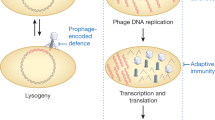
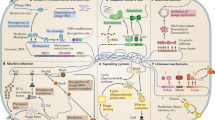
Abstract
To contend with the diversity and ubiquity of bacteriophages and other mobile genetic elements, bacteria have developed an arsenal of immune defence mechanisms. Bacterial defences include CRISPR–Cas, restriction–modification and a growing list of mechanistically diverse systems, which constitute the bacterial ‘immune system’. As a response, bacteriophages and mobile genetic elements have evolved direct and indirect mechanisms to circumvent or block bacterial defence pathways and ensure successful infection. Recent advances in methodological and computational approaches, as well as the increasing availability of genome sequences, have boosted the discovery of direct inhibitors of bacterial defence systems. In this Review, we discuss methods for the discovery of direct inhibitors, their diverse mechanisms of action and perspectives on their emerging applications in biotechnology and beyond.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Salmond, G. P. C. & Fineran, P. C. A century of the phage: past, present and future. Nat. Rev. Microbiol. 13, 777–786 (2015).
Suttle, C. A. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812 (2007).
Brüssow, H., Canchaya, C. & Hardt, W.-D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68, 560–602 (2004).
Mayo-Muñoz, D., Pinilla-Redondo, R., Birkholz, N. & Fineran, P. C. A host of armor: prokaryotic immune strategies against mobile genetic elements. Cell Rep. 42, 112672 (2023). This review provides a comprehensive overview of bacterial immune defences discovered to date.
Georjon, H. & Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 21, 686–700 (2023).
Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687 (2013).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Malone, L. M., Birkholz, N. & Fineran, P. C. Conquering CRISPR: how phages overcome bacterial adaptive immunity. Curr. Opin. Biotechnol. 68, 30–36 (2021).
Ibarra-Chávez, R., Hansen, M. F., Pinilla-Redondo, R., Seed, K. D. & Trivedi, U. Phage satellites and their emerging applications in biotechnology. FEMS Microbiol. Rev. 45, fuab031 (2021).
Barrangou, R., Sontheimer, E. J. & Marraffini, L. A. Crispr: Biology and Applications. (John Wiley & Sons, 2022).
Marino, N. D., Pinilla-Redondo, R., Csörgő, B. & Bondy-Denomy, J. Anti-CRISPR protein applications: natural brakes for CRISPR-Cas technologies. Nat. Methods 17, 471–479 (2020).
Studier, F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J. Mol. Biol. 94, 283–295 (1975). In this study, the first inhibitor of restriction–modification systems, Ocr, is identified through the isolation of sensitive T7 phage mutants.
Tock, M. R. & Dryden, D. T. F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Bondy-Denomy, J., Pawluk, A., Maxwell, K. L. & Davidson, A. R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493, 429–432 (2013). In this study, the first five Acrs are discovered in the genomes of bacteriophages infecting Pseudomonas aeruginosa.
Hobbs, S. J. et al. Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity. Nature 605, 522–526 (2022). This study describes the anti-CBASS and anti-Pycsar enzymes Acb1 and Apyc1, representing the first known inhibitors of signalling-based defences.
Leavitt, A. et al. Viruses inhibit TIR gcADPR signalling to overcome bacterial defence. Nature 611, 326–331 (2022). This study finds that the Thoeris inhibitor Tad1 sequesters the immune signalling molecule produced by TIR-domain proteins.
Yirmiya, E. et al. Phages overcome bacterial immunity via diverse anti-defence proteins. Nature https://doi.org/10.1038/s41586-023-06869-w (2023). In this study, inhibitors of Hachimann, Gabija and Thoeris defence are discovered through comparative genomics.
Athukoralage, J. S. & White, M. F. Cyclic nucleotide signaling in phage defense and counter-defense. Annu. Rev. Virol. 9, 451–468 (2022).
Sontheimer, E. J. & Davidson, A. R. Inhibition of CRISPR-Cas systems by mobile genetic elements. Curr. Opin. Microbiol. 37, 120–127 (2017).
Pinilla-Redondo, R. et al. Discovery of multiple anti-CRISPRs highlights anti-defense gene clustering in mobile genetic elements. Nat. Commun. 11, 5652 (2020). This study reports that anti-defence genes cluster together in MGEs.
Samuel, B. & Burstein, D. A diverse repertoire of anti-defense systems is encoded in the leading region of plasmids. Preprint at bioRxiv https://doi.org/10.1101/2023.02.15.528439 (2023).
Pawluk, A., Bondy-Denomy, J., Cheung, V. H. W., Maxwell, K. L. & Davidson, A. R. A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa. mBio 5, e00896 (2014).
Pawluk, A. et al. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat. Microbiol. 1, 16085 (2016).
