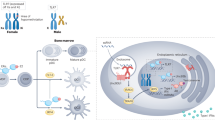
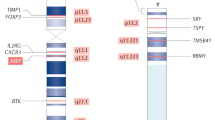
Abstract
There are notable sex-based differences in immune responses to pathogens and self-antigens, with female individuals exhibiting increased susceptibility to various autoimmune diseases, and male individuals displaying preferential susceptibility to some viral, bacterial, parasitic and fungal infections. Although sex hormones clearly contribute to sex differences in immune cell composition and function, the presence of two X chromosomes in female individuals suggests that differential gene expression of numerous X chromosome-linked immune-related genes may also influence sex-biased innate and adaptive immune cell function in health and disease. Here, we review the sex differences in immune system composition and function, examining how hormones and genetics influence the immune system. We focus on the genetic and epigenetic contributions responsible for altered X chromosome-linked gene expression, and how this impacts sex-biased immune responses in the context of pathogen infection and systemic autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Libert, C., Dejager, L. & Pinheiro, I. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 10, 594–604 (2010).
Pasche, B. et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect. Immun. 73, 5952–5960 (2005).
Natri, H., Garcia, A. R., Buetow, K. H., Trumble, B. C. & Wilson, M. A. The pregnancy pickle: evolved immune compensation due to pregnancy underlies sex differences in human diseases. Trends Genet. 35, 478–488 (2019).
Patin, E. et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 19, 302–314 (2018).
Clave, E. et al. Human thymopoiesis is influenced by a common genetic variant within the TCRA-TCRD locus. Sci. Transl. Med. 10, eaa02966 (2018).
Bongen, E. et al. Sex differences in the blood transcriptome identify robust changes in immune cell proportions with aging and influenza infection. Cell Rep. 29, 1961–1973.e4 (2019).
Abdullah, M. et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 272, 214–219 (2012).
Melzer, S. et al. Reference intervals for leukocyte subsets in adults: results from a population-based study using 10-color flow cytometry. Cytom. B Clin. Cytom. 88, 270–281 (2015).
Huang, Z. et al. Effects of sex and aging on the immune cell landscape as assessed by single-cell transcriptomic analysis. Proc. Natl Acad. Sci. USA 118, e2023216118 (2021).
Wikby, A., Mansson, I. A., Johansson, B., Strindhall, J. & Nilsson, S. E. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 9, 299–308 (2008).
Carr, E. J. et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nat. Immunol. 17, 461–468 (2016).
Khan, S. R. et al. Determinants of serum immunoglobulin levels: a systematic review and meta-analysis. Front. Immunol. 12, 664526 (2021).
Hensel, J. A., Khattar, V., Ashton, R. & Ponnazhagan, S. Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab. Invest. 99, 93–106 (2019).
Breznik, J. A., Schulz, C., Ma, J., Sloboda, D. M. & Bowdish, D. M. E. Biological sex, not reproductive cycle, influences peripheral blood immune cell prevalence in mice. J. Physiol. 599, 2169–2195 (2021).
Scotland, R. S., Stables, M. J., Madalli, S., Watson, P. & Gilroy, D. W. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118, 5918–5927 (2011).
Menees, K. B. et al. Sex- and age-dependent alterations of splenic immune cell profile and NK cell phenotypes and function in C57BL/6J mice. Immun. Ageing 18, 3 (2021).
Cheng, M. I. et al. The X-linked epigenetic regulator UTX controls NK cell-intrinsic sex differences. Nat. Immunol. 24, 780–791 (2023).
Schmiedel, B. J. et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell 175, 1701–1715.e16 (2018).
Durandy, A., Cantaert, T., Kracker, S. & Meffre, E. Potential roles of activation-induced cytidine deaminase in promotion or prevention of autoimmunity in humans. Autoimmunity 46, 148–156 (2013).
Young, N. A. et al. Estrogen modulation of endosome-associated Toll-like receptor 8: an IFNα-independent mechanism of sex-bias in systemic lupus erythematosus. Clin. Immunol. 151, 66–77 (2014).
