Abstract
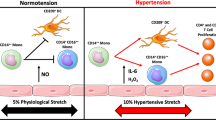
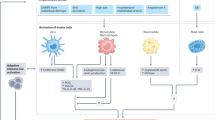
Hypertension is a global health problem, with >1.3 billion individuals with high blood pressure worldwide. In this Review, we present an inflammatory paradigm for hypertension, emphasizing the crucial roles of immune cells, cytokines and chemokines in disease initiation and progression. T cells, monocytes, macrophages, dendritic cells, B cells and natural killer cells are all implicated in hypertension. Neoantigens, the NLRP3 inflammasome and increased sympathetic outflow, as well as cytokines (including IL-6, IL-7, IL-15, IL-18 and IL-21) and a high-salt environment, can contribute to immune activation in hypertension. The activated immune cells migrate to target organs such as arteries (especially the perivascular fat and adventitia), kidneys, the heart and the brain, where they release effector cytokines that elevate blood pressure and cause vascular remodelling, renal damage, cardiac hypertrophy, cognitive impairment and dementia. IL-17 secreted by CD4+ T helper 17 cells and γδ T cells, and interferon-γ and tumour necrosis factor secreted by immunosenescent CD8+ T cells, exert crucial effector roles in hypertension, whereas IL-10 and regulatory T cells are protective. Effector mediators impair nitric oxide bioavailability, leading to endothelial dysfunction and increased vascular contractility. Inflammatory effector mediators also alter renal sodium and water balance and promote renal fibrosis. These mechanisms link hypertension with obesity, autoimmunity, periodontitis and COVID-19. A comprehensive understanding of the immune and inflammatory mechanisms of hypertension is crucial for safely and effectively translating the findings to clinical practice.
Key points
-
Hypertension is a global health challenge, affecting >1.3 billion people worldwide, and the development of this condition is closely associated with inflammatory dysregulation and immune activation.
-
Immune cells such as CD8+ T cells, T helper 17 cells, regulatory T cells, monocytes, macrophages and B cells, as well as chemokines and cytokines, such as IL-17, IL-18, interferon-γ and tumour necrosis factor, have crucial roles in the immune mechanisms underlying hypertension.
-
Relevant immune mechanisms can be grouped into two clusters: those involved in the regulation of the initial immune and inflammatory activation, and the immune effector mechanisms linking the immune system to end-organ mechanisms that regulate blood pressure.
-
Dendritic cells presenting neoantigens, NLRP3 inflammasome activity and neuroimmune activation all contribute to the immune activation process in hypertension.
-
Activated immune cells migrate to target organs, such as the arteries (especially the perivascular fat and adventitia), kidneys and brain, causing end-organ damage and elevating the blood pressure.
-
A deeper understanding of the immune crosstalk between the upstream regulators and downstream effectors in hypertension will enable the application of these findings effectively in clinical settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhou, B., Perel, P., Mensah, G. A. & Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 18, 785–802 (2021).
NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398, 957–980 (2021).
Schutte, A. E. et al. Addressing global disparities in blood pressure control: perspectives of the International Society of Hypertension. Cardiovasc. Res. 119, 381–409 (2022).
Harrison, D. G. et al. Inflammation, immunity, and hypertension. Hypertension 57, 132–140 (2011).
Freeman, M. W. et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N. Engl. J. Med. 388, 395–405 (2023).
Murray, E. C. et al. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc. Res. 117, 2589–2609 (2021).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Garshick, M. S., Ward, N. L., Krueger, J. G. & Berger, J. S. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J. Am. Coll. Cardiol. 77, 1670–1680 (2021).
Panoulas, V. F. et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology 46, 1477–1482 (2007).
Munoz Aguilera, E. et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc. Res. 116, 28–39 (2020).
Eke, P. I. et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J. Am. Dent. Assoc. 149, 576–588.e6 (2018).
Sharma, S. et al. Periodontal therapy and treatment of hypertension-alternative to the pharmacological approach. A systematic review and meta-analysis. Pharmacol. Res. 166, 105511 (2021).
Czesnikiewicz-Guzik, M. et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 40, 3459–3470 (2019).
Munoz Aguilera, E. et al. Is systemic inflammation a missing link between periodontitis and hypertension? Results from two large population-based surveys. J. Intern. Med. 289, 532–546 (2021).
Guzik, T. J. & Czesnikiewicz-Guzik, M. Mounting pressure of periodontitis. Hypertension 78, 552–554 (2021).
Abramson, J. L., Lewis, C., Murrah, N. V., Anderson, G. T. & Vaccarino, V. Relation of C-reactive protein and tumor necrosis factor-alpha to ambulatory blood pressure variability in healthy adults. Am. J. Cardiol. 98, 649–652 (2006).
Abramson, J. L., Weintraub, W. S. & Vaccarino, V. Association between pulse pressure and C-reactive protein among apparently healthy US adults. Hypertension 39, 197–202 (2002).
Bautista, L. E., Vera, L. M., Arenas, I. A. & Gamarra, G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-ɑ) and essential hypertension. J. Hum. Hypertens. 19, 149–154 (2005).
Sesso, H. D. et al. Plasma inflammatory markers and the risk of developing hypertension in men. J. Am. Heart Assoc. 4, e001802 (2015).
Wang, L. et al. Circulating inflammatory and endothelial markers and risk of hypertension in white and black postmenopausal women. Clin. Chem. 57, 729–736 (2011).
Zhang, H. et al. Role of the CCL2-CCR2 axis in cardiovascular disease: pathogenesis and clinical implications. Front. Immunol. 13, 975367 (2022).