Birkholz, N., Fagerlund, R. D., Smith, L. M., Jackson, S. A. & Fineran, P. C. The autoregulator Aca2 mediates anti-CRISPR repression. Nucleic Acids Res. 47, 9658–9665 (2019).
Stanley, S. Y. et al. Anti-CRISPR-associated proteins are crucial repressors of anti-CRISPR transcription. Cell 178, 1452–1464.e13 (2019).
Marino, N. D. et al. Discovery of widespread type I and type V CRISPR-Cas inhibitors. Science 362, 240–242 (2018).
Mahendra, C. et al. Broad-spectrum anti-CRISPR proteins facilitate horizontal gene transfer. Nat. Microbiol. 5, 620–629 (2020).
Roy, D., Huguet, K. T., Grenier, F. & Burrus, V. IncC conjugative plasmids and SXT/R391 elements repair double-strand breaks caused by CRISPR–Cas during conjugation. Nucleic Acids Res. 48, 8815–8827 (2020).
LeRoux, M. et al. The DarTG toxin-antitoxin system provides phage defence by ADP-ribosylating viral DNA. Nat. Microbiol. 7, 1028–1040 (2022).
Ho, P. et al. Bacteriophage antidefense genes that neutralize TIR and STING immune responses. Cell Rep. 42, 112305 (2023).
He, F. et al. Anti-CRISPR proteins encoded by archaeal lytic viruses inhibit subtype I-D immunity. Nat. Microbiol. 3, 461–469 (2018).
Val-Calvo, J. et al. Establishment genes present on pLS20 family of conjugative plasmids are regulated in two different ways. Microorganisms 9, 2465 (2021).
Peng, X., Mayo-Muñoz, D., Bhoobalan-Chitty, Y. & Martínez-Álvarez, L. Anti-CRISPR proteins in archaea. Trends Microbiol. 28, 913–921 (2020).
Silas, S. et al. Activation of programmed cell death and counter-defense functions of phage accessory genes. Preprint at bioRxiv https://doi.org/10.1101/2023.04.06.535777 (2023).
Gao, L. A. et al. Prokaryotic innate immunity through pattern recognition of conserved viral proteins. Science 377, eabm4096 (2022).
Stokar-Avihail, A. et al. Discovery of phage determinants that confer sensitivity to bacterial immune systems. Cell 186, 1863–1876.e16 (2023). This study systematically identifies triggers of defence systems and reveals common mechanisms of phage sensing by defence systems.
Blower, T. R., Evans, T. J., Przybilski, R., Fineran, P. C. & Salmond, G. P. C. Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism. PLoS Genet. 8, e1003023 (2012).
Srikant, S., Guegler, C. K. & Laub, M. T. The evolution of a counter-defense mechanism in a virus constrains its host range. eLife 11, e79549 (2022).
Huiting, E. et al. Bacteriophages inhibit and evade cGAS-like immune function in bacteria. Cell 186, 864–876.e21 (2023).
Garb, J. et al. Multiple phage resistance systems inhibit infection via SIR2-dependent NAD depletion. Nat. Microbiol. 7, 1849–1856 (2022). This study uses a phage mating approach to identify inhibitors of defence-associated sirtuins.
Uribe, R. V. et al. Discovery and characterization of Cas9 inhibitors disseminated across seven bacterial phyla. Cell Host Microbe 25, 233–241.e5 (2019). This study develops a high-throughput approach to screen for type II-A CRISPR–Cas inhibitors in metagenomic libraries.
Forsberg, K. J. et al. Functional metagenomics-guided discovery of potent Cas9 inhibitors in the human microbiome. eLife 8, e46540 (2019).
Bobonis, J. et al. Bacterial retrons encode phage-defending tripartite toxin–antitoxin systems. Nature 609, 144–150 (2022).
Bondy-Denomy, J. et al. A unified resource for tracking anti-CRISPR names. CRISPR J. 1, 304–305 (2018).
Eitzinger, S. et al. Machine learning predicts new anti-CRISPR proteins. Nucleic Acids Res. 48, 4698–4708 (2020). This study develops a machine learning-based method to aid the direct identification of new potential anti-CRISPRs.
Gussow, A. B. et al. Machine-learning approach expands the repertoire of anti-CRISPR protein families. Nat. Commun. 11, 3784 (2020).
Wandera, K. G. et al. Anti-CRISPR prediction using deep learning reveals an inhibitor of Cas13b nucleases. Mol. Cell 82, 2714–2726.e4 (2022).
Davidson, A. R. et al. Anti-CRISPRs: protein inhibitors of CRISPR-Cas systems. Annu. Rev. Biochem. 89, 309–332 (2020).