Lee, J. et al. Oestrogen up-regulates interleukin-21 production by CD4+ T lymphocytes in patients with systemic lupus erythematosus. Immunology 142, 573–580 (2014).
Lee, S. et al. Interleukin-23 drives expansion of Thelper 17 cells through epigenetic regulation by signal transducer and activators of transcription 3 in lupus patients. Rheumatology 59, 3058–3069 (2020).
Berghofer, B. et al. TLR7 ligands induce higher IFN-α production in females. J. Immunol. 177, 2088–2096 (2006).
Meier, A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 15, 955–959 (2009).
Seillet, C. et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 119, 454–464 (2012).
Regis, E. et al. Sex differences in innate anti-viral immune responses to respiratory viruses and in their clinical outcomes in a birth cohort study. Sci. Rep. 11, 23741 (2021).
Congy-Jolivet, N. et al. Monocytes are the main source of STING-mediated IFN-α production. EBioMedicine 80, 104047 (2022).
Griesbeck, M. et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J. Immunol. 195, 5327–5336 (2015).
Gal-Oz, S. T. et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat. Commun. 10, 4295 (2019).
Zeng, Z. et al. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat. Immunol. 19, 1100–1111 (2018).
McDonald, G. et al. Female bias in systemic lupus erythematosus is associated with the differential expression of X-linked Toll-like receptor 8. Front. Immunol. 6, 457 (2015).
Souyris, M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Aomatsu, M., Kato, T., Kasahara, E. & Kitagawa, S. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem. Biophys. Res. Commun. 441, 220–225 (2013).
Ho, C. H. et al. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. PLoS ONE 12, e0180244 (2017).
Fish, E. N. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 8, 737–744 (2008).
Liva, S. M. & Voskuhl, R. R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 167, 2060–2067 (2001).
Trigunaite, A., Dimo, J. & Jorgensen, T. N. Suppressive effects of androgens on the immune system. Cell. Immunol. 294, 87–94 (2015).
Wilhelmson, A. S. et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 9, 2067 (2018).
Zhao, R. et al. A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity. Nature 577, 416–420 (2020).
Kissick, H. T. et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl Acad. Sci. USA 111, 9887–9892 (2014).
Dragin, N. et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Invest. 126, 1525–1537 (2016).
Zhu, M. L. et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 7, 11350 (2016).
Walecki, M. et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol. Biol. Cell 26, 2845–2857 (2015).
Marahrens, Y., Panning, B., Dausman, J., Strauss, W. & Jaenisch, R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11, 156–166 (1997).
Anguera, M. C. et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell 11, 75–90 (2012).
Adrianse, R. L. et al. Perturbed maintenance of transcriptional repression on the inactive X-chromosome in the mouse brain after Xist deletion. Epigenetics Chromatin 11, 50 (2018).
Yang, L., Yildirim, E., Kirby, J. E., Press, W. & Lee, J. T. Widespread organ tolerance to Xist loss and X reactivation except under chronic stress in the gut. Proc. Natl Acad. Sci. USA 117, 4262–4272 (2020).
Yildirim, E. et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 152, 727–742 (2013).
Boggs, B. A. et al. Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes. Nat. Genet. 30, 73–76 (2002).
Plath, K. et al. Role of histone H3 lysine 27 methylation in X inactivation. Science 300, 131–135 (2003).
Costanzi, C., Stein, P., Worrad, D. M., Schultz, R. M. & Pehrson, J. R. Histone macroH2A1 is concentrated in the inactive X chromosome of female preimplantation mouse embryos. Development 127, 2283–2289 (2000).
Clemson, C. M., McNeil, J. A., Willard, H. F. & Lawrence, J. B. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132, 259–275 (1996).
Wang, J. et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl Acad. Sci. USA 113, E2029–E2038 (2016).
Syrett, C. M. et al. Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells. PLoS Genet. 13, e1007050 (2017).
