Abstract
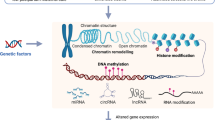
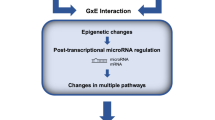
Stress is a primary risk factor for several neuropsychiatric disorders. Evidence from preclinical models and clinical studies of depression have revealed an array of structural and functional maladaptive changes, whereby adverse environmental factors shape the brain. These changes, observed from the molecular and transcriptional levels through to large-scale brain networks, to the behaviours reveal a complex matrix of interrelated pathophysiological processes that differ between sexes, providing insight into the potential underpinnings of the sex bias of neuropsychiatric disorders. Although many preclinical studies use chronic stress protocols, long-term changes are also induced by acute exposure to traumatic stress, opening a path to identify determinants of resilient versus susceptible responses to both acute and chronic stress. Epigenetic regulation of gene expression has emerged as a key player underlying the persistent impact of stress on the brain. Indeed, histone modification, DNA methylation and microRNAs are closely involved in many aspects of the stress response and reveal the glutamate system as a key player. The success of ketamine has stimulated a whole line of research and development on drugs directly or indirectly targeting glutamate function. However, the challenge of translating the emerging understanding of stress pathophysiology into effective clinical treatments remains a major challenge.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72 (2012).
Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012).
Popoli, M., Yan, Z., McEwen, B. S. & Sanacora, G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37 (2011). This review of stress-related effects on glutamatergic neurotransmitter function serves as the basis for this updated article.
McEwen, B. S. et al. Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363 (2015). This review highlights the broader roles of stress in modulating brain function.
Duman, R. S., Aghajanian, G. K., Sanacora, G. & Krystal, J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249 (2016).
Musazzi, L., Tornese, P., Sala, N. & Popoli, M. Acute or chronic? A stressful question. Trends Neurosci. 40, 525–535 (2017). This review summarizes recent findings on the outcomes of acute stress and proposes the use of acute stress protocols for distinguishing trajectories of resilience/susceptibility in the stress response.
Cannon, W. B. Organization for physiological homeostasis. Physiol. Rev. 9, 399–431 (1929).
Selye, H. The general adaptation syndrome and the diseases of adaptation. J. Allergy 6, 117–230 (1946).
McEwen, B. S. & Milner, T. A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 95, 24–39 (2017).
Karatsoreos, I. N. & McEwen, B. S. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn. Sci. 15, 576–584 (2011).
Russo, S. J., Murrough, J. W., Han, M. H., Charney, D. S. & Nestler, E. J. Neurobiology of resilience. Nat. Neurosci. 15, 1475–1484 (2012).
Tang, S. J. et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl Acad. Sci. USA 99, 467–472 (2002).
Ota, K. T. et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 20, 531–535 (2014).
Jernigan, C. S. et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1774–1779 (2011).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964 (2010). This study is among the first to demonstrate the relationship between ketamine’s ability to modulate neuroplasticity, especially through effects on the mTOR pathways and BDNF, and the drug’s antidepressant-like properties.
Duman, R. S. & Monteggia, L. M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127 (2006).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Lakshminarasimhan, H. & Chattarji, S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS ONE 7, e30481 (2012).
Koo, J. W., Chaudhury, D., Han, M. H. & Nestler, E. J. Role of mesolimbic brain-derived neurotrophic factor in depression. Biol. Psychiatry 86, 738–748 (2019).
Kallarackal, A. J. et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J. Neurosci. 33, 15669–15674 (2013).
Tornese, P. et al. Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine. Neurobiol. Stress 10, 100160 (2019). This article shows that maladaptive changes after chronic stress affect only susceptible animals and that ketamine reverses most behavioural and cellular changes.
Song, M., Martinowich, K. & Lee, F. S. BDNF at the synapse: why location matters. Mol. Psychiatry 22, 1370–1375 (2017). This excellent brief review illustrates why local synaptic synthesis of BDNF is important for homeostatic synaptic plasticity.
Baj, G. et al. Physical exercise and antidepressants enhance BDNF targeting in hippocampal CA3 dendrites: further evidence of a spatial code for BDNF splice variants. Neuropsychopharmacology 37, 1600–1611 (2012).
Mallei, A. et al. Expression and dendritic trafficking of BDNF-6 splice variant are impaired in knock-in mice carrying human BDNF Val66Met polymorphism. Int. J. Neuropsychopharmacol. 18, pyv069 (2015).
Yadid, G. & Friedman, A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog. Brain Res. 172, 265–286 (2008).
Belujon, P. & Grace, A. A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 20, 1036–1046 (2017).
