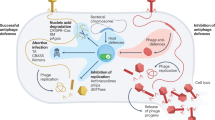
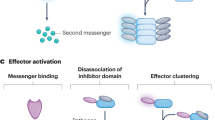
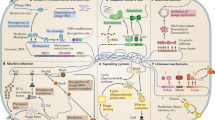
Abstract
Bacteria and their viruses have coevolved for billions of years. This ancient and still ongoing arms race has led bacteria to develop a vast antiphage arsenal. The development of high-throughput screening methods expanded our knowledge of defence systems from a handful to more than a hundred systems, unveiling many different molecular mechanisms. These findings reveal that bacterial immunity is much more complex than previously thought. In this Review, we explore recently discovered bacterial antiphage defence systems, with a particular focus on their molecular diversity, and discuss the ecological and evolutionary drivers and implications of the existing diversity of antiphage defence mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
18 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41579-023-00987-y
References
Ofir, G. & Sorek, R. Contemporary phage biology: from classic models to new insights. Cell 172, 1260–1270 (2018).
Suttle, C. A. Marine viruses — major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812 (2007).
Kever, L. et al. Aminoglycoside antibiotics inhibit phage infection by blocking an early step of the infection cycle. mBio 13, e0078322 (2022).
Kronheim, S. et al. A chemical defence against phage infection. Nature 564, 283–286 (2018). This study demonstrates that some bacteria produce small antiphage molecules.
Cohen, D. et al. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature 574, 691–695 (2019). This study shows that homologues of the eukaryotic cGAS–STING pathway are bacterial signalling antiphage systems.
Ofir, G. et al. Antiviral activity of bacterial TIR domains via immune signalling molecules. Nature 600, 116–120 (2021). In this study, the authors discover the molecular mechanism of Thoeris, an antiphage signalling system that comprises TIR domains known to be involved in immune signalling in plants.
Tal, N. et al. Cyclic CMP and cyclic UMP mediate bacterial immunity against phages. Cell 184, 5728–5739.e16 (2021).
Gao, L. A. et al. Prokaryotic innate immunity through pattern recognition of conserved viral proteins. Science 377, eabm4096 (2022). This study shows that nucleotide binding oligomerization domain-like receptors (NLR), which are known to perform recognition of pathogen-associated molecular patterns in eukaryotes, can recognize conserved structural features of phage proteins to trigger immune response.
Chopin, M.-C., Chopin, A. & Bidnenko, E. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8, 473–479 (2005).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
LeRoux, M. & Laub, M. T. Toxin–antitoxin systems as phage defense elements. Annu. Rev. Microbiol. 76, 21–43 (2022).
Tesson, F. et al. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 13, 2561 (2022). In this study, the authors developed a tool to systematically detect antiphage systems in prokaryotic genomes and used it to describe the antiviral arsenal of bacteria.
Millman, A. et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 30, 1556–1569.e5 (2022).
Lopatina, A., Tal, N. & Sorek, R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 7, 371–384 (2020).
Tock, M. R. & Dryden, D. T. F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Goldfarb, T. et al. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 34, 169–183 (2015).
Gordeeva, J. et al. BREX system of Escherichia coli distinguishes self from non-self by methylation of a specific DNA site. Nucleic Acids Res. 47, 253–265 (2019).
Ofir, G. et al. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 3, 90–98 (2018).
Wang, L., Jiang, S., Deng, Z., Dedon, P. C. & Chen, S. DNA phosphorothioate modification — a new multi-functional epigenetic system in bacteria. FEMS Microbiol. Rev. 43, 109–122 (2019).
Xiong, L. et al. A new type of DNA phosphorothioation-based antiviral system in archaea. Nat. Commun. 10, 1688 (2019).
Wang, S. et al. SspABCD-SspFGH constitutes a new type of DNA phosphorothioate-based bacterial defense system. mBio 12, e00613–e00621 (2021).
Xiong, X. et al. SspABCD-SspE is a phosphorothioation-sensing bacterial defence system with broad anti-phage activities. Nat. Microbiol. 5, 917–928 (2020).
Thiaville, J. J. et al. Novel genomic island modifies DNA with 7-deazaguanine derivatives. Proc. Natl Acad. Sci. USA 113, E1452–E1459 (2016).