Syrett, C. M. et al. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight 4, e126751 (2019).
Syrett, C. M. et al. Diversity of epigenetic features of the inactive X-chromosome in NK cells, dendritic cells, and macrophages. Front. Immunol. 9, 3087 (2019).
Pyfrom, S. et al. The dynamic epigenetic regulation of the inactive X chromosome in healthy human B cells is dysregulated in lupus patients. Proc. Natl Acad. Sci. USA 118, e2024624118 (2021).
Syrett, C. M. et al. Diversity of epigenetic features of the inactive X-chromosome in NK cells, dendritic cells, and macrophages. Front. Immunol. 9, 3087 (2018).
Ramos-Casals, M. et al. Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun. Rev. 14, 670–679 (2015).
Jacobson, D. L., Gange, S. J., Rose, N. R. & Graham, N. M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243 (1997).
Beeson, P. B. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 96, 457–462 (1994).
Koch-Henriksen, N. & Sorensen, P. S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9, 520–532 (2010).
Syrett, C. M. & Anguera, M. C. When the balance is broken: X-linked gene dosage from two X chromosomes and female-biased autoimmunity. J. Leukoc. Biol. 106, 919–932 (2019).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369 (2014).
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 41, 778–799 (1998).
Meyer, A. et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology 54, 50–63 (2015).
Runmarker, B. & Andersen, O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 116, 117–134 (1993).
Peoples, C., Medsger, T. A. Jr, Lucas, M., Rosario, B. L. & Feghali-Bostwick, C. A. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J. Scleroderma Relat. Disord. 1, 177–240 (2016).
Crosslin, K. L. & Wiginton, K. L. Sex differences in disease severity among patients with systemic lupus erythematosus. Gend. Med. 8, 365–371 (2011).
Courvoisier, N. et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res. Ther. 10, R106 (2008).
Sierra, I. et al. Unusual X chromosome inactivation maintenance in female alveolar type 2 cells is correlated with increased numbers of X-linked escape genes and sex-biased gene expression. Stem Cell Rep. 18, 489–502 (2022).
Tukiainen, T. et al. Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 (2017).
Jiwrajka, N. et al. Impaired dynamic X-chromosome inactivation maintenance in T cells is a feature of spontaneous murine SLE that is exacerbated in female-biased models. J. Autoimmun. 139, 103084 (2023).
Syrett, C. M., Sierra, I., Beethem, Z. T., Dubin, A. H. & Anguera, M. C. Loss of epigenetic modifications on the inactive X chromosome and sex-biased gene expression profiles in B cells from NZB/W F1 mice with lupus-like disease. J. Autoimmun. 107, 102357 (2020).
Scofield, R. H. et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 58, 2511–2517 (2008).
Scofield, R. H. et al. 47XXY and 47XXX in scleroderma and myositis. ACR Open Rheumatol. 4, 528–533 (2022).
De Vries, G. J. et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 22, 9005–9014 (2002).
Smith-Bouvier, D. L. et al. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 205, 1099–1108 (2008).
David, A. et al. Intrinsic autoimmune capacities of hematopoietic cells from female New Zealand hybrid mice. Genes Immun. 15, 153–161 (2014).
Geha, R. S. et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J. Allergy Clin. Immunol. 120, 776–794 (2007).
Jiwrajka, N. & Anguera, M. C. The X in sex-biased immunity and autoimmune rheumatic disease. J. Exp. Med. 219, e20211487 (2022).
Desai-Mehta, A., Lu, L., Ramsey-Goldman, R. & Datta, S. K. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Invest. 97, 2063–2073 (1996).
Hewagama, A. et al. Overexpression of X-linked genes in T cells from women with lupus. J. Autoimmun. 41, 60–71 (2013).
Lian, X. et al. DNA demethylation of CD40l in CD4+ T cells from women with systemic sclerosis: a possible explanation for female susceptibility. Arthritis Rheum. 64, 2338–2345 (2012).