Wiegand, T., Karambelkar, S., Bondy-Denomy, J. & Wiedenheft, B. Structures and strategies of anti-CRISPR-mediated immune suppression. Annu. Rev. Microbiol. 74, 21–37 (2020).
Suresh, S. K., Murugan, K. & Sashital, D. G. Enzymatic anti-CRISPRs improve the bacteriophage arsenal. Nat. Struct. Mol. Biol. 26, 250–251 (2019).
Knott, G. J. et al. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a. Nat. Struct. Mol. Biol. 26, 315–321 (2019). This study discovers the first multiple-turnover enzymatic CRISPR–Cas inhibitor.
Azam, A. H. et al. Viruses encode tRNA and anti-retron to evade bacterial immunity. Preprint at bioRxiv https://doi.org/10.1101/2023.03.15.532788 (2023).
Burroughs, A. M., Zhang, D., Schäffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 43, 10633–10654 (2015).
Athukoralage, J. S. et al. An anti-CRISPR viral ring nuclease subverts type III CRISPR immunity. Nature 577, 572–575 (2020).
Birkholz, N. & Fineran, P. C. Turning down the (C)BASS: phage-encoded inhibitors jam bacterial immune signaling. Mol. Cell 82, 2185–2187 (2022).
Dong, L. et al. An anti-CRISPR protein disables type V Cas12a by acetylation. Nat. Struct. Mol. Biol. 26, 308–314 (2019).
Jurenas, D., Fraikin, N., Goormaghtigh, F. & Van Melderen, L. Biology and evolution of bacterial toxin–antitoxin systems. Nat. Rev. Microbiol. 20, 335–350 (2022).
Bondy-Denomy, J. et al. Multiple mechanisms for CRISPR–Cas inhibition by anti-CRISPR proteins. Nature 526, 136–139 (2015).
Chowdhury, S. et al. Structure reveals mechanisms of viral suppressors that intercept a CRISPR RNA-guided surveillance complex. Cell 169, 47–57.e11 (2017).
Bhoobalan-Chitty, Y., Johansen, T. B., Di Cianni, N. & Peng, X. Inhibition of type III CRISPR-Cas immunity by an archaeal virus-encoded anti-CRISPR protein. Cell 179, 448–458.e11 (2019).
Antine, S. P. et al. Structural basis of Gabija anti-phage defence and viral immune evasion. Nature https://doi.org/10.1038/s41586-023-06855-2 (2023).
Harrington, L. B. et al. A broad-spectrum inhibitor of CRISPR-Cas9. Cell 170, 1224–1233.e15 (2017).
Pawluk, A. et al. Disabling a type I-E CRISPR-Cas nuclease with a bacteriophage-encoded anti-CRISPR protein. mBio 8, e01751-17 (2017).
Penner, M., Morad, I., Snyder, L. & Kaufmann, G. Phage T4-coded Stp: double-edged effector of coupled DNA and tRNA-restriction systems. J. Mol. Biol. 249, 857–868 (1995).
Otsuka, Y. & Yonesaki, T. Dmd of bacteriophage T4 functions as an antitoxin against Escherichia coli LsoA and RnlA toxins. Mol. Microbiol. 83, 669–681 (2012).
Ramage, H. R., Connolly, L. E. & Cox, J. S. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution. PLoS Genet. 5, e1000767 (2009).
Yang, L. et al. Insights into the inhibition of type I-F CRISPR-Cas system by a multifunctional anti-CRISPR protein AcrIF24. Nat. Commun. 13, 1931 (2022).
Fuchsbauer, O. et al. Cas9 allosteric inhibition by the anti-CRISPR protein AcrIIA6. Mol. Cell 76, 922–937.e7 (2019).
Marino, N. D. et al. Translation-dependent downregulation of Cas12a mRNA by an anti-CRISPR protein. Preprint at bioRxiv https://doi.org/10.1101/2022.11.29.518452 (2022).
Osuna, B. A. et al. Listeria phages induce cas9 degradation to protect lysogenic genomes. Cell Host Microbe 28, 31–40.e9 (2020).
Li, Y. & Bondy-Denomy, J. Anti-CRISPRs go viral: the infection biology of CRISPR-Cas inhibitors. Cell Host Microbe 29, 704–714 (2021).
Jenson, J. M., Li, T., Du, F., Ea, C.-K. & Chen, Z. J. Ubiquitin-like conjugation by bacterial cGAS enhances anti-phage defence. Nature 616, 326–331 (2023).
Court, R., Cook, N., Saikrishnan, K. & Wigley, D. The crystal structure of λ-Gam protein suggests a model for RecBCD inhibition. J. Mol. Biol. 371, 25–33 (2007).
Wilkinson, M. et al. Structural basis for the inhibition of RecBCD by Gam and its synergistic antibacterial effect with quinolones. eLife 5, e22963 (2016).