Rincón-Cortés, M. & Grace, A. A. Antidepressant effects of ketamine on depression-related phenotypes and dopamine dysfunction in rodent models of stress. Behav. Brain Res. 379, 112367 (2020).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Friedman, A. K. et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319 (2014).
Lammel, S., Tye, K. M. & Warden, M. R. Progress in understanding mood disorders: optogenetic dissection of neural circuits. Genes Brain Behav. 13, 38–51 (2014).
Chaudhury, D., Liu, H. & Han, M. H. Neuronal correlates of depression. Cell Mol. Life Sci. 72, 4825–4848 (2015).
Knowland, D. & Lim, B. K. Circuit-based frameworks of depressive behaviors: the role of reward circuitry and beyond. Pharmacol. Biochem. Behav. 174, 42–52 (2018).
Shabel, S. J., Proulx, C. D., Piriz, J. & Malinow, R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science 345, 1494–1498 (2014).
Yang, Y., Wang, H., Hu, J. & Hu, H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 48, 90–96 (2018).
Cerniauskas, I. et al. Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron 104, 899–915.e8 (2019).
Hu, H., Cui, Y. & Yang, Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 21, 277–295 (2020). This work is an excellent review on the role of the LHb in health, disease and the action of chronic stress.
Moreines, J. L., Owrutsky, Z. L. & Grace, A. A. Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 42, 904–913 (2017).
Schmidt, F. M. et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur. Arch. Psychiatry Clin. Neurosci. 267, 107–115 (2017).
Morris, J. S., Smith, K. A., Cowen, P. J., Friston, K. J. & Dolan, R. J. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage 10, 163–172 (1999).
Sanacora, G., Treccani, G. & Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77 (2012).
Sousa, N. & Almeida, O. F. Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends Neurosci. 35, 742–751 (2012).
Abdallah, C. G., Sanacora, G., Duman, R. S. & Krystal, J. H. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 66, 509–523 (2015).
Duman, R. S., Sanacora, G. & Krystal, J. H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90 (2019). This review draws comparisons of chronic stress exposure and MDD related to neuronal atrophy, altered connectivity and network function, highlighting findings showing alterations of the principle, excitatory glutamate neurons, as well as inhibitory GABA interneurons across studies.
Banasr, M. et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 15, 501–511 (2010).
Koolschijn, P. C., van Haren, N. E., Lensvelt-Mulders, G. J., Hulshoff Pol, H. E. & Kahn, R. S. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 30, 3719–3735 (2009).
Kempton, M. J. et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry 68, 675–690 (2011).
Schmaal, L. et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol. Psychiatry 21, 806–812 (2016).
Soetanto, A. et al. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch. Gen. Psychiatry 67, 448–457 (2010).
Kang, H. J. et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 18, 1413–1417 (2012). This seminal article shows that synapse number and function are decreased in the dorsolateral PFC of patients with major depression, linking neuroarchitectural changes with volumetric loss evidenced with neuroimaging.
Schmaal, L. et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl. Psychiatry 10, 172 (2020).
Knowland, D. et al. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170, 284–297.e18 (2017).
McIntosh, A. L., Gormley, S., Tozzi, L., Frodl, T. & Harkin, A. Recent advances in translational magnetic resonance imaging in animal models of stress and depression. Front. Cell Neurosci. 11, 150 (2017).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 (2015). This meta-analysis of resting-state functional connectivity studies in humans shows that major depression is characterized by altered connectivity among large-scale brain networks.
van Oort, J. et al. How the brain connects in response to acute stress: a review at the human brain systems level. Neurosci. Biobehav. Rev. 83, 281–297 (2017).
Siegmund, A. & Wotjak, C. T. Toward an animal model of posttraumatic stress disorder. Ann. NY Acad. Sci. 1071, 324–334 (2006).
Abdallah, C. G. et al. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu. Rev. Pharmacol. Toxicol. 59, 171–189 (2019).
Musazzi, L., Tornese, P., Sala, N. & Popoli, M. What acute stress protocols can tell us about PTSD and stress-related neuropsychiatric disorders. Front. Pharmacol. 9, 758 (2018).
Chen, Y. et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl Acad. Sci. USA 107, 13123–13128 (2010).
Izquierdo, A., Wellman, C. L. & Holmes, A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 26, 5733–5738 (2006).
Hajszan, T. et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol. Psychiatry 65, 392–400 (2009).
Nava, N. et al. Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cereb. Cortex 27, 694–705 (2017).
Li, S. H. & Graham, B. M. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 4, 73–82 (2017).
Eid, R. S., Gobinath, A. R. & Galea, L. A. M. Sex differences in depression: insights from clinical and preclinical studies. Prog. Neurobiol. 176, 86–102 (2019).
Hodes, G. E. & Epperson, C. N. Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatry 86, 421–432 (2019).