Perez-Melgosa, M., Hollenbaugh, D. & Wilson, C. B. Cutting edge: CD40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J. Immunol. 163, 1123–1127 (1999).
Enghard, P. et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 60, 199–206 (2009).
Ogawa, N., Ping, L., Zhenjun, L., Takada, Y. & Sugai, S. Involvement of the interferon-γ-induced T cell-attracting chemokines, interferon-γ-inducible 10-kd protein (CXCL10) and monokine induced by interferon-γ (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis Rheum. 46, 2730–2741 (2002).
Kong, W. et al. Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin. Rheumatol. 37, 43–49 (2018).
Kil, L. P. et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012).
Katewa, A. et al. Btk-specific inhibition blocks pathogenic plasma cell signatures and myeloid cell-associated damage in IFNα-driven lupus nephritis. JCI Insight 2, e90111 (2017).
Valencia, X. & Lipsky, P. E. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 3, 619–626 (2007).
Wang, Y. Y. et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br. J. Dermatol. 171, 39–47 (2014).
Bonelli, M., von Dalwigk, K., Savitskaya, A., Smolen, J. S. & Scheinecker, C. Foxp3 expression in CD4+ T cells of patients with systemic lupus erythematosus: a comparative phenotypic analysis. Ann. Rheum. Dis. 67, 664–671 (2008).
MacDonald, K. G. et al. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J. Allergy Clin. Immunol. 135, 946–955.e9 (2015).
Yang, C., Huang, X. R., Fung, E., Liu, H. F. & Lan, H. Y. The regulatory T-cell transcription factor Foxp3 protects against crescentic glomerulonephritis. Sci. Rep. 7, 1481 (2017).
Garcia-Ortiz, H. et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann. Rheum. Dis. 69, 1861–1865 (2010).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
Deane, J. A. et al. Control of Toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
Pisitkun, P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006).
Harris, V. M. et al. Characterization of cxorf21 provides molecular insight into female-bias immune response in SLE pathogenesis. Front. Immunol. 10, 2160 (2019).
Kelvin, E. A. et al. The association of host age and gender with inflammation around neurocysticercosis cysts. Ann. Trop. Med. Parasitol. 103, 487–499 (2009).
Wright, J. E., Werkman, M., Dunn, J. C. & Anderson, R. M. Current epidemiological evidence for predisposition to high or low intensity human helminth infection: a systematic review. Parasit. Vectors 11, 65 (2018).
Tchuem Tchuenté, L. A., Behnke, J. M., Gilbert, F. S., Southgate, V. R. & Vercruysse, J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop. Med. Int. Health 8, 975–986 (2003).
Nicolosi, A. et al. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus. Epidemiology 5, 570–575 (1994).
WHO Ebola Response Team et al. Ebola virus disease among male and female persons in West Africa. N. Engl. J. Med. 374, 96–98 (2016).
Hertz, D. & Schneider, B. Sex differences in tuberculosis. Semin. Immunopathol. 41, 225–237 (2019).
Shaheen, A. A. M., Somayaji, R., Myers, R. & Mody, C. H. Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: a population-based study. Int. J. STD AIDS 29, 453–460 (2018).
Gemmati, D. et al. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int. J. Mol. Sci. 21, 3474 (2020).
Devaux, C. A., Rolain, J.-M. & Raoult, D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. 53, 425–435 (2020).
Channappanavar, R. et al. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 198, 4046–4053 (2017).
Stelzig, K. E. et al. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L1280–L1281 (2020).
Oghumu, S. et al. Cutting edge: CXCR3 escapes X chromosome inactivation in T cells during infection: potential implications for sex differences in immune responses. J. Immunol. 203, 789–794 (2019).
Soulat, D. et al. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J. 27, 2135–2146 (2008).
Szappanos, D. et al. The RNA helicase DDX3X is an essential mediator of innate antimicrobial immunity. PLoS Pathog. 14, e1007397 (2018).
Bustamante, J. et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat. Immunol. 12, 213–221 (2011).
Filipe-Santos, O. et al. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 18, 347–361 (2006).
