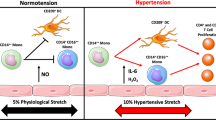
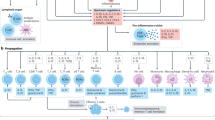
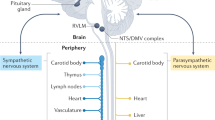
Abstract
Hypertension is a leading risk factor for morbidity and mortality worldwide. Despite current anti-hypertensive therapies, most individuals with hypertension fail to achieve adequate blood pressure control. Moreover, even with adequate control, a residual risk of cardiovascular events and associated organ damage remains. These findings suggest that current treatment modalities are not addressing a key element of the underlying pathology. Emerging evidence implicates immune cells as key mediators in the development and progression of hypertension. In this Review, we discuss our current understanding of the diverse roles of innate and adaptive immune cells in hypertension, highlighting key findings from human and rodent studies. We explore mechanisms by which these immune cells promote hypertensive pathophysiology, shedding light on their multifaceted involvement. In addition, we highlight advances in our understanding of autoimmunity, HIV and immune checkpoints that provide valuable insight into mechanisms of chronic and dysregulated inflammation in hypertension.
Key points
-
Emerging evidence from human and rodent studies suggests an important role for both innate and adaptive immune cells in the pathogenesis of hypertension.
-
The release of inflammatory cytokines, production of reactive oxygen species, and neoantigen presentation by innate immune cells results in vascular damage, renal injury, inflammation and elevated blood pressure.
-
Adaptive immune cells shift towards pro-inflammatory roles in hypertension, resulting in enhanced cytotoxicity and inflammatory cytokine production that leads to impaired vascular and renal natriuretic functions.
-
Immune cells coordinate as an integrated system, suggesting that targeting regulatory nodes of the inflammatory response might be beneficial for treating hypertension.
-
Current evidence supports a model whereby immune activation and resultant hypertension-related organ dysfunction act synergistically to amplify blood pressure and promote end-organ damage in hypertension.
-
Advances in our understanding of autoimmunity, HIV and immune checkpoint inhibitor therapies provide key insights into the effects of immune activation and chronic inflammation in the development of hypertension.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1659–1724 (2016).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426–e483 (2018).
Muntner, P. et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA 324, 1190–1200 (2020).
Newton, K. & Dixit, V. M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4, a006049 (2012).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Marko, L. et al. Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60, 1430–1436 (2012).
Wei, S. Y. et al. Multiple mechanisms are involved in salt-sensitive hypertension-induced renal injury and interstitial fibrosis. Sci. Rep. 7, 45952 (2017).
Xiao, L. et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ. Res. 117, 547–557 (2015).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Wenzel, P. Monocytes as immune targets in arterial hypertension. Br. J. Pharmacol. 176, 1966–1977 (2019).
Parissis, J. T., Korovesis, S., Giazitzoglou, E., Kalivas, P. & Katritsis, D. Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int. J. Cardiol. 83, 13–21 (2002).
Alexander, M. R. et al. Human monocyte transcriptional profiling identifies IL-18 receptor accessory protein and lactoferrin as novel immune targets in hypertension. Br. J. Pharmacol. 176, 2015–2027 (2019).
Kirabo, A. et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 124, 4642–4656 (2014).
Loperena, R. et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc. Res. 114, 1547–1563 (2018).
Wenzel, P. et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381 (2011).
Kossmann, S. et al. Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. J. Biol. Chem. 289, 27540–27550 (2014).
Gkaliagkousi, E. et al. Decreased platelet nitric oxide contributes to increased circulating monocyte-platelet aggregates in hypertension. Eur. Heart J. 30, 3048–3054 (2009).
Zaldivia, M. T. et al. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 69, 323–331 (2017).
Han, P. et al. Platelet P-selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. Sci. Adv. 6, eaaz1580 (2020).
Gebhard, S. et al. Angiotensin II-dependent hypertension causes reversible changes in the platelet proteome. J. Hypertens. 29, 2126–2137 (2011).
Hilt, Z. T. et al. Platelet-derived β2M regulates monocyte inflammatory responses. JCI Insight 4, e122943 (2019).
Hughson, M. D. et al. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am. J. Kidney Dis. 52, 18–28 (2008).
De Ciuceis, C. et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler. Thromb. Vasc. Biol. 25, 2106–2113 (2005).
Oh, J. et al. Macrophage secretion of miR-106b-5p causes renin-dependent hypertension. Nat. Commun. 11, 4798 (2020).