Hille, F. et al. The biology of CRISPR–Cas: backward and forward. Cell 172, 1239–1259 (2018).
Garb, J. et al. Multiple phage resistance systems inhibit infection via SIR2-dependent NAD+ depletion. Nat. Microbiol. https://doi.org/10.1038/s41564-022-01207-8 (2022).
Zaremba, M. et al. Short prokaryotic argonautes provide defence against incoming mobile genetic elements through NAD+ depletion. Nat. Microbiol. 7, 1857–1869 (2022).
Zeng, Z. et al. A short prokaryotic argonaute activates membrane effector to confer antiviral defense. Cell Host Microbe 30, 930–943.e6 (2022).
Deep, A. et al. The SMC-family wadjet complex protects bacteria from plasmid transformation by recognition and cleavage of closed-circular DNA. Mol. Cell 82, 4145–4159.e7 (2022).
Dalton, V. B. et al. Bacterial cGAS senses a viral RNA to initiate immunity. Preprint at bioRxiv https://doi.org/10.1101/2023.03.07.531596 (2023).
Depardieu, F. et al. A eukaryotic-like serine/threonine kinase protects staphylococci against phages. Cell Host Microbe 20, 471–481 (2016).
Zhang, T. et al. Direct activation of a bacterial innate immune system by a viral capsid protein. Nature 612, 132–140 (2022).
Rousset, F. et al. Phages and their satellites encode hotspots of antiviral systems. Cell Host Microbe 30, 740–753.e5 (2022). This study uncovered a new method to bioinformatically predict antiphage systems by identifying defensive hotspots in phages and phage satellites and demonstrates that antiphage systems of satellites can benefit their helper phage.
Parma, D. H. et al. The Rex system of bacteriophage lambda: tolerance and altruistic cell death. Genes Dev. 6, 497–510 (1992).
Durmaz, E. & Klaenhammer, T. R. Abortive phage resistance mechanism abiz speeds the lysis clock to cause premature lysis of phage-infected lactococcus lactis. J. Bacteriol. 189, 1417–1425 (2007).
LeRoux, M. et al. The DarTG toxin–antitoxin system provides phage defence by ADP-ribosylating viral DNA. Nat. Microbiol. 7, 1028–1040 (2022).
Guegler, C. K. & Laub, M. T. Shutoff of host transcription triggers a toxin–antitoxin system to cleave phage RNA and abort infection. Mol. Cell 81, 2361–2373.e9 (2021). This study describes a toxin–antitoxin system type III with antiphage activity, which encodes an endoribonuclease toxin that degrades viral transcript.
Gao, L. et al. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 369, 1077–1084 (2020). This study uses a bioinformatic prediction method to validates 29 novel defence systems.
Millman, A. et al. Bacterial retrons function in anti-phage defense. Cell 183, 1551–1561.e12 (2020). This study shows that retrons can function as antiphage sensors that guard the RecBCD complex and trigger toxic effectors when activated.
Bobonis, J. et al. Bacterial retrons encode phage-defending tripartite toxin–antitoxin systems. Nature 609, 144–150 (2022).
Tal, N. et al. Bacteria deplete deoxynucleotides to defend against bacteriophage infection. Nat. Microbiol. 7, 1200–1209 (2022).
Kaufmann, G. Anticodon nucleases. Trends Biochem. Sci. 25, 70–74 (2000).
Penner, M., Morad, I., Snyder, L. & Kaufmann, G. Phage T4-coded Stp: double-edged effector of coupled DNA and tRNA-restriction systems. J. Mol. Biol. 249, 857–868 (1995).
Hsueh, B. Y. et al. Phage defence by deaminase-mediated depletion of deoxynucleotides in bacteria. Nat. Microbiol. 7, 1210–1220 (2022).
Koga, M., Otsuka, Y., Lemire, S. & Yonesaki, T. Escherichia coli rnlA and rnlB compose a novel toxin–antitoxin system. Genetics 187, 123–130 (2011).
Uzan, M. & Miller, E. S. Post-transcriptional control by bacteriophage T4: mRNA decay and inhibition of translation initiation. Virol. J. 7, 360 (2010).