Klein, S. L., Marriott, I. & Fish, E. N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 109, 9–15 (2015).
Fink, A. L., Engle, K., Ursin, R. L., Tang, W. Y. & Klein, S. L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl Acad. Sci. USA 115, 12477–12482 (2018).
Asano, T. et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6, eabl4348 (2021).
Onodi, F. et al. SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J. Exp. Med. 218, e20201387 (2021).
Fallerini, C. et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case–control study. eLife 10, e67569 (2021).
Solanich, X. et al. Genetic screening for TLR7 variants in young and previously healthy men with severe COVID-19. Front. Immunol. 12, 719115 (2021).
van der Made, C. I. et al. Presence of genetic variants among young men with severe COVID-19. JAMA 324, 663–673 (2020).
Green, M. S. et al. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines 10, 233 (2022).
Tsilingiris, D., Vallianou, N. G., Karampela, I. & Dalamaga, M. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metab. Open 11, 100101 (2021).
Ling, R. R. et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir. Med. 10, 679–688 (2022).
Florez-Vargas, O. et al. Bias in the reporting of sex and age in biomedical research on mouse models. eLife 5, e13615 (2016).
Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 (2011).
Guan, X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 606, 791–796 (2022).
Rees, F., Doherty, M., Grainge, M. J., Lanyon, P. & Zhang, W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology 56, 1945–1961 (2017).
Maciel, G., Crowson, C. S., Matteson, E. L. & Cornec, D. Prevalence of primary Sjogren’s syndrome in a US population-based cohort. Arthritis Care Res. 69, 1612–1616 (2017).
Pillemer, S. R. et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin. Proc. 76, 593–599 (2001).
Mayes, M. D. et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 48, 2246–2255 (2003).
Hughes, M. et al. Gender-related differences in systemic sclerosis. Autoimmun. Rev. 19, 102494 (2020).
Gabriel, S. E., Crowson, C. S. & O’Fallon, W. M. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 42, 415–420 (1999).
Zhou, Y. et al. T cell CD40LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clin. Immunol. 132, 362–370 (2009).
Balaton, B. P., Cotton, A. M. & Brown, C. J. Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biol. Sex Differ. 6, 35 (2015).
Lu, Q. et al. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J. Immunol. 179, 6352–6358 (2007).
Clegg, C. H. et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int. Immunol. 9, 1111–1122 (1997).
Le Coz, C. et al. CD40LG duplication-associated autoimmune disease is silenced by nonrandom X-chromosome inactivation. J. Allergy Clin. Immunol. 141, 2308–2311.e7 (2018).
Groom, J. R. & Luster, A. D. CXCR3 in T cell function. Exp. Cell Res. 317, 620–631 (2011).
Flier, J. et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J. Pathol. 194, 398–405 (2001).
Eriksson, C., Eneslätt, K., Ivanoff, J., Rantapää-Dahlqvist, S. & Sundqvist, K. G. Abnormal expression of chemokine receptors on T-cells from patients with systemic lupus erythematosus. Lupus 12, 766–774 (2003).
Rankin, A. L. et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J. Immunol. 191, 4540–4550 (2013).
Cornacchia, E. et al. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J. Immunol. 140, 2197–2200 (1988).
Golks, A., Tran, T. T., Goetschy, J. F. & Guerini, D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 26, 4368–4379 (2007).
Wu, J. L. et al. O-GlcNAcylation is required for B cell homeostasis and antibody responses. Nat. Commun. 8, 1854 (2017).
Odhams, C. A. et al. Interferon inducible X-linked gene CXorf21 may contribute to sexual dimorphism in systemic lupus erythematosus. Nat. Commun. 10, 2164 (2019).
Heinz, L. X. et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7–9. Nature 581, 316–322 (2020).
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47, 1457–1464 (2015).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis E Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Abbas, F. et al. HIV-1 infection enhances innate function and TLR7 expression in female plasmacytoid dendritic cells. Life Sci. Alliance 5, e202201452 (2022).