Levy, A. et al. CRISPR adaptation biases explain preference for acquisition of foreign DNA. Nature 520, 505–510 (2015).
Kuzmenko, A. et al. DNA targeting and interference by a bacterial Argonaute nuclease. Nature 587, 632–637 (2020).
Walkinshaw, M. D. et al. Structure of Ocr from bacteriophage T7, a protein that mimics B-form DNA. Mol. Cell 9, 187–194 (2002).
Isaev, A. et al. Phage T7 DNA mimic protein Ocr is a potent inhibitor of BREX defence. Nucleic Acids Res. 48, 5397–5406 (2020).
Hong, S. et al. CRISPR RNA and anti-CRISPR protein binding to the Xanthomonas albilineans Csy1-Csy2 heterodimer in the type I-F CRISPR-Cas system. J. Biol. Chem. 293, 2744–2754 (2018).
Dong, D. et al. Structural basis of CRISPR–SpyCas9 inhibition by an anti-CRISPR protein. Nature 546, 436–439 (2017).
Shin, J. et al. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci. Adv. 3, e1701620 (2017).
Koonin, E. V. & Krupovic, M. The depths of virus exaptation. Curr. Opin. Virol. 31, 1–8 (2018).
Faure, G. et al. CRISPR–Cas in mobile genetic elements: counter-defence and beyond. Nat. Rev. Microbiol. 17, 513–525 (2019).
Wang, X. et al. Structural basis of Cas3 inhibition by the bacteriophage protein AcrF3. Nat. Struct. Mol. Biol. 23, 868–870 (2016).
Pinilla-Redondo, R. et al. CRISPR-Cas systems are widespread accessory elements across bacterial and archaeal plasmids. Nucleic Acids Res. 50, 4315–4328 (2022).
Altae-Tran, H. et al. Uncovering the functional diversity of rare CRISPR-Cas systems with deep terascale clustering. Science 382, eadi1910 (2023).
Camara-Wilpert, S. et al. Bacteriophages suppress CRISPR–Cas immunity using RNA-based anti-CRISPRs. Nature 623, 601–607 (2023). This study uncovers the first RNA anti-CRISPRs, which act as mimics of CRISPR repeats to interact with Cas proteins and block immunity.
Katz, M. A. et al. Diverse viral cas genes antagonize CRISPR immunity. Preprint at bioRxiv https://doi.org/10.1101/2023.06.24.545427 (2023).
Mahler, M., Costa, A. R., van Beljouw, S. P. B., Fineran, P. C. & Brouns, S. J. J. Approaches for bacteriophage genome engineering. Trends Biotechnol. 41, 669–685 (2023).
Mayo-Muñoz, D. et al. Anti-CRISPR-based and CRISPR-based genome editing of Sulfolobus islandicus rod-shaped virus 2. Viruses 10, 695 (2018). This study uses Acrs as a counter-selection strategy for viral engineering.
Guan, J. et al. Bacteriophage genome engineering with CRISPR–Cas13a. Nat. Microbiol. 7, 1956–1966 (2022).
Malone, L. M. et al. A jumbo phage that forms a nucleus-like structure evades CRISPR–Cas DNA targeting but is vulnerable to type III RNA-based immunity. Nat. Microbiol. 5, 48–55 (2020).
Mendoza, S. D. et al. A bacteriophage nucleus-like compartment shields DNA from CRISPR nucleases. Nature 577, 244–248 (2020).
Dedrick, R. M. et al. Phage therapy of Mycobacterium infections: compassionate-use of phages in twenty patients with drug-resistant mycobacterial disease. Clin. Infect. Dis. 76, 103–112 (2023).
Harada, L. K. et al. Biotechnological applications of bacteriophages: state of the art. Microbiol. Res. 212–213, 38–58 (2018).
Nora, L. C. et al. The art of vector engineering: towards the construction of next-generation genetic tools. Microb. Biotechnol. 12, 125–147 (2019).
Bikard, D. & Barrangou, R. Using CRISPR-Cas systems as antimicrobials. Curr. Opin. Microbiol. 37, 155–160 (2017).
Birkholz, N., Jackson, S. A., Fagerlund, R. D. & Fineran, P. C. A mobile restriction–modification system provides phage defence and resolves an epigenetic conflict with an antagonistic endonuclease. Nucleic Acids Res. 50, 3348–3361 (2022).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Liang, M. et al. AcrIIA5 suppresses base editors and reduces their off-target effects. Cells 9, 1786 (2020).
Nakamura, M. et al. Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat. Commun. 10, 194 (2019).
Luo, M. L., Mullis, A. S., Leenay, R. T. & Beisel, C. L. Repurposing endogenous type I CRISPR-Cas systems for programmable gene repression. Nucleic Acids Res. 43, 674–681 (2015).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 184, 844 (2021).