Rabkin, S. W. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat. Clin. Pract. Cardiovasc. Med. 6, 192–199 (2009).
Thomas, J. M. et al. IL-18 (Interleukin-18) produced by renal tubular epithelial cells promotes renal inflammation and injury during deoxycorticosterone/salt-induced hypertension in mice. Hypertension 78, 1296–1309 (2021).
Krishnan, S. M., Sobey, C. G., Latz, E., Mansell, A. & Drummond, G. R. IL-1beta and IL-18: inflammatory markers or mediators of hypertension? Br. J. Pharmacol. 171, 5589–5602 (2014).
Sesso, H. D., Wang, L., Buring, J. E., Ridker, P. M. & Gaziano, J. M. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 49, 304–310 (2007).
Siedlinski, M. et al. White blood cells and blood pressure: a mendelian randomization study. Circulation 141, 1307–1317 (2020).
Liu, X. et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am. J. Hypertens. 28, 1339–1346 (2015).
Jhuang, Y. H. et al. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan. Hypertens. Res. 42, 1209–1214 (2019).
Xu, J. P. et al. Systemic inflammation markers and the prevalence of hypertension: a NHANES cross-sectional study. Hypertens. Res. 46, 1009–1019 (2023).
Itani, H. A. et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68, 123–132 (2016).
Loperena, R. et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc. Res. 114, 1547–1563 (2018).
Mikolajczyk, T. P. et al. Heterogeneity of peripheral blood monocytes, endothelial dysfunction and subclinical atherosclerosis in patients with systemic lupus erythematosus. Lupus 25, 18–27 (2016).
Youn, J. C. et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133 (2013).
Delaney, J. A. C. et al. Natural killer cells, gamma delta T cells and classical monocytes are associated with systolic blood pressure in the multi-ethnic study of atherosclerosis (MESA). BMC Cardiovasc. Disord. 21, 45 (2021).
Gackowska, L. et al. Loss of CD31 receptor in CD4+ and CD8+ T-cell subsets in children with primary hypertension is associated with hypertension severity and hypertensive target organ damage. J. Hypertens. 36, 2148–2156 (2018).
Ehret, G. B., O’Connor, A. A., Weder, A., Cooper, R. S. & Chakravarti, A. Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur. J. Hum. Genet. 17, 1650–1657 (2009).
Pineda, B. et al. Polymorphisms in genes involved in T-cell co-stimulation are associated with blood pressure in women. Gene 754, 144838 (2020).
Huan, T. et al. Integrative network analysis reveals molecular mechanisms of blood pressure regulation. Mol. Syst. Biol. 11, 799 (2015).
The International Consortium for Blood Pressure Genome-Wide Association Studies Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Devalliere, J. & Charreau, B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem. Pharmacol. 82, 1391–1402 (2011).
Saleh, M. A. et al. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J. Clin. Invest. 125, 1189–1202 (2015).
Huan, T. et al. A meta-analysis of gene expression signatures of blood pressure and hypertension. PLoS Genet. 11, e1005035 (2015).
Eales, J. M. et al. Uncovering genetic mechanisms of hypertension through multi-omic analysis of the kidney. Nat. Genet. 53, 630–637 (2021).
Zucker, R., Kovalerchik, M. & Linial, M. Gene-based association study reveals a distinct female genetic signal in primary hypertension. Hum. Genet. 142, 863–878 (2023).
McMaster, W. G., Kirabo, A., Madhur, M. S. & Harrison, D. G. Inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 116, 1022–1033 (2015).
Kirabo, A. et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 124, 4642–4656 (2014).
Amador, C. A. et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63, 797–803 (2014).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Caillon, A. et al. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation 135, 2155–2162 (2017).
Taherzadeh, Z. et al. Strain-dependent susceptibility for hypertension in mice resides in the natural killer gene complex. Am. J. Physiol. Heart Circ. Physiol. 298, H1273–H1282 (2010).
Hidalgo, A. et al. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc. Res. 118, 2737–2753 (2021).
Sreejit, G. et al. Neutrophils in cardiovascular disease: warmongers, peacemakers, or both? Cardiovasc. Res. 118, 2596–2609 (2022).
Barhoumi, T. et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57, 469–476 (2011).
Marvar, P. J. et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ. Res. 107, 263–270 (2010).
Vital, S. A., Terao, S., Nagai, M. & Granger, D. N. Mechanisms underlying the cerebral microvascular responses to angiotensin II-induced hypertension. Microcirculation 17, 641–649 (2010).
Pollow, D. P. et al. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64, 384–390 (2014).
Marvar, P. J. et al. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol. Psychiatry 71, 774–782 (2012).
Wu, J. et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 114, 616–625 (2014).
Wu, J. et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Invest. 126, 50–67 (2016).
Dinh, Q. N. et al. Aldosterone-induced hypertension is sex-dependent, mediated by T cells and sensitive to GPER activation. Cardiovasc. Res. 117, 960–970 (2021).
Mattson, D. L. et al. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R407–R414 (2013).
Wade, B., Petrova, G. & Mattson, D. L. Role of immune factors in angiotensin II-induced hypertension and renal damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R323–R333 (2018).
Abais-Battad, J. M., Lund, H., Fehrenbach, D. J. & Dasinger, J. H. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R28–R35 (2018).
Crowley, S. D. et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1089–R1097 (2010).
Ji, H. et al. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64, 573–582 (2014).
Ji, H. et al. Loss of resistance to angiotensin II-induced hypertension in the Jackson Laboratory recombination-activating gene null mouse on the C57BL/6J background. Hypertension 69, 1121–1127 (2017).