Bale, T. L. & Epperson, C. N. Sex differences and stress across the lifespan. Nat. Neurosci. 18, 1413–1420 (2015).
Wellman, C. L. et al. Sex differences in risk and resilience: stress effects on the neural substrates of emotion and motivation. J. Neurosci. 38, 9423–9432 (2018).
McEwen, B. S. & Morrison, J. H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29 (2013).
Kokras, N. & Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 171, 4595–4619 (2014).
Bondar, N. P., Lepeshko, A. A. & Reshetnikov, V. V. Effects of early-life stress on social and anxiety-like behaviors in adult mice: sex-specific effects. Behav. Neurol. 2018, 1538931 (2018).
Toledo-Rodriguez, M. & Sandi, C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007, 71203 (2007).
Yuen, E. Y. et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977 (2012).
Wei, J. et al. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatry 19, 588–598 (2014). This excellent study shows the sex-specific effects of repeated stress on glutamatergic transmission in prefrontal cortex and cognitive behaviours, as well as the protective role of oestrogen.
Papilloud, A. et al. Peripubertal stress-induced heightened aggression: modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology 44, 674–682 (2019).
Wei, J., Zhong, P., Qin, L., Tan, T. & Yan, Z. Chemicogenetic restoration of the prefrontal cortex to amygdala pathway ameliorates stress-induced deficits. Cereb. Cortex 28, 1980–1990 (2018).
Zelikowsky, M. et al. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173, 1265–1279.e19 (2018).
Pohl, J., Olmstead, M. C., Wynne-Edwards, K. E., Harkness, K. & Menard, J. L. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav. Neurosci. 121, 462–474 (2007).
Hodes, G. E. et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 35, 16362–16376 (2015).
Tan, T. et al. Neural circuits and activity dynamics underlying sex-specific effects of chronic social isolation stress. Cell Rep. 34, 108874 (2021).
Rincón-Cortés, M. & Grace, A. A. Sex-dependent effects of stress on immobility behavior and VTA dopamine neuron activity: modulation by ketamine. Int. J. Neuropsychopharmacol. 20, 823–832 (2017).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Golden, S. A., Covington, H. E. 3rd, Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Iñiguez, S. D. et al. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol. Psychiatry 83, 9–17 (2018).
Shors, T. J., Chua, C. & Falduto, J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 21, 6292–6297 (2001).
Goodwill, H. L. et al. Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell Rep. 25, e2294 (2018).
Farrell, M. R., Holland, F. H., Shansky, R. M. & Brenhouse, H. C. Sex-specific effects of early life stress on social interaction and prefrontal cortex dendritic morphology in young rats. Behav. Brain Res. 310, 119–125 (2016).
Sarkar, A. & Kabbaj, M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol. Psychiatry 80, 448–456 (2016).
Labonté, B. et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111 (2017). This work presents comprehensive genetic profiling that reveals marked sexual dimorphism at the transcriptional level in depressed humans and stressed mice, highlighting the importance of studying sex-specific treatments for depression.
Peña, C. J. et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 10, 5098 (2019).
Seney, M. L. et al. Opposite molecular signatures of depression in men and women. Biol. Psychiatry 84, 18–27 (2018).
Nugent, B. M., O’Donnell, C. M., Epperson, C. N. & Bale, T. L. Placental H3K27me3 establishes female resilience to prenatal insults. Nat. Commun. 9, 2555 (2018).
Elliott, E. et al. Dnmt3a in the medial prefrontal cortex regulates anxiety-like behavior in adult mice. J. Neurosci. 36, 730–740 (2016).
Peña, C. J. et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188 (2017).
Duclot, F. & Kabbaj, M. The estrous cycle surpasses sex differences in regulating the transcriptome in the rat medial prefrontal cortex and reveals an underlying role of early growth response 1. Genome Biol. 16, 256 (2015).
Stack, A. et al. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology 35, 570–580 (2010).
Heck, A. L. & Handa, R. J. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology 44, 45–58 (2019).
Lorsch, Z. S. et al. Estrogen receptor α drives pro-resilient transcription in mouse models of depression. Nat. Commun. 9, 1116 (2018).
Gold, P. W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 20, 32–47 (2015).
Bangasser, D. A. et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15, 896–904 (2010).
Harris, G. C. & Aston-Jones, G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 29, 571–577 (2006).
Soya, S. et al. Orexin modulates behavioral fear expression through the locus coeruleus. Nat. Commun. 8, 1606 (2017).
Flores, Á., Saravia, R., Maldonado, R. & Berrendero, F. Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci. 38, 550–559 (2015).