Johnston, R. K. et al. Use of anti-CRISPR protein AcrIIA4 as a capture ligand for CRISPR/Cas9 detection. Biosens. Bioelectron. 141, 111361 (2019).
Wein, T. & Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. 22, 629–638 (2022).
Seet, B. T. et al. Poxviruses and immune evasion. Annu. Rev. Immunol. 21, 377–423 (2003).
Patel, P. H. & Maxwell, K. L. Prophages provide a rich source of antiphage defense systems. Curr. Opin. Microbiol. 73, 102321 (2023).
Sahakyan, H., Makarova, K. S. & Koonin, E. V. Search for origins of anti-CRISPR proteins by structure comparison. CRISPR J. 6, 222–231 (2023).
Rousset, F. & Sorek, R. The evolutionary success of regulated cell death in bacterial immunity. Curr. Opin. Microbiol. 74, 102312 (2023).
LeGault, K. N. et al. Temporal shifts in antibiotic resistance elements govern phage-pathogen conflicts. Science 373, eabg2166 (2021).
Zhang, Z., Pan, S., Liu, T., Li, Y. & Peng, N. Cas4 nucleases can effect specific integration of CRISPR spacers. J. Bacteriol. 201, e00747-18 (2019).
León, L. M., Park, A. E., Borges, A. L., Zhang, J. Y. & Bondy-Denomy, J. Mobile element warfare via CRISPR and anti-CRISPR in Pseudomonas aeruginosa. Nucleic Acids Res. 49, 2114–2125 (2021).
Lin, P. et al. CRISPR-Cas13 inhibitors block RNA editing in bacteria and mammalian cells. Mol. Cell 78, 850–861.e5 (2020).
Muzyukina, P. et al. Identification of an anti-CRISPR protein that inhibits the CRISPR-Cas type I-B system in Clostridioides difficile. mSphere https://doi.org/10.1128/msphere.00401-23 (2023).
Rauch, B. J. et al. Inhibition of CRISPR-Cas9 with bacteriophage proteins. Cell 168, 150–158.e10 (2017).
Hynes, A. P. et al. An anti-CRISPR from a virulent streptococcal phage inhibits Streptococcus pyogenes Cas9. Nat. Microbiol. 2, 1374–1380 (2017).
Hynes, A. P. et al. Widespread anti-CRISPR proteins in virulent bacteriophages inhibit a range of Cas9 proteins. Nat. Commun. 9, 2919 (2018).
Watters, K. E. et al. Potent CRISPR-Cas9 inhibitors from Staphylococcus genomes. Proc. Natl Acad. Sci. USA 117, 6531–6539 (2020).
Forsberg, K. J. et al. The novel anti-CRISPR AcrIIA22 relieves DNA torsion in target plasmids and impairs SpyCas9 activity. PLoS Biol. 19, e3001428 (2021).
Varble, A. et al. Prophage integration into CRISPR loci enables evasion of antiviral immunity in Streptococcus pyogenes. Nat. Microbiol. 6, 1516–1525 (2021).
Lee, J. et al. Potent Cas9 inhibition in bacterial and human cells by AcrIIC4 and AcrIIC5 anti-CRISPR proteins. mBio 9, e02321-18 (2018).
Song, G. et al. Discovery of potent and versatile CRISPR–Cas9 inhibitors engineered for chemically controllable genome editing. Nucleic Acids Res. 50, 2836–2853 (2022).
Liu, J. et al. An archaeal virus-encoded anti-CRISPR protein inhibits type III-B immunity by inhibiting Cas RNP complex turnover. Nucleic Acids Res. https://doi.org/10.1093/nar/gkad804 (2023).
Lin, J., Alfastsen, L., Bhoobalan-Chitty, Y. & Peng, X. Molecular basis for inhibition of type III-B CRISPR-Cas by an archaeal viral anti-CRISPR protein. Cell Host Microbe 31, 1837–1849.e5 (2023).
Watters, K. E., Fellmann, C., Bai, H. B., Ren, S. M. & Doudna, J. A. Systematic discovery of natural CRISPR-Cas12a inhibitors. Science 362, 236–239 (2018).
Meeske, A. J. et al. A phage-encoded anti-CRISPR enables complete evasion of type VI-A CRISPR-Cas immunity. Science 369, 54–59 (2020).
Piel, D. et al. Phage–host coevolution in natural populations. Nat. Microbiol. 7, 1075–1086 (2022).
Bobay, L. M., Touchon, M. & Rocha, E. P. Manipulating or superseding host recombination functions: a dilemma that shapes phage evolvability. PLoS Genet. 9, e1003825 (2013).