Seniuk, A. et al. B6.Rag1 knockout mice generated at the Jackson Laboratory in 2009 show a robust wild-type hypertensive phenotype in response to Ang II (Angiotensin II). Hypertension 75, 1110–1116 (2020).
Uchida, H. A. et al. Total lymphocyte deficiency attenuates AngII-induced atherosclerosis in males but not abdominal aortic aneurysms in apoE deficient mice. Atherosclerosis 211, 399–403 (2010).
Sylvester, M. A. et al. Splenocyte transfer from hypertensive donors eliminates premenopausal female protection from ANG II-induced hypertension. Am. J. Physiol. Ren. Physiol. 322, F245–F257 (2022).
Madhur, M. S., Kirabo, A., Guzik, T. J. & Harrison, D. G. From rags to riches: moving beyond RAG1 in studies of hypertension. Hypertension 75, 930–934 (2020).
Nosalski, R. et al. T-cell-derived miRNA-214 mediates perivascular fibrosis in hypertension. Circ. Res. 126, 988–1003 (2020).
Vinh, A. et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122, 2529–2537 (2010).
Rudemiller, N. et al. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63, 559–564 (2014).
Rodriguez-Iturbe, B., Pons, H. & Johnson, R. J. Role of the immune system in hypertension. Physiol. Rev. 97, 1127–1164 (2017).
Pons, H. et al. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am. J. Physiol. Ren. Physiol. 304, F289–F299 (2013).
Colvert, C. A. et al. Endothelial mechanical stretch regulates the immunological synapse interface of renal endothelial cells in a sex-dependent manner. Am. J. Physiol. Ren. Physiol. 325, F22–F37 (2023).
Pober, J. S., Merola, J., Liu, R. & Manes, T. D. Antigen presentation by vascular cells. Front. Immunol. 8, 1907 (2017).
Aydin, S. et al. Antigen recognition detains CD8+ T cells at the blood-brain barrier and contributes to its breakdown. Nat. Commun. 14, 3106 (2023).
Taflin, C. et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc. Natl Acad. Sci. USA 108, 2891–2896 (2011).
Ngwenyama, N. et al. Antigen presentation by cardiac fibroblasts promotes cardiac dysfunction. Nat. Cardiovasc. Res. 1, 761–774 (2022).
Biancardi, V. C., Bomfim, G. F., Reis, W. L., Al-Gassimi, S. & Nunes, K. P. The interplay between angiotensin II, TLR4 and hypertension. Pharmacol. Res. 120, 88–96 (2017).
Bomfim, G. F. et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin. Sci. 122, 535–543 (2012).
McCarthy, C. G. et al. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc. Res. 107, 119–130 (2015).
Drummond, G. R., Vinh, A., Guzik, T. J. & Sobey, C. G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 19, 517–532 (2019).
Takahashi, M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 118, 372–385 (2022).
Krishnan, S. M. et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 115, 776–787 (2019).
Krishnan, S. M. et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br. J. Pharmacol. 173, 752–765 (2016).
Zhang, J. et al. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab. 23, 360–368 (2016).
Xiao, L., do Carmo, L. S., Foss, J. D., Chen, W. & Harrison, D. G. Sympathetic enhancement of memory T-cell homing and hypertension sensitization. Circ. Res. 126, 708–721 (2020).
Calvillo, L., Gironacci, M. M., Crotti, L., Meroni, P. L. & Parati, G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat. Rev. Cardiol. 16, 476–490 (2019).
Carnevale, D. Neuroimmune axis of cardiovascular control: mechanisms and therapeutic implications. Nat. Rev. Cardiol. 19, 379–394 (2022).
Carnevale, D. et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 41, 737–752 (2014).
Carnevale, L. et al. Celiac vagus nerve stimulation recapitulates angiotensin II-induced splenic noradrenergic activation, driving egress of CD8 effector cells. Cell Rep. 33, 108494 (2020).
Carnevale, D. et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun. 7, 13035 (2016).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Olofsson, P. S. et al. ɑ7 nicotinic acetylcholine receptor (ɑ7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med. 18, 539–543 (2012).
Xiao, L. et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ. Res. 117, 547–557 (2015).
Banek, C. T. et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68, 1415–1423 (2016).
Mohanta, S. K. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022).
Munakata, M. Clinical significance of stress-related increase in blood pressure: current evidence in office and out-of-office settings. Hypertens. Res. 41, 553–569 (2018).
Maaliki, D., Itani, M. M. & Itani, H. A. Pathophysiology and genetics of salt-sensitive hypertension. Front. Physiol. 13, 1001434 (2022).
Ruggeri Barbaro, N. et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc. Res. 117, 1358–1371 (2021).
Jantsch, J. et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 21, 493–501 (2015).
Binger, K. J. et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J. Clin. Invest. 125, 4223–4238 (2015).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Kleinewietfeld, M. & Hafler, D. A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 25, 305–312 (2013).
Norlander, A. E. et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2, e92801 (2017).
Van Beusecum, J. P. et al. High salt activates CD11c+ antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 74, 555–563 (2019).
Barbaro, N. R. et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 21, 1009–1020 (2017).
Pitzer, A. et al. DC ENaC-dependent inflammasome activation contributes to salt-sensitive hypertension. Circ. Res. 131, 328–344 (2022).
Kopp, C. et al. 23Na magnetic resonance imaging of tissue sodium. Hypertension 59, 167–172 (2012).
Schneider, M. P. et al. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J. Am. Soc. Nephrol. 28, 1867–1876 (2017).
Chachaj, A. et al. Role of the lymphatic system in the pathogenesis of hypertension in humans. Lymphat. Res. Biol. 16, 140–146 (2018).