Hagen, S. H. et al. Heterogeneous escape from X chromosome inactivation results in sex differences in type I IFN responses at the single human pDC level. Cell Rep. 33, 108485 (2020).
Fink, A. L. & Klein, S. L. Sex and gender impact immune responses to vaccines among the elderly. Physiology 30, 408–416 (2015).
Fischinger, S., Boudreau, C. M., Butler, A. L., Streeck, H. & Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 41, 239–249 (2019).
Klein, S. L., Jedlicka, A. & Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 10, 338–349 (2010).
Morgan, R. & Klein, S. L. The intersection of sex and gender in the treatment of influenza. Curr. Opin. Virol. 35, 35–41 (2019).
Potluri, T. et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 4, 29 (2019).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. USA 111, 869–874 (2014).
Kennedy, R. B. et al. Gender effects on humoral immune responses to smallpox vaccine. Vaccine 27, 3319–3323 (2009).
Troy, J. D., Hill, H. R., Ewell, M. G. & Frey, S. E. Sex difference in immune response to vaccination: a participant-level meta-analysis of randomized trials of IMVAMUNE smallpox vaccine. Vaccine 33, 5425–5431 (2015).
Trevisan, A. et al. Sex disparity in response to hepatitis B vaccine related to the age of vaccination. Int. J. Environ. Res. Public Health 17, 327 (2020).
Domínguez, A. et al. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur. J. Clin. Microbiol. Infect. Dis. 25, 310–317 (2006).
Riggenbach, M. M. et al. Mumps virus-specific immune response outcomes and sex-based differences in a cohort of healthy adolescents. Clin. Immunol. 234, 108912 (2022).
Mossong, J., O’Callaghan, C. J. & Ratnam, S. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine 19, 523–529 (2000).
Cheung, F. et al. Sex and prior exposure jointly shape innate immune responses to a live herpesvirus vaccine. eLife 12, e80652 (2023).
Lindsey, N. P. et al. Adverse event reports following yellow fever vaccination. Vaccine 26, 6077–6082 (2008).
Monath, T. P. et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am. J. Trop. Med. Hyg. 66, 533–541 (2002).
Andersen, A., Bjerregaard-Andersen, M., Rodrigues, A., Umbasse, P. & Fisker, A. B. Sex-differential effects of diphtheria-tetanus-pertussis vaccine for the outcome of paediatric admissions? A hospital based observational study from Guinea-Bissau. Vaccine 35, 7018–7025 (2017).
Aldakak, L., Huber, V. M., Ruhli, F. & Bender, N. Sex difference in the immunogenicity of the quadrivalent human papilloma virus vaccine: systematic review and meta-analysis. Vaccine 39, 1680–1686 (2021).
Zhu, Z., Xu, L. & Chen, G. Is there a difference in the efficacy of COVID-19 vaccine in males and females? — A systematic review and meta-analysis. Hum. Vaccin. Immunother. 17, 4741–4746 (2021).
Brown, M. A. & Su, M. A. An inconvenient variable: sex hormones and their impact on T cell responses. J. Immunol. 202, 1927–1933 (2019).
Singh, R. P., Hahn, B. H. & Bischoff, D. S. Interferon genes are influenced by 17β-estradiol in SLE. Front. Immunol. 12, 725325 (2021).
Straub, R. H. The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574 (2007).
Mohammad, I. et al. Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci. Signal. 11, eaap9412 (2018).
Kim, D. H. et al. Estrogen receptor α in T cells suppresses follicular helper T cell responses and prevents autoimmunity. Exp. Mol. Med. 51, 1–9 (2019).
Goodman, W. A. et al. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc. Natl Acad. Sci. USA 117, 17166–17176 (2020).
Aggelakopoulou, M., Kourepini, E., Paschalidis, N. & Panoutsakopoulou, V. ERβ in CD4+ T cells is crucial for ligand-mediated suppression of central nervous system autoimmunity. J. Immunol. 196, 4947–4956 (2016).