Athukoralage, J. S. & White, M. F. Cyclic nucleotide signaling in phage defense and counter-defense. Annu. Rev. Virol. 9, 451–468 (2022).
Steens, J. A., Salazar, C. R. P. & Staals, R. H. J. The diverse arsenal of type III CRISPR–Cas-associated CARF and SAVED effectors. Biochem. Soc. Trans. 50, 1353–1364 (2022).
Millman, A., Melamed, S., Amitai, G. & Sorek, R. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat. Microbiol. 5, 1608–1615 (2020).
Whiteley, A. T. et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567, 194–199 (2019).
Leavitt, A. et al. Viruses inhibit TIR gcADPR signaling to overcome bacterial defense. Nature 611, 326–331 (2022).
Athukoralage, J. S. et al. The dynamic interplay of host and viral enzymes in type III CRISPR-mediated cyclic nucleotide signalling. eLife 9, e55852 (2020).
Nussenzweig, P. M. & Marraffini, L. A. Molecular mechanisms of CRISPR–Cas immunity in bacteria. Annu. Rev. Genet. 54, 93–120 (2020).
LeGault, K. N., Barth, Z. K., DePaola, P. & Seed, K. D. A phage parasite deploys a nicking nuclease effector to inhibit viral host replication. Nucleic Acids Res. https://doi.org/10.1093/nar/gkac002 (2022).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018). This study develops a bioinformatic prediction method and experimentally validates nine novel antiphage systems.
Jaskólska, M., Adams, D. W. & Blokesch, M. Two defence systems eliminate plasmids from seventh pandemic vibrio cholerae. Nature 604, 323–329 (2022).
Lau, R. K. et al. Structure and mechanism of a cyclic trinucleotide-activated bacterial endonuclease mediating bacteriophage immunity. Mol. Cell 77, 723–733.e6 (2020).
Cheng, R. et al. A nucleotide-sensing endonuclease from the Gabija bacterial defense system. Nucleic Acids Res. 49, 5216–5229 (2021).
Davidov, E. & Kaufmann, G. RloC: a wobble nucleotide-excising and zinc-responsive bacterial tRNase. Mol. Microbiol. 69, 1560–1574 (2008).
Williams, M. C. et al. Restriction endonuclease cleavage of phage DNA enables resuscitation from Cas13-induced bacterial dormancy. Nat. Microbiol. 8, 400–409 (2023).
Bari, S. M. N. et al. A unique mode of nucleic acid immunity performed by a multifunctional bacterial enzyme. Cell Host Microbe 30, 570–582.e7 (2022).
Morehouse, B. R. et al. STING cyclic dinucleotide sensing originated in bacteria. Nature 586, 429–433 (2020).
Gao, Y. et al. Molecular basis of RADAR anti-phage supramolecular assemblies. Cell 186, 999–1012.e20 (2023).
Duncan-Lowey, B. et al. Cryo-EM structure of the RADAR supramolecular anti-phage defense complex. Cell 186, 987–998.e15 (2023).
Bernheim, A. et al. Prokaryotic viperins produce diverse antiviral molecules. Nature 589, 120–124 (2021).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022). This study uncovers the existence of bacterial Gasdermins that, similar to eukaryotic ones, form pores leading to immunity-related cell death.
VanderWal, A. R., Park, J.-U., Polevoda, B., Kellogg, E. H. & O’Connell, M. R. CRISPR-Csx28 forms a Cas13b-activated membrane pore required for robust CRISPR-Cas adaptive immunity. Preprint at bioRxiv https://doi.org/10.1101/2021.11.02.466367 (2021).
Cheng, X., Wang, W. & Molineux, I. J. F exclusion of bacteriophage T7 occurs at the cell membrane. Virology 326, 340–352 (2004).
Schmitt, C. K., Kemp, P. & Molineux, I. J. Genes 1.2 and 10 of bacteriophages T3 and T7 determine the permeability lesions observed in infected cells of Escherichia coli expressing the F plasmid gene pifA. J. Bacteriol. 173, 6507–6514 (1991).
Duncan-Lowey, B., McNamara-Bordewick, N. K., Tal, N., Sorek, R. & Kranzusch, P. J. Effector-mediated membrane disruption controls cell death in CBASS antiphage defense. Mol. Cell 81, 5039–5051.e5 (2021).