Zhuang, T. et al. A2AR-mediated lymphangiogenesis via VEGFR2 signaling prevents salt-sensitive hypertension. Eur. Heart J. 44, 2730–2742 (2023).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Balasubbramanian, D. et al. Augmenting renal lymphatic density prevents angiotensin II-induced hypertension in male and female mice. Am. J. Hypertens. 33, 61–69 (2020).
Wiig, H. et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Invest. 123, 2803–2815 (2013).
Goodlett, B. L. et al. Genetically inducing renal lymphangiogenesis attenuates hypertension in mice. Clin. Sci. 136, 1759–1772 (2022).
Nosalski, R. & Guzik, T. J. Skin sodium, lymphatics, and blood pressure: a non-canonical mechanism of salt-sensitive hypertension. Eur. Heart J. 44, 2743–2745 (2023).
Lee, H., Jeong, S. & Shin, E. C. Significance of bystander T cell activation in microbial infection. Nat. Immunol. 23, 13–22 (2022).
Trott, D. W. et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115 (2014).
Pacheco, Y. et al. Bystander activation and autoimmunity. J. Autoimmun. 103, 102301 (2019).
Thomas, J. M., Huuskes, B. M., Sobey, C. G., Drummond, G. R. & Vinh, A. The IL-18/IL-18R1 signalling axis: diagnostic and therapeutic potential in hypertension and chronic kidney disease. Pharmacol. Ther. 239, 108191 (2022).
Valente, A. J. et al. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am. J. Physiol. Heart Circ. Physiol. 303, H282–H296 (2012).
Sahar, S. et al. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: a novel cross-talk in the pathogenesis of atherosclerosis. Circ. Res. 96, 1064–1071 (2005).
Morel, J. C., Park, C. C., Woods, J. M. & Koch, A. E. A novel role for interleukin-18 in adhesion molecule induction through NFκB and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J. Biol. Chem. 276, 37069–37075 (2001).
Hyodo, Y. et al. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J. Immunol. 162, 1662–1668 (1999).
Gunturk, E. E., Gunturk, I., Topuz, A. N., Akkaya, H. & Topuz, M. Serum interleukin-18 levels are associated with non-dipping pattern in newly diagnosed hypertensive patients. Blood Press. Monit. 26, 87–92 (2021).
Thorand, B. et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984-2002. Diabetes 54, 2932–2938 (2005).
Evans, J. et al. The association of interleukin-18 genotype and serum levels with metabolic risk factors for cardiovascular disease. Eur. J. Endocrinol. 157, 633–640 (2007).
Ren, H. M., Lukacher, A. E., Rahman, Z. S. M. & Olsen, N. J. New developments implicating IL-21 in autoimmune disease. J. Autoimmun. 122, 102689 (2021).
Spolski, R. & Leonard, W. J. Interleukin-21: a double-edged sword with therapeutic potential. Nat. Rev. Drug. Discov. 13, 379–395 (2014).
Dale, B. L. et al. Critical role of Interleukin 21 and T follicular helper cells in hypertension and vascular dysfunction. JCI Insight 5, e129278 (2019).
McInnes, I. B., Leung, B. P., Sturrock, R. D., Field, M. & Liew, F. Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-ɑ production in rheumatoid arthritis. Nat. Med. 3, 189–195 (1997).
Laurent, C. et al. Interleukin-15 enhances proinflammatory T-cell responses in patients with MS and EAE. Neurol. Neuroimmunol. Neuroinflamm. 8, e931 (2021).
Villadsen, L. S. et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112, 1571–1580 (2003).
Aringer, M. et al. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology 40, 876–881 (2001).
Stumpf, C. et al. Serum levels of the Th1 chemoattractant interferon-gamma-inducible protein (IP) 10 are elevated in patients with essential hypertension. Hypertens. Res. 34, 484–488 (2011).
Li, R. et al. Interleukin-7 induces recruitment of monocytes/macrophages to endothelium. Eur. Heart J. 33, 3114–3123 (2012).
Bullenkamp, J. et al. Interleukin-7 and interleukin-15 drive CD4+CD28null T lymphocyte expansion and function in patients with acute coronary syndrome. Cardiovasc. Res. 117, 1935–1948 (2021).
Nosalski, R. & Guzik, T. J. IL-15 and IL-7: keys to dysregulated inflammation in acute coronary syndromes. Cardiovasc. Res. 117, 1806–1808 (2021).
Paiva, R. A., Ramos, C. V., Leiria, G. & Martins, V. C. IL-7 receptor drives early T lineage progenitor expansion. J. Immunol. 209, 1942–1949 (2022).
Pachynski, R. K. et al. IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 9, e002903 (2021).
Rosenberg, S. A. et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 29, 313–319 (2006).
Hartgring, S. A., Willis, C. R., Bijlsma, J. W., Lafeber, F. P. & van Roon, J. A. Interleukin-7-aggravated joint inflammation and tissue destruction in collagen-induced arthritis is associated with T-cell and B-cell activation. Arthritis Res. Ther. 14, R137 (2012).
Wuttge, D. M., Eriksson, P., Sirsjo, A., Hansson, G. K. & Stemme, S. Expression of interleukin-15 in mouse and human atherosclerotic lesions. Am. J. Pathol. 159, 417–423 (2001).
Gokkusu, C. et al. Influences of genetic variants in interleukin-15 gene and serum interleukin-15 levels on coronary heart disease. Cytokine 49, 58–63 (2010).
Hu, W., Wang, H., Wang, Z., Huang, H. & Dong, M. Elevated serum levels of interleukin-15 and interleukin-16 in preeclampsia. J. Reprod. Immunol. 73, 166–171 (2007).