Ip, W. K. & Medzhitov, R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat. Commun. 6, 6931 (2015).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Thowsen, I. M. et al. Genetic engineering of lymphangiogenesis in skin does not affect blood pressure in mouse models of salt-sensitive hypertension. Hypertension 79, 2451–2462 (2022).
Shah, K. H. et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ. Res. 117, 858–869 (2015).
Vinh, A. et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122, 2529–2537 (2010).
Barbaro, N. R. et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 21, 1009–1020 (2017).
Van Beusecum, J. P. et al. High salt activates CD11c+ antigen-presenting cells via SGK (Serum Glucocorticoid Kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 74, 555–563 (2019).
Van Beusecum, J. P. et al. Growth arrest specific-6 and Axl coordinate inflammation and hypertension. Circ. Res. 129, 975–991 (2021).
Thiam, H. R., Wong, S. L., Wagner, D. D. & Waterman, C. M. Cellular mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 36, 191–218 (2020).
Tatsukawa, Y. et al. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens. Res. 31, 1391–1397 (2008).
Siedlinski, M. et al. White blood cells and blood pressure: a Mendelian randomization study. Circulation 141, 1307–1317 (2020).
Ramasamy, R., Maqbool, M., Mohamed, A. L. & Noah, R. M. Elevated neutrophil respiratory burst activity in essential hypertensive patients. Cell Immunol. 263, 230–234 (2010).
Folco, E. J. et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1ɑ and cathepsin G. Arterioscler. Thromb. Vasc. Biol. 38, 1901–1912 (2018).
Kahlenberg, J. M., Carmona-Rivera, C., Smith, C. K. & Kaplan, M. J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 190, 1217–1226 (2013).
Chrysanthopoulou, A. et al. Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension. JCI Insight 6, e148668 (2021).
Krishnan, J. et al. IsoLGs (Isolevuglandins) drive neutrophil migration in hypertension and are essential for the formation of neutrophil extracellular traps. Hypertension 79, 1644–1655 (2022).
Ge, W. et al. The role of immunoglobulin E and mast cells in hypertension. Cardiovasc. Res. 118, 2985–2999 (2022).
Abais-Battad, J. M., Lund, H., Fehrenbach, D. J., Dasinger, J. H. & Mattson, D. L. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R28–r35 (2018).
Rudemiller, N., Lund, H., Jacob, H. J., Geurts, A. M. & Mattson, D. L. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63, 559–564 (2014).
Crowley, S. D. et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1089–R1097 (2010).
Trott, D. W. et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115 (2014).
Sun, X. N. et al. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-γ. Circ. Res. 120, 1584–1597 (2017).
Strioga, M., Pasukoniene, V. & Characiejus, D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 134, 17–32 (2011).
Guan, Y. et al. CD28null T cells in aging and diseases: from biology to assessment and intervention. Int. Immunopharmacol. 131, 111807 (2024).
Dale, B. L. et al. Critical role of Interleukin 21 and T follicular helper cells in hypertension and vascular dysfunction. JCI Insight 5, e129278 (2019).
Itani, H. A. et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68, 123–132 (2016).
Higaki, A., Mahmoud, A. U. M., Paradis, P. & Schiffrin, E. L. Role of interleukin-23/interleukin-17 axis in T-cell-mediated actions in hypertension. Cardiovasc. Res. 117, 1274–1283 (2021).
Kamat, N. V. et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension 65, 569–576 (2015).
Liu, Y. et al. CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat. Commun. 8, 14037 (2017).
Yao, W., Sun, Y., Wang, X. & Niu, K. Elevated serum level of interleukin 17 in a population with prehypertension. J. Clin. Hypertens. 17, 770–774 (2015).
Nguyen, H. et al. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res. 97, 696–704 (2013).
Wu, J. et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 114, 616–625 (2014).
Orejudo, M. et al. Interleukin-17A induces vascular remodeling of small arteries and blood pressure elevation. Clin. Sci. 134, 513–527 (2020).
Orejudo, M. et al. Interleukin 17A participates in renal inflammation associated to experimental and human hypertension. Front. Pharmacol. 10, 1015 (2019).
Norlander, A. E. et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68, 167–174 (2016).
Boehm, U., Klamp, T., Groot, M. & Howard, J. C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15, 749–795 (1997).
Garcia, A. G. et al. Interferon-γ ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am. J. Physiol. Heart Circ. Physiol. 303, H587–H596 (2012).
