel biallelic TRNT1 mutations resulting in sideroblastic anemia, combined B and T cell defects, hypogammaglobulinemia, recurrent infections, hypertrophic cardiomyopathy and developmental delay. Clin. Immunol. 188, 20–22 (2018).
DeLuca, A. P. et al. Hypomorphic mutations in TRNT1 cause retinitis pigmentosa with erythrocytic microcytosis. Hum. Mol. Genet. 25, 44–56 (2015).
Hull, S. et al. Expanding the phenotype of TRNT1-related immunodeficiency to include childhood cataract and inner retinal dysfunction. JAMA Ophthalmol. 134, 1049–1053 (2016).
Beck, D. B. et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 383, 2628–2638 (2020).
Gruber, C. N. et al. Complex autoinflammatory syndrome unveils fundamental principles of JAK1 kinase transcriptional and biochemical function. Immunity 53, 672–684.e11 (2020).
UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Acknowledgements
The authors would like to thank David Beck and Christina Kozycki for the critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks M. Gattorno, S. Ozen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Monogenic
-
A phenotype or disease that is caused by variation in a single gene and has well-defined inheritance pattern.
- Phenotype
-
An organism’s observable traits such as height, hair colour or blood type.
- Null alleles
-
Genetic changes that cause a complete lack of protein expression or can notably alter protein function.
- Somatic variants
-
Genetic alterations that occur post-zygotic in somatic cells (for example, leukocytes and keratinocytes) and are not passed on to children.
- Genotype
-
An organism’s complete set of genetic material; this term can be used to refer to the variants of a single gene or a set of variants in multiple genes.
- Mendelian
-
The manner by which genes and traits are passed from parents to their offspring, described by Gregor Mendel.
- Oligogenic
-
A phenotype or disease that is dependent on a few genes and is an intermediate between monogenic and polygenic inheritance.
- Dominant
-
The type of inheritance pattern referring to when a single copy of the altered gene is sufficient to cause disease or express the trait.
- Recessive
-
A type of inheritance pattern referring to when both copies of a gene are required for the phenotype or disease expressivity.
- Biallellic
-
A term used to refer to both alleles of a single gene or gene locus (both paternal and maternal).
- Missense variants
-
Genetic changes in a single nucleotide that might or might not result in the substitution of one amino acid for another in the protein.
- Hypermorphic variants
-
A type of genetic change (also known as a gain-of-function mutation) where the altered gene product has an increased level of activity or is expressed at higher levels. These mutations are typically dominantly inherited.
- Monoallelic
-
A term used to refer to when only one of the two copies of a particular gene (alleles) is actively expressed and the other allele is silent.
- Heterozygous
-
An individual who has two different alleles of a particular gene.
- Polygenic
-
A phenotype or disease that is influenced by several genes and often by environmental factors.
- Prenylation
-
A post-translational modification that involves covalent attachment of a lipid consisting of either three (farnesyl) or four (geranylgeranyl) isoprene units to a cysteine residue at or near the C terminus of a protein.
- Hypomorphic variants
-
A type of genetic change (also known as loss-of-function mutation) where the altered gene product has decreased activity or expression. These mutations are typically recessively inherited.
- Nonsense mutation
-
A genetic change that causes the translation of a protein to terminate earlier than would occur with the wild-type gene.
- Homozygous
-
An individual who has inherited the identical alleles of a particular gene from both parents.
- De novo
-
A genetic change that arises in a germ cell or fertilized egg and is not inherited from the parents.
- Disease expressivity
-
The extent to which a genotype shows its phenotypic expression in different people with the same genetic disease.
- Digenic
-
A phenotype or disorder that is expressed only when two non-allelic controlling genes interact.
Rights and permissions
About this article
Cite this article
Aksentijevich, I., Schnappauf, O. Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases. Nat Rev Rheumatol 17, 405–425 (2021). https://doi.org/10.1038/s41584-021-00614-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00614-1
This article is cited by
-
Genetically transitional disease: conceptual understanding and applicability to rheumatic disease
Nature Reviews Rheumatology (2024)
-
Investigation of immune-related diseases using patient-derived induced pluripotent stem cells
Inflammation and Regeneration (2023)
-
Targeting G-CSF to treat autoinflammation
Nature Immunology (2023)
-
The assessment of autoinflammatory disease classification criteria (Eurofever/PRINTO) in a real-life cohort
Clinical Rheumatology (2023)
-
Gain-of-function of MEFV Mutation Causes Very Early Onset Inflammatory Bowel Disease
Journal of Clinical Immunology (2023)