Murphy, K. C., Fenton, A. C. & Poteete, A. R. Sequence of the bacteriophage P22 anti-RecBCD (abc) genes and properties of P22 abc region deletion mutants. Virology 160, 456–464 (1987).
Murphy, K. C. Bacteriophage P22 Abc2 protein binds to RecC increases the 5′ strand nicking activity of RecBCD and together with λ Bet, promotes Chi-independent recombination. J. Mol. Biol. 296, 385–401 (2000).
Lin, L. Study of Bacteriophage T7 Gene 5.9 and Gene 5.5. PhD thesis, State Univ. of New York at Stony Brook (1992).
Wilkinson, M. et al. Structures of RecBCD in complex with phage-encoded inhibitor proteins reveal distinctive strategies for evasion of a bacterial immunity hub. eLife 11, e83409 (2022).
Chen, K. et al. ArdA proteins from different mobile genetic elements can bind to the EcoKI Type I DNA methyltransferase of E. coli K12. Biochim. Biophys. Acta 1844, 505–511 (2014).
Belogurov, A. A., Delver, E. P. & Rodzevich, O. V. Plasmid pKM101 encodes two nonhomologous antirestriction proteins (ArdA and ArdB) whose expression is controlled by homologous regulatory sequences. J. Bacteriol. 175, 4843–4850 (1993).
Serfiotis-Mitsa, D. et al. The structure of the KlcA and ArdB proteins reveals a novel fold and antirestriction activity against Type I DNA restriction systems in vivo but not in vitro. Nucleic Acids Res. 38, 1723–1737 (2010).
Dharmalingam, K., Revel, H. R. & Goldberg, E. B. Physical mapping and cloning of bacteriophage T4 anti-restriction endonuclease gene. J. Bacteriol. 149, 694–699 (1982).
Ho, C. H., Wang, H. C., Ko, T. P., Chang, Y. C. & Wang, A. H. The T4 phage DNA mimic protein Arn inhibits the DNA binding activity of the bacterial histone-like protein H-NS. J. Biol. Chem. 289, 27046–27054 (2014).
Rifat, D., Wright, N. T., Varney, K. M., Weber, D. J. & Black, L. W. Restriction endonuclease inhibitor IPI* of bacteriophage T4: a novel structure for a dedicated target. J. Mol. Biol. 375, 720–734 (2008).
Li, D. et al. Single phage proteins sequester TIR- and cGAS-generated signaling molecules. Preprint at bioRxiv https://doi.org/10.1101/2023.11.15.567273 (2023).
Gordeeva, J. et al. BREX system of Escherichia coli distinguishes self from non-self by methylation of a specific DNA site. Nucleic Acids Res. 47, 253–265 (2019).
Hille, F. et al. The biology of CRISPR-Cas: backward and forward. Cell 172, 1239–1259 (2018).
Makarova, K. S., Wolf, Y. I., van der Oost, J. & Koonin, E. V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol. Direct 4, 29 (2009).
Shabalina, S. A. & Koonin, E. V. Origins and evolution of eukaryotic RNA interference. Trends Ecol. Evol. 23, 578–587 (2008).
Swarts, D. C. et al. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 21, 743–753 (2014).
Bobadilla Ugarte, P., Barendse, P. & Swarts, D. C. Argonaute proteins confer immunity in all domains of life. Curr. Opin. Microbiol. 74, 102313 (2023).
Cheng, R. et al. A nucleotide-sensing endonuclease from the Gabija bacterial defense system. Nucleic Acids Res. 49, 5216–5229 (2021).
Dillingham, M. S. & Kowalczykowski, S. C. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 72, 642–671 (2008).
LeRoux, M. & Laub, M. T. Toxin-antitoxin systems as phage defense elements. Annu. Rev. Microbiol. 76, 21–43 (2022).
Millman, A., Melamed, S., Amitai, G. & Sorek, R. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat. Microbiol. 5, 1608–1615 (2020).
Whiteley, A. T. et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567, 194–199 (2019).
Tal, N. et al. Cyclic CMP and cyclic UMP mediate bacterial immunity against phages. Cell 184, 5728–5739.e16 (2021).
Ofir, G. et al. Antiviral activity of bacterial TIR domains via immune signalling molecules. Nature 600, 116–120 (2021).
Mestre, M. R., González-Delgado, A., Gutiérrez-Rus, L. I., Martínez-Abarca, F. & Toro, N. Systematic prediction of genes functionally associated with bacterial retrons and classification of the encoded tripartite systems. Nucleic Acids Res. 48, 12632–12647 (2020).
Pleška, M. & Guet, C. C. Effects of mutations in phage restriction sites during escape from restriction–modification. Biol. Lett. 13, 20170646 (2017).