Ji, M. J., Zhang, X. Y., Chen, Z., Wang, J. J. & Zhu, J. N. Orexin prevents depressive-like behavior by promoting stress resilience. Mol. Psychiatry 24, 282–293 (2019).
Brundin, L., Björkqvist, M., Petersén, A. & Träskman-Bendz, L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur. Neuropsychopharmacol. 17, 573–579 (2007).
Strawn, J. R. et al. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology 35, 1001–1007 (2010).
Johnson, P. L. et al. A key role for orexin in panic anxiety. Nat. Med. 16, 111–115 (2010).
Grafe, L. A., Cornfeld, A., Luz, S., Valentino, R. & Bhatnagar, S. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol. Psychiatry 81, 683–692 (2017).
Cohen, S. et al. Significance of the orexinergic system in modulating stress-related responses in an animal model of post-traumatic stress disorder. Transl. Psychiatry 10, 10 (2020).
Klengel, T. & Binder, E. B. Epigenetics of stress-related psychiatric disorders and gene × environment interactions. Neuron 86, 1343–1357 (2015). This work is an excellent review on epigenetic mechanisms associated with stress responses and the development of stress-related psychiatric disorders.
Gray, J. D., Kogan, J. F., Marrocco, J. & McEwen, B. S. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 13, 661–673 (2017).
Lee, J. B. et al. Histone deacetylase 6 gates the synaptic action of acute stress in prefrontal cortex. J. Physiol. 590, 1535–1546 (2012).
Lee, R. S. & Sawa, A. Environmental stressors and epigenetic control of the hypothalamic–pituitary–adrenal axis. Neuroendocrinology 100, 278–287 (2014).
Yuen, E. Y. et al. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl Acad. Sci. USA 106, 14075–14079 (2009).
Yuen, E. Y. et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatry 16, 156–170 (2011).
Wei, J. et al. Histone modification of Nedd4 ubiquitin ligase controls the loss of AMPA receptors and cognitive impairment induced by repeated stress. J. Neurosci. 36, 2119–2130 (2016).
Wei, J. et al. DNA methyltransferase 3A is involved in the sustained effects of chronic stress on synaptic functions and behaviors. Cereb. Cortex (2020).
Vialou, V., Feng, J., Robison, A. J. & Nestler, E. J. Epigenetic mechanisms of depression and antidepressant action. Annu. Rev. Pharmacol. Toxicol. 53, 59–87 (2013).
Golden, S. A. et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat. Med. 19, 337–344 (2013).
Bahari-Javan, S. et al. HDAC1 links early life stress to schizophrenia-like phenotypes. Proc. Natl Acad. Sci. USA 114, E4686–E4694 (2017).
Kim, H. D. et al. SIRT1 mediates depression-like behaviors in the nucleus accumbens. J. Neurosci. 36, 8441–8452 (2016).
Consortium, C. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 523, 588–591 (2015).
Renthal, W. et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56, 517–529 (2007).
Covington, H. E. III et al. Antidepressant actions of histone deacetylase inhibitors. J. Neurosci. 29, 11451–11460 (2009).
Jochems, J. et al. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol. Psychiatry 77, 345–355 (2015).
Covington, H. E. III et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron 71, 656–670 (2011).
Tsankova, N. M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 9, 519–525 (2006).
Mallei, A., Ieraci, A. & Popoli, M. Chronic social defeat stress differentially regulates the expression of BDNF transcripts and epigenetic modifying enzymes in susceptible and resilient mice. World J. Biol. Psychiatry 20, 555–566 (2019).
Barbier, E. et al. Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2. Mol. Psychiatry 22, 1746–1758 (2017).
Rusconi, F. et al. LSD1 modulates stress-evoked transcription of immediate early genes and emotional behavior. Proc. Natl Acad. Sci. USA 113, 3651–3656 (2016).
Zannas, A. S. & West, A. E. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 264, 157–170 (2014).
Turecki, G. & Meaney, M. J. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol. Psychiatry 79, 87–96 (2016).
Efstathopoulos, P. et al. NR3C1 hypermethylation in depressed and bullied adolescents. Transl. Psychiatry 8, 121 (2018).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 (2004).
Weaver, I. C. et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J. Neurosci. 25, 11045–11054 (2005).
Singh-Taylor, A. et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol. Psychiatry 23, 648–657 (2018).
Vogel Ciernia, A. et al. Experience-dependent neuroplasticity of the developing hypothalamus: integrative epigenomic approaches. Epigenetics 13, 318–330 (2018).
Elliott, E., Ezra-Nevo, G., Regev, L., Neufeld-Cohen, A. & Chen, A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 13, 1351–1353 (2010).
Niwa, M. et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 339, 335–339 (2013).