Kimura, A. et al. Protective roles of interferon-γ in cardiac hypertrophy induced by sustained pressure overload. J. Am. Heart Assoc. 7, e008145 (2018).
Benson, L. N. et al. The IFNγ-PDL1 pathway enhances CD8T-DCT interaction to promote hypertension. Circ. Res. 130, 1550–1564 (2022).
Saleh, M. A. et al. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J. Clin. Invest. 125, 1189–1202 (2015).
Zhang, J. et al. Tumor necrosis factor-ɑ produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64, 1275–1281 (2014).
Hsieh, C. S., Macatonia, S. E., O’Garra, A. & Murphy, K. M. T cell genetic background determines default T helper phenotype development in vitro. J. Exp. Med. 181, 713–721 (1995).
Wang, X. et al. Relationship of serum immunoglobulin levels to blood pressure and hypertension in an adult population. J. Hum. Hypertens. 32, 212–218 (2018).
Chen, Y. et al. Class switching and high-affinity immunoglobulin G production by B cells is dispensable for the development of hypertension in mice. Cardiovasc. Res. 117, 1217–1228 (2021). PMC7983008.
Chan, C. T. et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015).
Lei, L., Zhong, X. N., He, Z. Y., Zhao, C. & Sun, X. J. IL-21 induction of CD4+ T cell differentiation into Th17 cells contributes to bleomycin-induced fibrosis in mice. Cell Biol. Int. 39, 388–399 (2015).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Rudensky, A. Y. Regulatory T cells and Foxp3. Immunol. Rev. 241, 260–268 (2011).
Valencia, X. & Lipsky, P. E. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 3, 619–626 (2007).
Kvakan, H. et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119, 2904–2912 (2009).
Barhoumi, T. et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57, 469–476 (2011).
Tipton, A. J., Baban, B. & Sullivan, J. C. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R359–R367 (2012).
Gillis, E. E., Musall, J. B., Baban, B. & Sullivan, J. C. IL-10 treatment decreases blood pressure in male, but not female, spontaneously hypertensive rats. Am. J. Physiol. Renal Physiol. 319, F359–F365 (2020).
Mian, M. O., Barhoumi, T., Briet, M., Paradis, P. & Schiffrin, E. L. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J. Hypertens. 34, 97–108 (2016).
Emmerson, A. et al. Nox2 in regulatory T cells promotes angiotensin II-induced cardiovascular remodeling. J. Clin. Invest. 128, 3088–3101 (2018).
Kasal, D. A. et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59, 324–330 (2012).
Matrougui, K. et al. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am. J. Pathol. 178, 434–441 (2011).
Gackowska, L. et al. Regulatory T-cell subset distribution in children with primary hypertension is associated with hypertension severity and hypertensive target organ damage. J. Hypertens. 38, 692–700 (2020).
Sawant, D. V. & Vignali, D. A. Once a Treg, always a Treg? Immunol. Rev. 259, 173–191 (2014).
Hernandez, A. L. et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Invest. 125, 4212–4222 (2015).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Alexander, M. R. et al. Immune profiling reveals decreases in circulating regulatory and exhausted T cells in human hypertension. JACC Basic. Transl. Sci. 8, 319–336 (2023).
Fabbiano, S. et al. Immunosuppression-independent role of regulatory T cells against hypertension-driven renal dysfunctions. Mol. Cell Biol. 35, 3528–3546 (2015).
Shokoples, B. G. et al. P2RX7 gene knockout or antagonism reduces angiotensin II-induced hypertension, vascular injury and immune cell activation. J. Hypertens. 41, 1701–1712 (2023).
Junger, W. G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 (2011).
Zhao, T. V. et al. ATP release drives heightened immune responses associated with hypertension. Sci. Immunol. 4, eaau6426 (2019).
Schenk, U. et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 4, ra12 (2011).
MacLeod, M. K., Kappler, J. W. & Marrack, P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology 130, 10–15 (2010).
Itani, H. A. et al. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res. 118, 1233–1243 (2016).
Xiao, L., do Carmo, L. S., Foss, J. D., Chen, W. & Harrison, D. G. Sympathetic enhancement of memory T-cell homing and hypertension sensitization. Circ. Res. 126, 708–721 (2020).
Ribot, J. C., Lopes, N. & Silva-Santos, B.γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021).
Caillon, A. et al. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation 135, 2155–2162 (2017).