Piel, D. et al. Phage–host coevolution in natural populations. Nat. Microbiol. 7, 1075–1086 (2022).
Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687 (2013).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010).
Puigbò, P., Makarova, K. S., Kristensen, D. M., Wolf, Y. I. & Koonin, E. V. Reconstruction of the evolution of microbial defense systems. BMC Evol. Biol. 17, 94 (2017).
Payne, L. J. et al. Identification and classification of antiviral defence systems in bacteria and archaea with PADLOC reveals new system types. Nucleic Acids Res. 49, 10868–10878 (2021).
Fillol-Salom, A. et al. Bacteriophages benefit from mobilizing pathogenicity islands encoding immune systems against competitors. Cell 185, 3248–3262.e20 (2022).
Vassallo, C. N., Doering, C. R., Littlehale, M. L., Teodoro, G. I. C. & Laub, M. T. A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome. Nat. Microbiol. 7, 1568–1579 (2022). In this study, the authors developed a new high-throughput method for the discovery of antiphage systems through random cloning of genomic fragments of E. coli isolates, uncovering 21 novel systems.
Benler, S. et al. Cargo genes of Tn7-like transposons comprise an enormous diversity of defense systems, mobile genetic elements, and antibiotic resistance genes. mBio 12, e0293821 (2021).
Hochhauser, D., Millman, A. & Sorek, R. The defense island repertoire of the Escherichia coli pan-genome. PLoS Genet. 19, e1010694 (2023).
LeGault, K. N. et al. Temporal shifts in antibiotic resistance elements govern phage-pathogen conflicts. Science 373, eabg2166 (2021). This study demonstrates that V. cholerae resistance to phages is determined by the turnover of SXT integrative and conjugative elements, which often encode both antibiotic resistance genes and antiphage systems.
Picton, D. M. et al. The phage defence island of a multidrug resistant plasmid uses both BREX and type IV restriction for complementary protection from viruses. Nucleic Acids Res. 49, 11257–11273 (2021).
Wu, Y. et al. Defence systems provide synergistic anti-phage activity in E. coli. Preprint at bioRxiv https://doi.org/10.1101/2022.08.21.504612 (2022).
Rocha, E. P. C. & Bikard, D. Microbial defenses against mobile genetic elements and viruses: who defends whom from what? PLoS Biol. 20, e3001514 (2022).
Hussain, F. A. et al. Rapid evolutionary turnover of mobile genetic elements drives bacterial resistance to phages. Science 374, 488–492 (2021). This study shows that in a set of nearly clonal V. lentus strains, phage host range is largely explained by the rapid turnover of phage defence elements.
Bernheim, A. & Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119 (2020).
Dedrick, R. M. et al. Prophage-mediated defence against viral attack and viral counter-defence. Nat. Microbiol. 2, 16251 (2017).
Gentile, G. M. et al. More evidence of collusion: a new prophage-mediated viral defense system encoded by mycobacteriophage Sbash. mBio 10, e00196-19 (2019).
Montgomery, M. T., Guerrero Bustamante, C. A., Dedrick, R. M., Jacobs-Sera, D. & Hatfull, G. F. Yet more evidence of collusion: a new viral defense system encoded by Gordonia Phage CarolAnn. mBio 10, e02417–e02418 (2019).
Owen, S. V. et al. Prophages encode phage-defense systems with cognate self-immunity. Cell Host Microbe 29, 1620–1633.e8 (2021).
Ali, Y. et al. Temperate Streptococcus thermophilus phages expressing superinfection exclusion proteins of the Ltp type. Front. Microbiol. 5, 98 (2014).
Yu, Y. T. & Snyder, L. Translation elongation factor Tu cleaved by a phage-exclusion system. Proc. Natl Acad. Sci. USA 91, 802–806 (1994).
Koonin, E. V., Makarova, K. S., Wolf, Y. I. & Krupovic, M. Evolutionary entanglement of mobile genetic elements and host defence systems: guns for hire. Nat. Rev. Genet. 21, 119–131 (2020).
Anantharaman, V., Makarova, K. S., Burroughs, A. M., Koonin, E. V. & Aravind, L. Comprehensive analysis of the HEPN superfamily: identification of novel roles in intra-genomic conflicts, defense, pathogenesis and RNA processing. Biol. Direct 8, 15 (2013).