Roth, T. L., Zoladz, P. R., Sweatt, J. D. & Diamond, D. M. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J. Psychiatr. Res. 45, 919–926 (2011).
Boersma, G. J. et al. Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics 9, 437–447 (2014).
Saunderson, E. A. et al. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc. Natl Acad. Sci. USA 113, 4830–4835 (2016).
Shen, X. F. et al. Role of DNA hypomethylation in lateral habenular nucleus in the development of depressive-like behavior in rats. J. Affect. Disord. 252, 373–381 (2019).
Yao, B. et al. DNA N6-methyladenine is dynamically regulated in the mouse brain following environmental stress. Nat. Commun. 8, 1122 (2017).
Engel, M. et al. The role of m6A/m-RNA methylation in stress response regulation. Neuron 99, 389–403.e9 (2018).
Zhu, Y. et al. Genome-wide profiling of DNA methylome and transcriptome in peripheral blood monocytes for major depression: a monozygotic discordant twin study. Transl. Psychiatry 9, 215 (2019).
Higuchi, F. et al. Hippocampal microRNA-124 enhances chronic stress resilience in mice. J. Neurosci. 36, 7253–7267 (2016).
Roy, B., Dunbar, M., Shelton, R. C. & Dwivedi, Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology 42, 864–875 (2017).
Sillivan, S. E. et al. MicroRNA regulation of persistent stress-enhanced memory. Mol. Psychiatry 25, 965–976 (2020).
Murphy, C. P. & Singewald, N. Role of microRNAs in anxiety and anxiety-related disorders. Curr. Top. Behav. Neurosci. 42, 185–219 (2019).
Dwyer, J. B. et al. Hormonal treatments for major depressive disorder: state of the art. Am. J. Psychiatry 177, 686–705 (2020).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000).
Krystal, J. H., Abdallah, C. G., Sanacora, G., Charney, D. S. & Duman, R. S. Ketamine: a paradigm shift for depression research and treatment. Neuron 101, 774–778 (2019).
Skolnick, P. Antidepressants for the new millennium. Eur. J. Pharmacol. 375, 31–40 (1999).
Newport, D. J. et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 172, 950–966 (2015).
US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified (2019).
Athira, K., Mohan, A. S. V. & Chakravarty, S. Rapid acting antidepressants in the mTOR pathway: current evidence. Brain Res. Bull. 163, 170–177 (2020).
Chowdhury, G. M. et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol. Psychiatry 22, 120–126 (2017).
Moghaddam, B., Adams, B., Verma, A. & Daly, D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927 (1997).
Maeng, S. et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry 63, 349–352 (2008).
Elhussiny, M. E. A. et al. Modulation by chronic stress and ketamine of ionotropic AMPA/NMDA and metabotropic glutamate receptors in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 104, 110033 (2021).
Pothula, S. et al. Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol. Psychiatry 26, 5097–5111 (2020).
Gerhard, D. M. et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J. Clin. Invest. 130, 1336–1349 (2020).
Perszyk, R. E. et al. GluN2D-containing N-methyl-d-aspartate receptors mediate synaptic transmission in hippocampal interneurons and regulate interneuron activity. Mol. Pharmacol. 90, 689–702 (2016).
Suzuki, K. & Monteggia, L. M. The role of eEF2 kinase in the rapid antidepressant actions of ketamine. Adv. Pharmacol. 89, 79–99 (2020).
Tang, X. H. et al. Extrasynaptic CaMKIIα is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model. J. Neuroinflammation 17, 181 (2020).
Zanos, P. & Gould, T. D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23, 801–811 (2018).
Kadriu, B. et al. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. 22, 119–135 (2019).
Casarotto, P. C. et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 184, 1299–1313.e19 (2021).
Laje, G. et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry 72, e27–e28 (2012).
Su, T. P. et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42, 2482–2492 (2017).
Li, Q. S., Wajs, E., Ochs-Ross, R., Singh, J. & Drevets, W. C. Genome-wide association study and polygenic risk score analysis of esketamine treatment response. Sci. Rep. 10, 12649 (2020).
Chen, M. H. et al. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: a double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J. Affect. Disord. 259, 15–20 (2019).
Abdallah, C. G. et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45, 990–997 (2020).
Duncan, G. E., Moy, S. S., Knapp, D. J., Mueller, R. A. & Breese, G. R. Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Res. 787, 181–190 (1998).
Gass, N. et al. Differences between ketamine’s short-term and long-term effects on brain circuitry in depression. Transl. Psychiatry 9, 172 (2019).
Belujon, P. & Grace, A. A. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol. Psychiatry 76, 927–936 (2014).
Ionescu, D. F. et al. Ketamine-associated brain changes: a review of the neuroimaging literature. Harv. Rev. Psychiatry 26, 320–339 (2018).