Comeau, K., Shokoples, B., Caillon, A., Paradis, P. & Schiffrin, E. L. Angiotensin II-induced memory γδ T cells sensitize mice to a mild hypertensive stimulus. Am. J. Hypertens. 36, 619–628 (2023).
Mamedov, M. R. et al. CRISPR screens decode cancer cell pathways that trigger γδ T cell detection. Nature 621, 188–195 (2023).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120, 2269–2279 (2012).
Murakata, Y., Fujimaki, T. & Yamada, Y. Association of a butyrophilin, subfamily 2, member A1 gene polymorphism with hypertension. Biomed. Rep. 2, 818–822 (2014).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Sonnenberg, G. F. & Hepworth, M. R. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 19, 599–613 (2019).
Shah, A. S. V. et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 138, 1100–1112 (2018).
Peck, R. N. et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 12, 125 (2014).
Reis, K. G. et al. Blood pressure, T cells, and mortality in people with HIV in Tanzania during the first 2 years of antiretroviral therapy. J. Clin. Hypertens. 22, 1554–1562 (2020).
Brenchley, J. M., Price, D. A. & Douek, D. C. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7, 235–239 (2006).
Wang, Y. et al. HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells. Nat. Immunol. 21, 274–286 (2020).
Cerwenka, A. & Lanier, L. L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 16, 112–123 (2016).
van den Boorn, J. G. et al. Inflammasome-dependent induction of adaptive NK cell memory. Immunity 44, 1406–1421 (2016).
Pitzer, A. et al. DC ENaC-dependent inflammasome activation contributes to salt-sensitive hypertension. Circ. Res. 131, 328–344 (2022).
Omi, T. et al. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur. J. Hum. Genet. 14, 1295–1305 (2006).
Pontillo, A. et al. Polymorphisms in inflammasome genes and susceptibility to HIV-1 infection. J. Acquir. Immune Defic. Syndr. 59, 121–125 (2012).
Weng, N. P., Akbar, A. N. & Goronzy, J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 (2009).
Tassiopoulos, K. et al. CD28-negative CD4+ and CD8+ T cells in antiretroviral therapy-naive HIV-infected adults enrolled in adult clinical trials group studies. J. Infect. Dis. 205, 1730–1738 (2012).
Morris, S. R. et al. Inflammescent CX3CR1+CD57+CD8+ T cells are generated and expanded by IL-15. JCI Insight 5, e132963 (2020).
Youn, J. C. et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133 (2013).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Drobni, Z. D. et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142, 2299–2311 (2020).
Turker, I., Sharma, A., Huang, S., Johnson, D. B. & Alexander, M. R. Combination immune checkpoint inhibitor therapy is associated with increased blood pressure in melanoma patients. Hypertension 80, e43–e45 (2022).
Panoulas, V. F. et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology 46, 1477–1482 (2007).
Mirghani, H. et al. The association of psoriasis, diabetes mellitus, and hypertension: a meta-analysis. Cureus 15, e48855 (2023).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Patrick, D. M. et al. Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus. JCI Insight 7, e136678 (2022).
Mathis, K. W. et al. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59, 673–679 (2012).
Rudofsky, U. H. et al. Differences in the occurrence of hypertension among (NZB X NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am. J. Pathol. 116, 107–114 (1984).
Clemmer, J. S., Hillegass, W. B. & Taylor, E. B. Antihypertensive effects of immunosuppressive therapy in autoimmune disease. J. Hum. Hypertens. 37, 300–306 (2023).
Herrera, J., Ferrebuz, A., MacGregor, E. G. & Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 17, S218–S225 (2006).
Yoshida, S. et al. Infliximab, a TNF-ɑ inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J. Hum. Hypertens. 28, 165–169 (2014).
Madhur, M. S. et al. Hypertension: do inflammation and immunity hold the key to solving this epidemic? Circ. Res. 128, 908–933 (2021).
Lieb, W., Enserro, D. M., Sullivan, L. M. & Vasan, R. S. Residual cardiovascular risk in individuals on blood pressure-lowering treatment. J. Am. Heart Assoc. 4, e002155 (2015).
Blacher, J. et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME study. J. Hum. Hypertens. 24, 19–26 (2010).
Bellamy, L., Casas, J.-P., Hingorani, A. D. & Williams, D. J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335, 974 (2007).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Ernesto Schiffrin, Philip Wenzel, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, B.A., Alexander, M.R. & Harrison, D.G. Immune mechanisms in the pathophysiology of hypertension. Nat Rev Nephrol (2024). https://doi.org/10.1038/s41581-024-00838-w
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-024-00838-w