Lowey, B. et al. CBASS immunity uses CARF-related effectors to sense 3′–5′- and 2′–5′-linked cyclic oligonucleotide signals and protect bacteria from phage infection. Cell 182, 38–49.e17 (2020).
Francois, R. et al. A conserved family of immune effectors cleaves cellular ATP upon viral infection. Preprint at bioRxiv https://doi.org/10.1101/2023.01.24.525353 (2023).
Aravind, L., Iyer, L. M. & Burroughs, A. M. Discovering biological conflict systems through genome analysis: evolutionary principles and biochemical novelty. Annu. Rev. Biomed. Data Sci. 5, 367–391 (2022).
Stern, A. & Sorek, R. The phage-host arms race: shaping the evolution of microbes. Bioessays 33, 43–51 (2011).
Dupuis, M.-È., Villion, M., Magadán, A. H. & Moineau, S. CRISPR–Cas and restriction–modification systems are compatible and increase phage resistance. Nat. Commun. 4, 2087 (2013).
Costa, A. R. et al. Accumulation of defense systems drives panphage resistance in Pseudomonas aeruginosa. Preprint at bioRxiv https://doi.org/10.1101/2022.08.12.503731 (2022).
Srikant, S., Guegler, C. K. & Laub, M. T. The evolution of a counter-defense mechanism in a virus constrains its host range. eLife 11, e79549 (2022).
Maguin, P., Varble, A., Modell, J. W. & Marraffini, L. A. Cleavage of viral DNA by restriction endonucleases stimulates the type II CRISPR–Cas immune response. Mol. Cell 82, 907–919.e7 (2022).
Zheng, Z. et al. The CRISPR–Cas systems were selectively inactivated during evolution of Bacillus cereus group for adaptation to diverse environments. ISME J. 14, 1479–1493 (2020).
Vasu, K. & Nagaraja, V. Diverse functions of restriction–modification systems in addition to cellular defense. Microbiol. Mol. Biol. Rev. 77, 53–72 (2013).
Varble, A., Meaden, S., Barrangou, R., Westra, E. R. & Marraffini, L. A. Recombination between phages and CRISPR–Cas loci facilitates horizontal gene transfer in staphylococci. Nat. Microbiol. 4, 956–963 (2019).
Watson, B. N. J., Staals, R. H. J. & Fineran, P. C. CRISPR-Cas-mediated phage resistance enhances horizontal gene transfer by transduction. mBio 9, e02406–e02417 (2018).
Hsu, B. B. et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 25, 803–814.e5 (2019).
Braga, L. P. P. et al. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome 8, 52 (2020).
Kellner, M. J., Koob, J. G., Gootenberg, J. S., Abudayyeh, O. O. & Zhang, F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat. Protoc. 14, 2986–3012 (2019).
Loenen, W. A. M., Dryden, D. T. F., Raleigh, E. A., Wilson, G. G. & Murray, N. E. Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res. 42, 3–19 (2014).
Lopez, S. C., Crawford, K. D., Lear, S. K., Bhattarai-Kline, S. & Shipman, S. L. Precise genome editing across kingdoms of life using retron-derived DNA. Nat. Chem. Biol. 18, 199–206 (2022).
Pennisi, E. The CRISPR craze. Science 341, 833–836 (2013).
Schubert, M. G. et al. High-throughput functional variant screens via in vivo production of single-stranded DNA. Proc. Natl Acad. Sci. USA 118, e2018181118 (2021).
Alseth, E. O. et al. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance. Nature 574, 549–552 (2019).
Shaer Tamar, E. & Kishony, R. Multistep diversification in spatiotemporal bacterial-phage coevolution. Nat. Commun. 13, 7971 (2022).
Jacob, F. Evolution and tinkering. Science 196, 1161–1166 (1977).
Kibby, E. M. et al. Bacterial NLR-related proteins protect against phage. Cell 186, 2410–2424.e18 (2023).
Wein, T. & Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. 186, 2410–2424.e18 (2022).
Cury, J. et al. Conservation of antiviral systems across domains of life reveals novel immune mechanisms in humans. Preprint at bioRxiv https://doi.org/10.1101/2022.12.12.520048 (2022).