El-Baradie, S. M., Mahmoud, M. & Makhlouf, H. H. Elevated serum levels of interleukin-15, interleukin-16, and human chorionic gonadotropin in women with preeclampsia. J. Obstet. Gynaecol. Can. 31, 142–148 (2009).
Kalantar, F. et al. Serum levels of tumor necrosis factor-ɑ, interleukin-15 and interleukin-10 in patients with pre-eclampsia in comparison with normotensive pregnant women. Iran. J. Nurs. Midwifery Res. 18, 463–466 (2013).
Kaibe, M. et al. Serum interleukin-15 concentration in patients with essential hypertension. Am. J. Hypertens. 18, 1019–1025 (2005).
Ferrante, G. & Condorelli, G. Interleukin-6 trans-signalling and risk of future cardiovascular events: a new avenue for atheroprotection? Cardiovasc. Res. 115, 8–9 (2019).
Didion, S. P. Cellular and oxidative mechanisms associated with interleukin-6 signaling in the vasculature. Int. J. Mol. Sci. 18, 2563 (2017).
Molitor, M. et al. Aircraft noise exposure induces pro-inflammatory vascular conditioning and amplifies vascular dysfunction and impairment of cardiac function after myocardial infarction. Cardiovasc. Res. 119, 1416–1426 (2023).
Tanaka, T., Narazaki, M. & Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014).
Korn, T. & Hiltensperger, M. Role of IL-6 in the commitment of T cell subsets. Cytokine 146, 155654 (2021).
Manhiani, M. M. et al. The role of IL-6 in the physiologic versus hypertensive blood pressure actions of angiotensin II. Physiol. Rep. 3, e12595 (2015).
Itani, H. A. et al. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ. Res. 118, 1233–1243 (2016).
Wang, X. et al. Single-cell transcriptome profiling reveals enriched memory T-cell subpopulations in hypertension. Front. Cell Dev. Biol. 11, 1132040 (2023).
Fukuda, S., Tsuchikura, S. & Iida, H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp. Anim. 53, 67–72 (2004).
Svendsen, U. G. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol. Microbiol. Scand. A 84, 523–528 (1976).
Ba, D., Takeichi, N., Kodama, T. & Kobayashi, H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J. Immunol. 128, 1211–1216 (1982).
Olsen, F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol. Microbiol. Scand. C. 88, 1–5 (1980).
Ventola, D. A. & Strausser, H. R. Evaluation of T cell subpopulation and function in thymosin treated spontaneously hypertensive rats. Thymus 6, 129–141 (1984).
Okuda, T. & Grollman, A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med. 25, 257–264 (1967).
Santisteban, M. M. et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ. Res. 117, 178–191 (2015).
Wang, L. et al. Genetic and pharmacologic inhibition of the chemokine receptor CXCR2 prevents experimental hypertension and vascular dysfunction. Circulation 134, 1353–1368 (2016).
Rudemiller, N. P. et al. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65, 1111–1117 (2015).
Rohde, D. et al. Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nat. Cardiovasc. Res. 1, 28–44 (2022).
Wu, J. et al. Origin of matrix-producing cells that contribute to aortic fibrosis in hypertension. Hypertension 67, 461–468 (2016).
Itani, M. M. et al. Sphingosine 1 phosphate promotes hypertension specific memory T cell trafficking in response to repeated hypertensive challenges. Front. Physiol. 13, 930487 (2022).
Nosalski, R. & Guzik, T. J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 174, 3496–3513 (2017).
Mikolajczyk, T. P. et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 30, 1987–1999 (2016).
Mikolajczyk, T. P. et al. Role of inflammatory chemokines in hypertension. Pharmacol. Ther. 223, 107799 (2021).
Piqueras, L. & Sanz, M. J. Angiotensin II and leukocyte trafficking: new insights for an old vascular mediator. Role of redox-signaling pathways. Free. Radic. Biol. Med. 157, 38–54 (2020).
Lopez Gelston, C. A. et al. Enhancing renal lymphatic expansion prevents hypertension in mice. Circ. Res. 122, 1094–1101 (2018).
Beaini, S. et al. VEGF-C attenuates renal damage in salt-sensitive hypertension. J. Cell Physiol. 234, 9616–9630 (2019).
Hevia, D. et al. Myeloid CD11c+ antigen-presenting cells ablation prevents hypertension in response to angiotensin II plus high-salt diet. Hypertension 71, 709–718 (2018).
Norlander, A. E. et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68, 167–174 (2016).
Alexander, Y. et al. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 117, 29–42 (2021).
Drummond, G. R., Selemidis, S., Griendling, K. K. & Sobey, C. G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug. Discov. 10, 453–471 (2011).
Faraco, G. et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 21, 240–249 (2018).
Murray, E. C. et al. Vascular phenotypes in early hypertension. J. Hum. Hypertens. 37, 898–906 (2022).
Rizzoni, D. et al. Immune system and microvascular remodeling in humans. Hypertension 79, 691–705 (2022).
Fernandez-Castelo, S. et al. Angiotensin II regulates interferon-γ production. J. Interferon Res. 7, 261–268 (1987).
Kamat, N. V. et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension 65, 569–576 (2015).
Benson, L. N. et al. The IFNγ-PDL1 pathway enhances CD8T-DCT interaction to promote hypertension. Circ. Res. 130, 1550–1564 (2022).
Kossmann, S. et al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler. Thromb. Vasc. Biol. 33, 1313–1319 (2013).
Zhang, J. D. et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ. Res. 110, 1604–1617 (2012).
Sun, X. N. et al. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ. Res. 120, 1584–1597 (2017).
Marko, L. et al. Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60, 1430–1436 (2012).
