Abstract
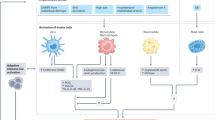
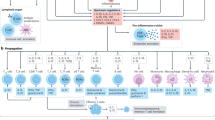
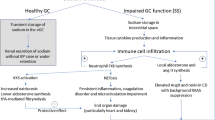
Emerging evidence has supported a role of inflammation and immunity in the genesis of hypertension. In humans and experimental models of hypertension, cells of the innate and adaptive immune system enter target tissues, including vessels and the kidney, and release powerful mediators including cytokines, matrix metalloproteinases and reactive oxygen species that cause tissue damage, fibrosis and dysfunction. These events augment the blood pressure elevations in hypertension and promote end-organ damage. Factors that activate immune cells include sympathetic outflow, increased sodium within microenvironments where these cells reside, and signals received from the vasculature. In particular, the activated endothelium releases reactive oxygen species and interleukin (IL)-6 which in turn stimulate transformation of monocytes to become antigen presenting cells and produce cytokines like IL-1β and IL-23, which further affect T cell function to produce IL-17A. Genetic deletion or neutralization of these cytokines ameliorates hypertension and end-organ damage. In this review, we will consider in depth features of the hypertensive milieu that lead to these events and consider new treatment approaches to limit the untoward effects of inflammation in hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019;140:e596–e646.
Harrison DG, Coffman TM, Wilcox CS. Pathophysiology of hypertension. Circ Res. 2021;128:847–863.
Madhur MS, Elijovich F, Alexander MR, Pitzer A, Ishimwe J, Beusecum JPV, et al. Hypertension: do inflammation and immunity hold the key to solving this epidemic? Circ Res. 2021;128:908–933.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460.
Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33.
Crowley SD, Song Y-S, Lin EE, Griffiths R, Kim H-S, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–R1097.
Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–R414.
Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537.
Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, et al. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res. 2016;118:1233–1243.
Caillon A, Mian MOR, Fraulob-Aquino JC, Huo K-G, Barhoumi T, Ouerd S, et al. γ/δ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation. 2017;135:2155–2162.
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II–induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507.
Saleh MA, Norlander AE, Madhur MS. Inhibition of interleukin-17A, but not interleukin-17F, signaling lowers blood pressure, and reduces end-organ inflammation in angiotensin II–induced hypertension. Jacc Basic Transl Sci. 2016;1:606–616.
Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II–induced hypertension. Hypertension. 2018;68:167–174.
Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704.
Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, et al. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension. 2016;67:1218–1227.
Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor–dependent mechanism. Hypertension. 2006;48:1050–1057.
Lee DL, Sturgis LC, Labazi H, Osborne JB, Fleming C, Pollock JS, et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ. 2006;290:H935–H940.
Alexander MR, Norlander AE, Elijovich F, Atreya RV, Gaye A, Gnecco JS, et al. Human monocyte transcriptional profiling identifies IL‐18 receptor accessory protein and lactoferrin as novel immune targets in hypertension. Brit J Pharm. 2019;176:2015–2027.
Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, et al. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515.
Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KRC, Xiao L, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:50–67.
Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, et al. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol-Heart Circ. 2005;288:37–42.
Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, et al. Nox1 overexpression potentiates angiotensin ii-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676.
Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, et al. Nox1 is involved in angiotensin II–mediated hypertension. Circulation. 2005;112:2677–2685.
Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, et al. Decreased blood pressure in NOX1‐deficient mice. Febs Lett. 2006;580:497–504.
Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arteriosclerosis Thrombosis Vasc Biol. 2005;25:274–278.
Kirabo A, Fontana V, Faria APC, de, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656.
Ciuceis CD, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II–infused macrophage colony-stimulating factor–deficient mice. Arteriosclerosis Thrombosis Vasc Biol. 2005;25:2106–2113.
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, et al. Lysozyme M–positive monocytes mediate angiotensin II–induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381.
Kossmann S, Hu H, Steven S, Schönfelder T, Fraccarollo D, Mikhed Y, et al. Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. J Biol Chem. 2014;289:27540–27550.
Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804.
Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483.
Loperena R, Beusecum JPV, Itani HA, Engel N, Laroumanie F, Xiao L, et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 2018;114:1547–1563.
Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–1020.
Barbaro NR, Beusecum JV, Xiao L, Carmo LD, Pitzer A, Loperena R. et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res. 2021;117:1358–1371.