Garnier, L. et al. Estrogen signaling in bystander Foxp3neg CD4+ T cells suppresses cognate Th17 differentiation in trans and protects from central nervous system autoimmunity. J. Immunol. 201, 3218–3228 (2018).
Xiong, Y. et al. Estradiol resolves pneumonia via ERβ in regulatory T cells. JCI Insight 6, e133251 (2021).
Tabor, D. E. & Gould, K. A. Estrogen receptor α promotes lupus in (NZBxNZW)F1 mice in a B cell intrinsic manner. Clin. Immunol. 174, 41–52 (2017).
Carlsten, H. et al. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell. Immunol. 144, 190–202 (1992).
Herrmann, M., Scholmerich, J. & Straub, R. H. Influence of cytokines and growth factors on distinct steroidogenic enzymes in vitro: a short tabular data collection. Ann. N. Y. Acad. Sci. 966, 166–186 (2002).
de Man, Y. A., Dolhain, R. J., van de Geijn, F. E., Willemsen, S. P. & Hazes, J. M. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 59, 1241–1248 (2008).
Baer, A. N., Witter, F. R. & Petri, M. Lupus and pregnancy. Obstet. Gynecol. Surv. 66, 639–653 (2011).
Cutolo, M. & Straub, R. H. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat. Rev. Rheumatol. 16, 628–644 (2020).
Gubbels Bupp, M. R. & Jorgensen, T. N. Androgen-induced immunosuppression. Front. Immunol. 9, 794 (2018).
Traish, A., Bolanos, J., Nair, S., Saad, F. & Morgentaler, A. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. J. Clin. Med. 7, 549 (2018).
Lyon, M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190, 372–373 (1961).
Brown, C. J. et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349, 38–44 (1991).
Brown, C. J. et al. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71, 527–542 (1992).
Colognori, D., Sunwoo, H., Kriz, A. J., Wang, C. Y. & Lee, J. T. Xist deletional analysis reveals an interdependency between Xist RNA and Polycomb complexes for spreading along the inactive X. Mol. Cell 74, 101–117.e10 (2019).
Simon, M. D. et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature 504, 465–469 (2013).
Żylicz, J. J. et al. The implication of early chromatin changes in X chromosome inactivation. Cell 176, 182–197.23 (2019).
Engreitz, J. M. et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, 1237973 (2013).
Żylicz, J. J. et al. The implication of early chromatin changes in X chromosome inactivation. Cell 176, 182–197.e23 (2019).
Costanzi, C. & Pehrson, J. R. Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393, 599–601 (1998).
Gendrel, A.-V. et al. Smchd1-dependent and -independent pathways determine developmental dynamics of CpG Island methylation on the inactive X chromosome. Dev. Cell 23, 265–279 (2012).
Lock, L. F., Takagi, N. & Martin, G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell 48, 39–46 (1987).
Norris, D. P., Brockdorff, N. & Rastan, S. Methylation status of CpG-rich islands on active and inactive mouse X chromosomes. Mamm. Genome 1, 78–83 (1991).
Sharp, A. J. et al. DNA methylation profiles of human active and inactive X chromosomes. Genome Res. 21, 1592–1600 (2011).
Cotton, A. M. et al. Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Hum. Mol. Genet. 24, 1528–1539 (2015).
Carrel, L. & Willard, H. F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 (2005).
Berletch, J. B. et al. Escape from X inactivation varies in mouse tissues. PLoS Genet. 11, e1005079 (2015).
Yang, F., Babak, T., Shendure, J. & Disteche, C. M. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 20, 614–622 (2010).
Berletch, J. B., Yang, F., Xu, J., Carrel, L. & Disteche, C. M. Genes that escape from X inactivation. Hum. Genet. 130, 237–245 (2011).
Aguet, F. et al. The impact of sex on gene expression across human tissues. Science 369, eaba3066 (2020).
Zhang, Y. et al. Genes that escape X-inactivation in humans have high intraspecific variability in expression, are associated with mental impairment but are not slow evolving. Mol. Biol. Evol. 30, 2588–2601 (2013).