Satou, R. & Gonzalez-Villalobos, R. A. JAK-STAT and the renin-angiotensin system: the role of the JAK-STAT pathway in blood pressure and intrarenal renin-angiotensin system regulation. JAKSTAT 1, 250–256 (2012).
Shokoples, B. G. et al. Angiotensin II-induced a steeper blood pressure elevation in IL-23 receptor-deficient mice: role of interferon-γ-producing T cells. Hypertens. Res. 46, 40–49 (2023).
Saleh, M. A., Norlander, A. E. & Madhur, M. S. Inhibition of interleukin 17-A but not interleukin-17F signaling lowers blood pressure and reduces end-organ inflammation in angiotensin II-induced hypertension. JACC Basic. Transl. Sci. 1, 606–616 (2016).
Higaki, A., Mahmoud, A. U. M., Paradis, P. & Schiffrin, E. L. Role of interleukin-23/interleukin-17 axis in T-cell-mediated actions in hypertension. Cardiovasc. Res. 117, 1274–1283 (2021).
Karbach, S. et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 34, 2658–2668 (2014).
Platten, M. et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc. Natl Acad. Sci. USA 106, 14948–14953 (2009).
Orejudo, M. et al. Interleukin-17A induces vascular remodeling of small arteries and blood pressure elevation. Clin. Sci. 134, 513–527 (2020).
Nguyen, H. et al. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res. 97, 696–704 (2013).
Tipton, A. J., Baban, B. & Sullivan, J. C. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64, 557–564 (2014).
Singh, M. V. et al. Abnormal CD161+ immune cells and retinoic acid receptor-related orphan receptor γt-mediate enhanced IL-17F expression in the setting of genetic hypertension. J. Allergy Clin. Immunol. 140, 809–821.e3 (2017).
Cao, Y. et al. IL (interleukin)-17A acts in the brain to drive neuroinflammation, sympathetic activation, and hypertension. Hypertension 78, 1450–1462 (2021).
Chae, C. U., Lee, R. T., Rifai, N. & Ridker, P. M. Blood pressure and inflammation in apparently healthy men. Hypertension 38, 399–403 (2001).
Ridker, P. M., Rifai, N., Stampfer, M. J. & Hennekens, C. H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101, 1767–1772 (2000).
Mengozzi, A. et al. The importance of microvascular inflammation in ageing and age-related diseases: a position paper from the ESH working group on small arteries, section of microvascular inflammation. J. Hypertens. 41, 1521–1543 (2023).
Lee, D. L. et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 290, H935–H940 (2006).
Brands, M. W. et al. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56, 879–884 (2010).
Lee, D. L., Wilson, J. L., Duan, R., Hudson, T. & El-Marakby, A. Peroxisome proliferator-activated receptor-ɑ activation decreases mean arterial pressure, plasma interleukin-6, and COX-2 while increasing renal CYP4A expression in an acute model of DOCA-salt hypertension. PPAR Res. 2011, 502631 (2011).
Funakoshi, Y., Ichiki, T., Ito, K. & Takeshita, A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 34, 118–125 (1999).
Coles, B. et al. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am. J. Pathol. 171, 315–325 (2007).
Hashmat, S. et al. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am. J. Physiol. Ren. Physiol. 311, F555–F561 (2016).
Zhang, W. et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 59, 136–144 (2012).
Gonzalez, G. E. et al. Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J. Hypertens. 33, 144–152 (2015).
Schrader, L. I., Kinzenbaw, D. A., Johnson, A. W., Faraci, F. M. & Didion, S. P. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler. Thromb. Vasc. Biol. 27, 2576–2581 (2007).
Naya, M. et al. Plasma interleukin-6 and tumor necrosis factor-ɑ can predict coronary endothelial dysfunction in hypertensive patients. Hypertens. Res. 30, 541–548 (2007).
Wassmann, S. et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 94, 534–541 (2004).
Orshal, J. M. & Khalil, R. A. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R1013–R1023 (2004).
Hung, M. J., Cherng, W. J., Hung, M. Y., Wu, H. T. & Pang, J. H. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J. Hypertens. 28, 940–951 (2010).
Kranzhofer, R. et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 1623–1629 (1999).
Melendez, G. C. et al. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56, 225–231 (2010).
Yang, Y. et al. Interleukin-9 deletion relieves vascular dysfunction and decreases blood pressure via the STAT3 pathway in angiotensin II-treated mice. Mediators Inflamm. 2020, 5741047 (2020).
Zhuang, R. et al. Perivascular fibrosis is mediated by a KLF10-IL-9 signaling axis in CD4+ T cells. Circ. Res. 130, 1662–1681 (2022).
Li, Y. Y. Tumor necrosis factor-alpha g308alpha gene polymorphism and essential hypertension: a meta-analysis involving 2244 participants. PLoS ONE 7, e35408 (2012).
Sriramula, S., Haque, M., Majid, D. S. & Francis, J. Involvement of tumor necrosis factor-ɑ in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 51, 1345–1351 (2008).
Huang, B. et al. Renal tumor necrosis factor ɑ contributes to hypertension in Dahl salt-sensitive rats. Sci. Rep. 6, 21960 (2016).
Zhao, Q. et al. Association between anti-TNF therapy for rheumatoid arthritis and hypertension: a meta-analysis of randomized controlled trials. Medicine 94, e731 (2015).
Wang, H. X. et al. CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodelling via IL-10 signalling in mice. Cardiovasc. Res. 115, 83–93 (2019).
Didion, S. P., Kinzenbaw, D. A., Schrader, L. I., Chu, Y. & Faraci, F. M. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 54, 619–624 (2009).