Kovarik JJ, Morisawa N, Wild J, Marton A, Takase‐Minegishi K, Minegishi S, et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol. 2021;232:e13629.
Wild J, Jung R, Knopp T, Efentakis P, Benaki D, Grill A, et al. Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol. 2021;232:e13628.
Beusecum JPV, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, et al. High salt activates CD11c+ antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension. 2019;74:555–563.
Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Ren. 2019;317:F361–F374.
Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196.
Ferguson JF, Aden LA, Barbaro NR, Beusecum JPV, Xiao L, Simmons AJ, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019; 4. https://doi.org/10.1172/jci.insight.126241.
Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin ii–induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016; 5. https://doi.org/10.1161/jaha.116.003698.
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–4415.
Nakai M, Ribeiro RV, Stevens BR, Gill P, Muralitharan RR, Yiallourou S, et al. Essential hypertension is associated with changes in gut microbial metabolic pathways: a multisite analysis of ambulatory blood pressure. Hypertension. 2021;78:804–815.
Xiao L, Itani HA, Carmo LS, do, Carver LS, Breyer RM, Harrison DG. Central EP3 (E Prostanoid 3) receptors mediate salt-sensitive hypertension and immune activation. Hypertension. 2019;74:1507–1515.
Shah KH, Shi P, Giani JF, Janjulia T, Bernstein EA, Li Y, et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res. 2015;117:858–869.
Chiasson VL, Bounds KR, Chatterjee P, Manandhar L, Pakanati AR, Hernandez M, et al. Myeloid-derived suppressor cells ameliorate cyclosporine A–induced hypertension in mice. Hypertension. 2018;71:199–207.
Lu X, Rudemiller NP, Wen Y, Ren J, Hammer GE, Griffiths R, et al. A20 in myeloid cells protects against hypertension by inhibiting dendritic cell-mediated t-cell activation. Circ Res. 2019;125:1055–1066.
Shao B-Z, Xu Z-Q, Han B-Z, Su D-F, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharm. 2015;6:262.
Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GRIL‐1β. and IL‐18: inflammatory markers or mediators of hypertension? Brit J Pharm. 2014;171:5589–5602.
Dorffel Y, Latsch C, Stuhlmuller B, Schreiber S, Scholze S, Burmester GR, et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117.
Omi T, Kumada M, Kamesaki T, Okuda H, Munkhtulga L, Yanagisawa Y, et al. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur J Hum Genet. 2006;14:1295–1305.
Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res. 2018;115:776–787.
Ren X-S, Tong Y, Ling L, Chen D, Sun H-J, Zhou H, et al. NLRP3 gene deletion attenuates angiotensin II-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cell Physiol Biochem. 2018;44:2269–2280.
Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, et al. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation. 2016;134:1866–1880.
Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, et al. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk. Hypertension. 2019;75:477–482.
Siedlinski M, Jozefczuk E, Xu X, Teumer A, Evangelou E, Schnabel RB, et al. White blood cells and blood pressure: a Mendelian randomization study. Circulation. 2020;141:1307–1317.
Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, et al. Infliximab, a TNF-α inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2014;28:165–169.
Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225.
Pitchford LM, Driver PM, Fuller JC, Akers WS, Abumrad NN, Amarnath V, et al. Safety, tolerability, and pharmacokinetics of repeated oral doses of 2-hydroxybenzylamine acetate in healthy volunteers: a double-blind, randomized, placebo-controlled clinical trial. Bmc Pharm Toxicol. 2020;21:3.
Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625.
Author information
Authors and Affiliations
Contributions
JVB, HM and DGH composed and edited this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van Beusecum, J.P., Moreno, H. & Harrison, D.G. Innate immunity and clinical hypertension. J Hum Hypertens 36, 503–509 (2022). https://doi.org/10.1038/s41371-021-00627-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-021-00627-z
This article is cited by
-
Life is really simple, but we insist on making it complicated. (Confucius)
Hypertension Research (2023)
-
Lifestyle Medicine as a Treatment for Resistant Hypertension
Current Hypertension Reports (2023)
-
Sodium Homeostasis and Hypertension
Current Cardiology Reports (2023)
-
Treatment of male and female spontaneously hypertensive rats with TNF-α inhibitor etanercept increases markers of renal injury independent of an effect on blood pressure
Biology of Sex Differences (2022)