Meester, I. et al. SeXY chromosomes and the immune system: reflections after a comparative study. Biol. Sex Differ. 11, 3 (2020).
Berletch, J. B. et al. Identification of genes escaping X inactivation by allelic expression analysis in a novel hybrid mouse model. Data Brief. 5, 761–769 (2015).
Andrews, B. S. et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J. Exp. Med. 148, 1198–1215 (1978).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Fairhurst, A. M. et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 38, 1971–1978 (2008).
Haywood, M. E. et al. Identification of intervals on chromosomes 1, 3, and 13 linked to the development of lupus in BXSB mice. Arthritis Rheum. 43, 349–355 (2000).
Burnet, F. M. & Holmes, M. C. The natural history of the NZB/NZW F1 hybrid mouse: a laboratory model of systemic lupus erythematosus. Australas. Ann. Med. 14, 185–191 (1965).
Helyer, B. J. & Howie, J. B. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature 197, 197 (1963).
Kessler, H. S. A laboratory model for Sjogren’s syndrome. Am. J. Pathol. 52, 671–685 (1968).
Roubinian, J. R., Talal, N., Greenspan, J. S., Goodman, J. R. & Siiteri, P. K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J. Exp. Med. 147, 1568–1583 (1978).
Verheul, H. A., Verveld, M., Hoefakker, S. & Schuurs, A. H. Effects of ethinylestradiol on the course of spontaneous autoimmune disease in NZB/W and NOD mice. Immunopharmacol. Immunotoxicol. 17, 163–180 (1995).
Waters, S. T. et al. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin. Immunol. 100, 372–383 (2001).
Reeves, W. H., Lee, P. Y., Weinstein, J. S., Satoh, M. & Lu, L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 30, 455–464 (2009).
Satoh, M. et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin. Exp. Immunol. 121, 399–405 (2000).
Du, S. et al. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 111, 2806–2811 (2014).
Crawford, J. D. et al. The XIST lncRNA is a sex-specific reservoir of TLR7 ligands in SLE. JCI Insight 8, e169344 (2023).
Dou, D. R. et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell 187, 733–749.e16 (2024).
Dai, D. The transcription factor ZEB2 drives the formation of age-associated B cells. Science 383, 413–421 (2024).
Acknowledgements
The authors thank L. King and members of the Anguera laboratory for their input and feedback on the manuscript and figures. This work was supported by grants from the US National Institutes of Health (R01-AI134834 to M.C.A., T32 DK-07780 to K.S.F. and T32-AR076951-01 to N.J.), the Lupus Research Alliance TIL grant (to M.C.A.); the Rheumatology Research Foundation Future Physician Scientist Award (to C.D.L.), the Rheumatology Research Foundation Scientist Development Award (to N.J.), the Scleroderma Research Foundation Postdoctoral Award (to N.J.), the Penn Colton Center for Autoimmunity and the Institute for Immunology and Immune Health (to M.C.A. and N.J.), the Philadelphia Tri-State Chapter Goldie Simon Preceptorship Award (to C.D.L. and N.E.T.), the Lupus Foundation of America, Philadelphia Tri-State Chapter Gina M. Finzi Memorial Student Summer Awards (to C.D.L. and N.E.T.), and the H. Ralph Schumacher Rheumatology Research Fund (to N.J.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed substantially to discussion of the content. M.C.A. wrote the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Maureen A. Su, who co-reviewed with Nicole Wilkinson, Katy Flanagan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Forsyth, K.S., Jiwrajka, N., Lovell, C.D. et al. The conneXion between sex and immune responses. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-00996-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-00996-9
This article is cited by
-
Human in vivo evidence of associations between herpes simplex virus and cerebral amyloid-beta load in normal aging
Alzheimer's Research & Therapy (2024)
-
The miR-183/96/182 cluster regulates sensory innervation, resident myeloid cells and functions of the cornea through cell type-specific target genes
Scientific Reports (2024)