Kassan, M., Galan, M., Partyka, M., Trebak, M. & Matrougui, K. Interleukin-10 released by CD4+CD25+ natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler. Thromb. Vasc. Biol. 31, 2534–2542 (2011).
Zemse, S. M., Hilgers, R. H. & Webb, R. C. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am. J. Physiol. Heart Circ. Physiol. 292, H3103–H3108 (2007).
Yang, T. et al. Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340 (2015).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Avery, E. G. et al. Quantifying the impact of gut microbiota on inflammation and hypertensive organ damage. Cardiovasc. Res. 119, 1441–1452 (2023).
Jama, H. A. et al. Prebiotic intervention with HAMSAB in untreated essential hypertensive patients assessed in a phase II randomized trial. Nat. Cardiovasc. Res. 2, 35–43 (2023).
Hotez, P. J. Linking tropical infections to hypertension: new comorbid disease paradigms in our era of “blue marble health”. J. Am. Heart Assoc. 8, e03984 (2019).
Etyang, A. O. et al. Effect of previous exposure to malaria on blood pressure in Kilifi, Kenya: a Mendelian randomization study. J. Am. Heart Assoc. 8, e011771 (2019).
Etyang, A. O., Smeeth, L., Cruickshank, J. K. & Scott, J. A. The malaria-high blood pressure hypothesis. Circ. Res. 119, 36–40 (2016).
Gallego-Delgado, J., Walther, T. & Rodriguez, A. The high blood pressure-malaria protection hypothesis. Circ. Res. 119, 1071–1075 (2016).
Nwokocha, C. R., Bafor, E. E., Ajayi, O. I. & Ebeigbe, A. B. The malaria-high blood pressure hypothesis: revisited. Am. J. Hypertens. 33, 695–702 (2020).
Hui, J. et al. Association of cytomegalovirus infection with hypertension risk: a meta-analysis. Wien. Klin. Wochenschr. 128, 586–591 (2016).
Li, C., Samaranayake, N. R., Ong, K. L., Wong, H. K. & Cheung, B. M. Is human cytomegalovirus infection associated with hypertension? The United States National Health and Nutrition Examination Survey 1999-2002. PLoS ONE 7, e39760 (2012).
Cheng, J. et al. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 5, e1000427 (2009).
Laing, A. G. et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 26, 1623–1635 (2020).
Trump, S. et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 39, 705–716 (2021).
Lim, G. B. ACEi reduces hypertension-induced hyperinflammation in COVID-19. Nat. Rev. Cardiol. 18, 231 (2021).
Davis, K. et al. Association between HIV infection and hypertension: a global systematic review and meta-analysis of cross-sectional studies. BMC Med. 19, 105 (2021).
Masenga, S. K. et al. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr. Hypertens. Rep. 21, 56 (2019).
Regnault, V., Challande, P., Pinet, F., Li, Z. & Lacolley, P. Cell senescence: basic mechanisms and the need for computational networks in vascular ageing. Cardiovasc. Res. 117, 1841–1858 (2021).
Furman, D. et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 23, 174–184 (2017).
Rothman, A. M. et al. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension 75, 477–482 (2020).
Takamura, C. et al. Suppression of murine autoimmune myocarditis achieved with direct renin inhibition. J. Cardiol. 68, 253–260 (2016).
Mikolajczyk, T. P. et al. 1,2,3,4,6-Penta-O-galloyl-β-d-glucose modulates perivascular inflammation and prevents vascular dysfunction in angiotensin II-induced hypertension. Br. J. Pharmacol. 176, 1951–1965 (2019).
De Miguel, C., Das, S., Lund, H. & Mattson, D. L. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1136–R1142 (2010).
Batchu, N. et al. Role of Axl in T-lymphocyte survival in salt-dependent hypertension. Arterioscler. Thromb. Vasc. Biol. 36, 1638–1646 (2016).
Perrotta, M. et al. Deoxycorticosterone acetate-salt hypertension activates placental growth factor in the spleen to couple sympathetic drive and immune system activation. Cardiovasc. Res. 114, 456–467 (2018).
Chen, X. H. et al. Deficiency of complement C3a and C5a receptors prevents angiotensin II-induced hypertension via regulatory T cells. Circ. Res. 122, 970–983 (2018).
Kim, J. Y., Lee, E., Koo, S., Kim, C. W. & Kim, I. Transfer of Th17 from adult spontaneous hypertensive rats accelerates development of hypertension in juvenile spontaneous hypertensive rats. Biomed. Res. Int. 2021, 6633825 (2021).
De Ciuceis, C. et al. Relationship between different subpopulations of circulating CD4+ T-lymphocytes and microvascular structural alterations in humans. Am. J. Hypertens. 30, 51–60 (2017).
Ji, Q. et al. Circulating Th1, Th2, and Th17 levels in hypertensive patients. Dis. Markers 2017, 7146290 (2017).
Imiela, A. M. et al. Th17/Treg imbalance in patients with primary hyperaldosteronism and resistant hypertension. Pol. Arch. Intern. Med. 132, 16171 (2022).
Lu, X. et al. Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension 75, 131–138 (2020).
Alexander, M. R. et al. Immune profiling reveals decreases in circulating regulatory and exhausted T cells in human hypertension. JACC Basic. Transl. Sci. 8, 319–336 (2023).
Olofsson, P. S. et al. Blood pressure regulation by CD4+ lymphocytes expressing choline acetyltransferase. Nat. Biotechnol. 34, 1066–1071 (2016).
Kvakan, H. et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119, 2904–2912 (2009).
Kanellakis, P., Dinh, T. N., Agrotis, A. & Bobik, A. CD4+CD25+Foxp3+ regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J. Hypertens. 29, 1820–1828 (2011).