Grimm, O. et al. Acute ketamine challenge increases resting state prefrontal–hippocampal connectivity in both humans and rats. Psychopharmacology 232, 4231–4241 (2015).
Abdallah, C. G. et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 566–574 (2017).
Abdallah, C. G. et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42, 1210–1219 (2017).
Abdallah, C. G. et al. A robust and reproducible connectome fingerprint of ketamine is highly associated with the connectomic signature of antidepressants. Neuropsychopharmacology 46, 478–485 (2020).
Kraus, C. et al. Evaluating global brain connectivity as an imaging marker for depression: influence of preprocessing strategies and placebo-controlled ketamine treatment. Neuropsychopharmacology 45, 982–989 (2020).
Chaki, S., Koike, H. & Fukumoto, K. Targeting of metabotropic glutamate receptors for the development of novel antidepressants. Chronic Stress 3, 2470547019837712 (2019).
Joffe, M. E. & Conn, P. J. Antidepressant potential of metabotropic glutamate receptor mGlu2 and mGlu3 negative allosteric modulators. Neuropsychopharmacology 44, 214–236 (2019).
Joffe, M. E. et al. mGlu2 and mGlu3 negative allosteric modulators divergently enhance thalamocortical transmission and exert rapid antidepressant-like effects. Neuron 105, 46–59.e3 (2020).
Nasca, C., Bigio, B., Zelli, D., Nicoletti, F. & McEwen, B. S. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol. Psychiatry 20, 755–763 (2015).
Highland, J. N., Zanos, P., Georgiou, P. & Gould, T. D. Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology 44, 1788–1796 (2019).
Holz, A. et al. Enhanced mGlu5 signaling in excitatory neurons promotes rapid antidepressant effects via AMPA receptor activation. Neuron 104, 338–352.e7 (2019).
Palucha, A. et al. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol. Biochem. Behav. 81, 901–906 (2005).
Pal, B. Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell Mol. Life Sci. 75, 2917–2949 (2018).
Genoud, C. et al. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 4, e343 (2006).
Witcher, M. R., Kirov, S. A. & Harris, K. M. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55, 13–23 (2007).
Murphy-Royal, C. et al. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat. Neurosci. 18, 219–226 (2015).
Henneberger, C. et al. LTP induction boosts glutamate spillover by driving withdrawal of perisynaptic astroglia. Neuron 108, 919–936 (2020).
Ostroff, L. E., Manzur, M. K., Cain, C. K. & Ledoux, J. E. Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J. Comp. Neurol. 522, 2152–2163 (2014).
Alexander, L. et al. Over-activation of primate subgenual cingulate cortex enhances the cardiovascular, behavioral and neural responses to threat. Nat. Commun. 11, 5386 (2020).
Alam, M. A. & Datta, P. K. Epigenetic regulation of excitatory amino acid transporter 2 in neurological disorders. Front. Pharmacol. 10, 1510 (2019).
Tetreault, L. A., Zhu, M. P., Wilson, J. R., Karadimas, S. K. & Fehlings, M. G. The impact of riluzole on neurobehavioral outcomes in preclinical models of traumatic and nontraumatic spinal cord injury: results from a systematic review of the literature. Glob. Spine J. 10, 216–229 (2020).
Shimizu, E. N., Seifert, J. L., Johnson, K. J. & Romero-Ortega, M. I. Prophylactic riluzole attenuates oxidative stress damage in spinal cord distraction. J. Neurotrauma 35, 1319–1328 (2018).
Wu, Y., Satkunendrarajah, K. & Fehlings, M. G. Riluzole improves outcome following ischemia–reperfusion injury to the spinal cord by preventing delayed paraplegia. Neuroscience 265, 302–312 (2014).
Srinivas, S., Wali, A. R. & Pham, M. H. Efficacy of riluzole in the treatment of spinal cord injury: a systematic review of the literature. Neurosurg. Focus. 46, E6 (2019).
Kerckhove, N. et al. Effectiveness assessment of riluzole in the prevention of oxaliplatin-induced peripheral neuropathy: RILUZOX-01: protocol of a randomised, parallel, controlled, double-blind and multicentre study by the UNICANCER-AFSOS Supportive Care intergroup. BMJ Open 9, e027770 (2019).
Pittenger, C., Kelmendi, B., Wasylink, S., Bloch, M. H. & Coric, V. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a series of 13 cases, with long-term follow-up. J. Clin. Psychopharmacol. 28, 363–367 (2008).
Spangler, P. T. et al. Randomized controlled trial of riluzole augmentation for posttraumatic stress disorder: efficacy of a glutamatergic modulator for antidepressant-resistant symptoms. J. Clin. Psychiatry 81, 20m13233 (2020).