Rocha, E. P., Danchin, A. & Viari, A. Evolutionary role of restriction/modification systems as revealed by comparative genome analysis. Genome Res. 11, 946–958 (2001).
Rusinov, I. S., Ershova, A. S., Karyagina, A. S., Spirin, S. A. & Alexeevski, A. V. Avoidance of recognition sites of restriction-modification systems is a widespread but not universal anti-restriction strategy of prokaryotic viruses. BMC Genomics 19, 885 (2018).
van den Berg, D. F., van der Steen, B. A., Costa, A. R. & Brouns, S. J. J. Phage tRNAs evade tRNA-targeting host defenses through anticodon loop mutations. eLife 12, e85183 (2023).
Loenen, W. A. & Murray, N. E. Modification enhancement by the restriction alleviation protein (Ral) of bacteriophage lambda. J. Mol. Biol. 190, 11–22 (1986).
King, G. & Murray, N. E. Restriction alleviation and modification enhancement by the Rac prophage of Escherichia coli K-12. Mol. Microbiol. 16, 769–777 (1995).
Shaw, L. P., Rocha, E. P. C. & MacLean, R. C. Restriction-modification systems have shaped the evolution and distribution of plasmids across bacteria. Nucleic Acids Res. 51, 6806–6818 (2023).
Iida, S., Streiff, M. B., Bickle, T. A. & Arber, W. Two DNA antirestriction systems of bacteriophage P1, darA, and darB: characterization of darA− phages. Virology 157, 156–166 (1987).
González-Montes, L., Del Campo, I., Garcillán-Barcia, M. P., de la Cruz, F. & Moncalián, G. ArdC, a ssDNA-binding protein with a metalloprotease domain, overpasses the recipient hsdRMS restriction system broadening conjugation host range. PLoS Genet. 16, e1008750 (2020).
Millman, A. et al. Bacterial retrons function in anti-phage defense. Cell 183, 1551–1561.e12 (2020).
Rousset, F. et al. Phages and their satellites encode hotspots of antiviral systems. Cell Host Microbe 30, 740–753.e5 (2022).
Smith, L. M., Campa, A. R. & Fineran, P. C. in Crispr: Biology and Applications (eds Barrangou, R. et al.) Ch. 10 (John Wiley & Sons, 2022).
Patterson, A. G. et al. Quorum sensing controls adaptive immunity through the regulation of multiple CRISPR-Cas systems. Mol. Cell 64, 1102–1108 (2016).
Høyland-Kroghsbo, N. M. et al. Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc. Natl Acad. Sci. USA 114, 131–135 (2017).
Shah, M. et al. A phage-encoded anti-activator inhibits quorum sensing in Pseudomonas aeruginosa. Mol. Cell 81, 571–583.e6 (2021).
Campa, A. R. et al. The Rsm (Csr) post-transcriptional regulatory pathway coordinately controls multiple CRISPR–Cas immune systems. Nucleic Acids Res. 49, 9508–9525 (2021).
Borges, A. L. et al. Bacterial alginate regulators and phage homologs repress CRISPR–Cas immunity. Nat. Microbiol. 5, 679–687 (2020).
Skennerton, C. T. et al. Phage encoded H-NS: a potential Achilles heel in the bacterial defence system. PLoS ONE 6, e20095 (2011).
Shmakov, S. A. et al. Widespread CRISPR-derived RNA regulatory elements in CRISPR-Cas systems. Nucleic Acids Res. 51, 8150–8168 (2023).
Amitsur, M., Levitz, R. & Kaufmann, G. Bacteriophage T4 anticodon nuclease, polynucleotide kinase and RNA ligase reprocess the host lysine tRNA. EMBO J. 6, 2499–2503 (1987).
Hossain, A. A., McGinn, J., Meeske, A. J., Modell, J. W. & Marraffini, L. A. Viral recombination systems limit CRISPR-Cas targeting through the generation of escape mutations. Cell Host Microbe 29, 1482–1495.e12 (2021).
Skorupski, K., Tomaschewski, J., Rüger, W. & Simon, L. D. A bacteriophage T4 gene which functions to inhibit Escherichia coli Lon protease. J. Bacteriol. 170, 3016–3024 (1988).
Sberro, H. et al. Discovery of functional toxin/antitoxin systems in bacteria by shotgun cloning. Mol. Cell 50, 136–148 (2013).
Engelberg-Kulka, H. et al. rexB of bacteriophage λ is an anti-cell death gene. Proc. Natl Acad. Sci. USA 95, 15481–15486 (1998).
Studier, F. W. & Movva, N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J. Virol. 19, 136–145 (1976).
Andriianov, A. et al. Phage T3 overcomes the BREX defense through SAM cleavage and inhibition of SAM synthesis by SAM lyase. Cell Rep. 42, 112972 (2023).