Chan, C. T. et al. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66, 1023–1033 (2015).
Dingwell, L. S. et al. B-cell deficiency lowers blood pressure in mice. Hypertension 73, 561–570 (2019).
Chen, Y. et al. Class switching and high-affinity immunoglobulin G production by B cells is dispensable for the development of hypertension in mice. Cardiovasc. Res. 117, 1217–1228 (2021).
Nosalski, R. et al. Nox1/4 inhibition exacerbates age dependent perivascular inflammation and fibrosis in a model of spontaneous hypertension. Pharmacol. Res. 161, 105235 (2020).
Suryaprabha, P., Padma, T. & Rao, U. B. Increased serum IgG levels in essential hypertension. Immunol. Lett. 8, 143–145 (1984).
Hilme, E., Herlitz, H., Soderstrom, T. & Hansson, L. Increased secretion of immunoglobulins in malignant hypertension. J. Hypertens. 7, 91–95 (1989).
De Ciuceis, C. et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler. Thromb. Vasc. Biol. 25, 2106–2113 (2005).
Wenzel, P. et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124, 1370–1381 (2011).
Chan, C. T. et al. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 60, 1207–1212 (2012).
Moore, J. P. et al. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H906–H917 (2015).
Huang, L. et al. Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front. Physiol. 9, 473 (2018).
Czopek, A. et al. A novel role for myeloid endothelin-B receptors in hypertension. Eur. Heart J. 40, 768–784 (2019).
Guyonnet, L. et al. Deletion of the myeloid endothelin-B receptor confers long-term protection from angiotensin II-mediated kidney, eye and vessel injury. Kidney Int. 98, 1193–1209 (2020).
Weinberger, T. et al. Ontogeny of arterial macrophages defines their functions in homeostasis and inflammation. Nat. Commun. 11, 4549 (2020).
Ko, E. A. et al. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 292, H1789–H1795 (2007).
Frenis, K. et al. Ablation of lysozyme M-positive cells prevents aircraft noise-induced vascular damage without improving cerebral side effects. Basic. Res. Cardiol. 116, 31 (2021).
Alexander, M. R. et al. Human monocyte transcriptional profiling identifies IL-18 receptor accessory protein and lactoferrin as novel immune targets in hypertension. Br. J. Pharmacol. 176, 2015–2027 (2019).
Shah, K. H. et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ. Res. 117, 858–869 (2015).
Morton, J. et al. Circulating neutrophils maintain physiological blood pressure by suppressing bacteria and IFNγ-dependent iNOS expression in the vasculature of healthy mice. Blood 111, 5187–5194 (2008).
Yildirim, A., Russell, J., Yan, L. S., Senchenkova, E. Y. & Granger, D. N. Leukocyte-dependent responses of the microvasculature to chronic angiotensin II exposure. Hypertension 60, 1503–1509 (2012).
Khraibi, A. A., Smith, T. L., Hutchins, P. M., Lynch, C. D. & Dusseau, J. W. Thymectomy delays the development of hypertension in Okamoto spontaneously hypertensive rats. J. Hypertens. 5, 537–541 (1987).
Bataillard, A., Freiche, J. C., Vincent, M., Sassard, J. & Touraine, J. L. Antihypertensive effect of neonatal thymectomy in the genetically hypertensive LH rat. Thymus 8, 321–330 (1986).
Svendsen, U. G. The role of thymus for the development and prognosis of hypertension and hypertensive vascular disease in mice following renal infarction. Acta Pathol. Microbiol. Scand. A 84, 235–243 (1976).
Svendsen, U. G. Influence of neonatal thymectomy on blood pressure and hypertensive vascular disease in rats with renal hypertension. Acta Pathol. Microbiol. Scand. A 83, 199–205 (1975).
Olsen, F. Evidence for an immunological factor in the hypertensive vascular disease. Acta Pathol. Microbiol. Scand. A 79, 22–26 (1971).
Nosalski, R., McGinnigle, E., Siedlinski, M. & Guzik, T. J. Novel immune mechanisms in hypertension and cardiovascular risk. Curr. Cardiovasc. Risk Rep. 11, 12 (2017).
Muller, D. N. et al. NF-κB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 35, 193–201 (2000).
Muller, D. N. et al. Effect of bosentan on NF-κB, inflammation, and tissue factor in angiotensin II-induced end-organ damage. Hypertension 36, 282–290 (2000).
Krzywinski, M. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009).
Acknowledgements
T.J.G. is funded by the European Research Council (ERC and InflammaTENSION; ERC-CoG-726318), British Heart Foundation (FS/14/49/30838 and FS/4yPhD/F/20/34127A), as part of the British Heart Foundation Centre for Research Excellence at the University of Edinburgh (RE/18/5/34216), and CVD ERA-CVD (GutBrainImmune and ImmuneHyperCog, NCBiR, Poland). T.J.G. and P.M. are supported by the British Heart Foundation (PG/19/84/34771, FS/19/56/34893A, PG/21/10541 and PG/21/10634). R.N. is funded by the Chancellor’s Fellowship at the University of Edinburgh.
Author information
Authors and Affiliations
Contributions
T.J.G. and R.N. researched data for the article and wrote the manuscript. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Annet Kirabo, Ernesto L. Schiffrin, Philip Wenzel, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guzik, T.J., Nosalski, R., Maffia, P. et al. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol (2024). https://doi.org/10.1038/s41569-023-00964-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-023-00964-1
This article is cited by
-
Breaking the Barrier: The Role of Gut Epithelial Permeability in the Pathogenesis of Hypertension
Current Hypertension Reports (2024)