Sakurai, H. et al. Longer-term open-label study of adjunctive riluzole in treatment-resistant depression. J. Affect. Disord. 258, 102–108 (2019).
Mathew, S. J., Gueorguieva, R., Brandt, C., Fava, M. & Sanacora, G. A randomized, double-blind, placebo-controlled, sequential parallel comparison design trial of adjunctive riluzole for treatment-resistant major depressive disorder. Neuropsychopharmacology 42, 2567–2574 (2017).
Yao, R., Wang, H., Yuan, M., Wang, G. & Wu, C. Efficacy and safety of riluzole for depressive disorder: a systematic review and meta-analysis of randomized placebo-controlled trials. Psychiatry Res. 284, 112750 (2020).
Pereira, A. C. et al. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc. Natl Acad. Sci. USA 111, 18733–18738 (2014).
Mokhtari, Z., Baluchnejadmojarad, T., Nikbakht, F., Mansouri, M. & Roghani, M. Riluzole ameliorates learning and memory deficits in Aβ25–35-induced rat model of Alzheimer’s disease and is independent of cholinoceptor activation. Biomed. Pharmacother. 87, 135–144 (2017).
Okamoto, M. et al. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl. Psychiatry 8, 153 (2018).
Whitcomb, D. J. & Molnar, E. Is riluzole a new drug for Alzheimer’s disease? J. Neurochem. 135, 207–209 (2015).
Pereira, A. C. et al. Age and Alzheimer’s disease gene expression profiles reversed by the glutamate modulator riluzole. Mol. Psychiatry 22, 296–305 (2017).
Hunsberger, H. C. et al. Riluzole rescues glutamate alterations, cognitive deficits, and tau pathology associated with P301L tau expression. J. Neurochem. 135, 381–394 (2015).
Lesuis, S. L., Kaplick, P. M., Lucassen, P. J. & Krugers, H. J. Treatment with the glutamate modulator riluzole prevents early life stress-induced cognitive deficits and impairments in synaptic plasticity in APPswe/PS1dE9 mice. Neuropharmacology 150, 175–183 (2019).
Recourt, K. et al. The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl. Psychiatry 9, 216 (2019).
Gunduz-Bruce, H. et al. Trial of SAGE-217 in patients with major depressive disorder. N. Engl. J. Med. 381, 903–911 (2019).
Kato, T. et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J. Clin. Invest. 129, 2542–2554 (2019).
Azim, L. et al. Study protocol for a randomised placebo-controlled trial of pramipexole in addition to mood stabilisers for patients with treatment resistant bipolar depression (the PAX-BD study). BMC Psychiatry 21, 334 (2021).
Krystal, A. D. et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat. Med. 26, 760–768 (2020).
Maier, S. F. & Seligman, M. E. Learned helplessness at fifty: insights from neuroscience. Psychol. Rev. 123, 349–367 (2016).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
Lupien, S. J., McEwen, B. S., Gunnar, M. R. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
Meaney, M. J. et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev. Neurosci. 18, 49–72 (1996).
Moench, K. M., Breach, M. R. & Wellman, C. L. Chronic stress produces enduring sex- and region-specific alterations in novel stress-induced c-Fos expression. Neurobiol. Stress 10, 100147 (2019).
Arnau-Soler, A. et al. Genome-wide interaction study of a proxy for stress-sensitivity and its prediction of major depressive disorder. PLoS ONE 13, e0209160 (2018).
Malhi, G. S., Das, P., Bell, E., Mattingly, G. & Mannie, Z. Modelling resilience in adolescence and adversity: a novel framework to inform research and practice. Transl. Psychiatry 9, 316 (2019).
Bremner, J. D. Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectr. 7, 129–130 (2002).
Bennett, M. R., Hatton, S. N. & Lagopoulos, J. Stress, trauma and PTSD: translational insights into the core synaptic circuitry and its modulation. Brain Struct. Funct. 221, 2401–2426 (2016).
Slattery, D. A. & Cryan, J. F. Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology 234, 1451–1465 (2017).
Strekalova, T. et al. Update in the methodology of the chronic stress paradigm: internal control matters. Behav. Brain Funct. 7, 9 (2011).
Willner, P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. Stress 6, 78–93 (2017).
Watanabe, Y., Gould, E. & McEwen, B. S. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 588, 341–345 (1992).
Radley, J. J. et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125, 1–6 (2004).
Vollmayr, B. & Henn, F. A. Learned helplessness in the rat: improvements in validity and reliability. Brain Res. Protoc. 8, 1–7 (2001).
Bagley, J. & Moghaddam, B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience 77, 65–73 (1997).
Venero, C. & Borrell, J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur. J. Neurosci. 11, 2465–2473 (1999).