Martínez-Alvarez, L., Stickel, D., Salegi-Díez, A., Bhoobalan-Chitty, Y. & Peng, X. To be or not to be an anti-CRISPR: AcrIII-1 and the importance of working with native biological systems. Preprint at bioRxiv https://doi.org/10.1101/2023.01.10.523387 (2023).
Millman, A. et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 30, 1556–1569.e5 (2022).
Tesson, F. et al. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 13, 2561 (2022).
Borges, A. L. et al. Bacteriophage cooperation suppresses CRISPR-Cas3 and Cas9 immunity. Cell 174, 917–925.e10 (2018).
Landsberger, M. et al. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell 174, 908–916.e12 (2018).
Chevallereau, A. et al. Exploitation of the cooperative behaviors of anti-CRISPR phages. Cell Host Microbe 27, 189–198.e6 (2020).
Chevallereau, A., Pons, B. J., van Houte, S. & Westra, E. R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 20, 49–62 (2022).
Meaden, S. et al. High viral abundance and low diversity are associated with increased CRISPR-Cas prevalence across microbial ecosystems. Curr. Biol. 32, 220–227.e5 (2022).
Bryant, P., Pozzati, G. & Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 13, 1265 (2022).
Yu, D., Chojnowski, G., Rosenthal, M. & Kosinski, J. AlphaPulldown—a python package for protein–protein interaction screens using AlphaFold-Multimer. Bioinformatics 39, btac749 (2022).
Garmaeva, S. et al. Studying the gut virome in the metagenomic era: challenges and perspectives. BMC Biol. 17, 84 (2019).
Jørgensen, T. S., Kiil, A. S., Hansen, M. A., Sørensen, S. J. & Hansen, L. H. Current strategies for mobilome research. Front. Microbiol. 5, 750 (2015).
Wu, Y. et al. Synergistic anti-phage activity of bacterial defence systems. Preprint at bioRxiv https://doi.org/10.1101/2022.08.21.504612 (2023).
Yan, Y., Zheng, J., Zhang, X. & Yin, Y. dbAPIS: a database of anti-prokaryotic immune system genes. Nucleic Acids Res. 52, D419–D425 (2024).
Acknowledgements
We thank C. Smart, L. Payne and S. Crellin for helpful discussions. Research on phage defence and anti-defence systems conducted in the Fineran laboratory is supported by a University of Otago Research Grant, Bioprotection Aotearoa and the Marsden Fund, Royal Society of New Zealand (Te Pūtea Rangahau a Marsden, Te Apārangi). D.M.-M. was supported by a University of Otago Doctoral Scholarship and a Postgraduate Publishing Bursary. R.P.-R. was supported by a Lundbeck Foundation grant, MIMOSAS project [R347-2020-2346].
Author information
Authors and Affiliations
Contributions
D.M.-M., R.P.-R. and N.B. conceptualized the idea. D.M.-M. and R.P.-R. wrote the manuscript with contributions from S.C.-W., N.B. and P.C.F. D.M.-M. prepared Figs. 1 and 4, Table 1 and Box 1. R.P.-R. prepared Table 1 and Box 3. S.C.-W. prepared Fig. 2 and Box 2. N.B. prepared Fig. 3.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Asma Hatoum-Aslan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bacteriophages
-
(Phages). Viruses that infect bacteria.
- CRISPR RNAs
-
Small guide RNAs that assemble with one or multiple Cas proteins and guide them to complementary nucleic acids in invading mobile genetic elements for degradation.
- Direct inhibitor
-
Inhibitory component that specifically targets and interferes with the defence mechanisms that bacteria use to stop mobile genetic element invasion. Direct inhibitors enable mobile genetic elements to overcome host immunity.
- Escape mutant
-
A mobile genetic element that has evolved to evade defence targeting, typically through mutation.
- Jumbo phages
-
Bacteriophages with large genomes, typically over 200 kb.
- Mobile genetic element
-
(MGE). Genetic entity that requires a host cell to propagate and can move within or between cells. Examples include bacteriophages, plasmids and transposons.
- Plasmid
-
Cytoplasmic DNA element that can replicate independently of the bacterial chromosome and can often transfer horizontally to other bacterial cells.
- Protospacer adjacent motif
-
Short DNA sequence (usually 2–6 base pairs in length) that is immediately adjacent to the target sequence of a CRISPR–Cas system. It is essential for differentiating between self and non-self matches during interference.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mayo-Muñoz, D., Pinilla-Redondo, R., Camara-Wilpert, S. et al. Inhibitors of bacterial immune systems: discovery, mechanisms and applications. Nat Rev Genet 25, 237–254 (2024). https://doi.org/10.1038/s41576-023-00676-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-023-00676-9