Musazzi, L. et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS ONE 5, e8566 (2010).
Treccani, G. et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol. Psychiatry 19, 433–443 (2014).
Yang, C. H., Huang, C. C. & Hsu, K. S. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J. Neurosci. 25, 4288–4293 (2005).
Homiack, D., O’Cinneide, E., Hajmurad, S., Dohanich, G. P. & Schrader, L. A. Effect of acute alarm odor exposure and biological sex on generalized avoidance and glutamatergic signaling in the hippocampus of Wistar rats. Stress 21, 292–303 (2018).
Martin, K. P. & Wellman, C. L. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb. Cortex 21, 2366–2373 (2011).
Musazzi, L., Tornese, P., Sala, N. & Popoli, M. Acute stress is not acute: sustained enhancement of glutamate release after acute stress involves readily releasable pool size and synapsin I activation. Mol. Psychiatry 22, 1226–1227 (2017).
Marrocco, J. et al. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J. Neurosci. 34, 2015–2024 (2014).
Komatsuzaki, Y. et al. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS ONE 7, e34124 (2012).
Nava, N. et al. Chronic desipramine prevents acute stress-induced reorganization of medial prefrontal cortex architecture by blocking glutamate vesicle accumulation and excitatory synapse increase. Int. J. Neuropsychopharmacol. 18, pyu085 (2014).
Porsolt, R. D., Le Pichon, M. & Jalfre, M. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 (1977).
Commons, K. G., Cholanians, A. B., Babb, J. A. & Ehlinger, D. G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 8, 955–960 (2017).
Bali, A. & Jaggi, A. S. Electric foot shock stress adaptation: does it exist or not? Life Sci. 130, 97–102 (2015).
Flandreau, E. I. & Toth, M. Animal models of PTSD: a critical review. Curr. Top. Behav. Neurosci. 38, 47–68 (2018).
Acknowledgements
The authors dedicate this article to the memory of B. McEwen and R. Duman, two colleagues and friends who contributed with their thorough and passionate work to many of the findings illustrated here and to the development of knowledge on stress and mental illness. M.P. is indebted to former and present members of his group, in particular L. Musazzi, A. Mallei, A. Ieraci, G. Treccani and P. Tornese, for some of the works illustrated here. Z.Y. is grateful to her laboratory members who have worked on stress-related projects, past collaboration with B. McEwen and US National Institutes of Health (NIH) support (MH085774, MH108842, MH126443). G.S. is extremely appreciative of the mentorship of R. Duman and B. McEwen and the collaborations with M. Banasr. The authors thank A. Raiti for preparation of Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
G.S. has served as a consultant for Allergan, Alkermes, AstraZeneca, Avanier Pharmaceuticals, Axsome Therapeutics Biohaven Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Clexio, Denovo Biopharma, Engrail Therapeutics, EMA Wellness, Epiodyne, Intra-Cellular Therapies, Janssen, Lundbeck, Merck & Co., Navitor Pharmaceuticals, Neurocrine, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis Therapeutics, Sage Pharmaceuticals, Taisho Pharmaceuticals, Valeant, Vistagen Therapeutics, and XW Labs over the past 36 months; has received research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Hoffman La-Roche, Merck, Naurex, Servier and Usona over the past 36 months; holds equity in BioHaven Pharmaceuticals; and is a co-inventor on US Patent 8,778,979 held by Yale University and a co-inventor on US Provisional Patent Application No. 047162-7177P1 (00754) filed on 20 August, 2018 by Yale University Office of Cooperative Research. Z.Y. has no competing interests. M.P. has received research contracts from Rodin Therapeutics (now Alkermes) in the last 36 months; and has received research contracts from Merck, GlaxoSmithKline, Servier, Sigma-Tau, Fidia and Abbott.
Additional information
Peer review information
Nature Reviews Neuroscience thanks Anthony Grace, David Slattery and the other, anonymous, reviewer for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanacora, G., Yan, Z. & Popoli, M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci 23, 86–103 (2022). https://doi.org/10.1038/s41583-021-00540-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-021-00540-x
This article is cited by
-
Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: past, present, and future
Neuropsychopharmacology (2024)
-
Stress-related cellular pathophysiology as a crosstalk risk factor for neurocognitive and psychiatric disorders
BMC Neuroscience (2023)
-
Ginsenoside Rg1 in neurological diseases: From bench to bedside
Acta Pharmacologica Sinica (2023)
-
Changes at glutamate tripartite synapses in the prefrontal cortex of a new animal model of resilience/vulnerability to acute stress
Translational Psychiatry (2023)
-
Stress, microRNAs, and stress-related psychiatric disorders: an overview
Molecular Psychiatry (2023)