Warren, R. A. Modified bases in bacteriophage DNAs. Annu. Rev. Microbiol. 34, 137–158 (1980).
Liu, Y. et al. Covalent modifications of the bacteriophage genome confer a degree of resistance to bacterial CRISPR systems. J. Virol. 94, e01630-20 (2020).
Hobbs, S. J. et al. Phage anti-CBASS and anti-PYCSAR nucleases subvert bacterial immunity. Nature 605, 522–526 (2022).
Borges, A. L. et al. Bacteriophage cooperation suppresses CRISPR-Cas3 and Cas9 immunity. Cell 174, 917–925.e10 (2018).
Landsberger, M. et al. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell 174, 908–916.e12 (2018).
Blower, T. R., Evans, T. J., Przybilski, R., Fineran, P. C. & Salmond, G. P. C. Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism. PLoS Genet. 8, e1003023 (2012).
Makarova, K. S., Wolf, Y. I., Snir, S. & Koonin, E. V. Defense islands in bacterial and archaeal genomes and prediction of novel defense systems. J. Bacteriol. 193, 6039–6056 (2011).
Bondy-Denomy, J. et al. Prophages mediate defense against phage infection through diverse mechanisms. ISME J. 10, 2854–2866 (2016).
Scholl, D., Adhya, S. & Merril, C. Escherichia coli K1’s capsule is a barrier to bacteriophage T7. Appl. Environ. Microbiol. 71, 4872–4874 (2005).
Ohshima, Y., Schumacher-Perdreau, F., Peters, G. & Pulverer, G. The role of capsule as a barrier to bacteriophage adsorption in an encapsulated Staphylococcus simulans strain. Med. Microbiol. Immunol. 177, 229–233 (1988).
Scanlan, P. D. & Buckling, A. Co-evolution with lytic phage selects for the mucoid phenotype of Pseudomonas fluorescens SBW25. ISME J. 6, 1148–1158 (2012).
Seed, K. D. et al. Evolutionary consequences of intra-patient phage predation on microbial populations. eLife 3, e03497 (2014).
Harvey, H. et al. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation. Nat. Microbiol. 3, 47–52 (2018).
Tzipilevich, E., Pollak-Fiyaksel, O., Shraiteh, B. & Ben-Yehuda, S. Bacteria elicit a phage tolerance response subsequent to infection of their neighbors. EMBO J. 41, e109247 (2022).
Wohlfarth, J. C. et al. l-form conversion in Gram-positive bacteria enables escape from phage infection. Nat. Microbiol. 8, 387–399 (2023).
Ongenae, V. et al. Reversible bacteriophage resistance by shedding the bacterial cell wall. Open. Biol. 12, 210379 (2022).
Dillingham, M. S. & Kowalczykowski, S. C. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 72, 642–671 (2008).
Guo, L., Sattler, L., Shafqat, S., Graumann, P. L. & Bramkamp, M. A bacterial dynamin-like protein confers a novel phage resistance strategy on the population level in Bacillus subtilis. mBio https://doi.org/10.1128/mbio.03753-21 (2022).
Stokar-Avihail, A. et al. Discovery of phage determinants that confer sensitivity to bacterial immune systems. Cell 186, 1863–1876.e16 (2023). This study systematically uncovers triggers of antiphage defence systems and major trends of sensing mechanisms of antiphage immunity.
Athukoralage, J. S. et al. An anti-CRISPR viral ring nuclease subverts type III CRISPR immunity. Nature 577, 572–575 (2020).
Huiting, E. et al. Bacteriophages inhibit and evade cGAS-like immune function in bacteria. Cell 186, 864–876.e21 (2023).
Pawluk, A., Davidson, A. R. & Maxwell, K. L. Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 16, 12–17 (2018).
Acknowledgements
The authors are grateful to A. Millman, G. Ofir and F. Rousset for their very useful feedback on early versions of this manuscript. The authors also thank all members of the Molecular Diversity of Microbes Lab for their comments and suggestions during the writing process. H.G. and A.B. are supported by the ERC Starting Grant (PECAN 101040529). To promote gender equality and inclusivity in research, we are convinced of the importance of acknowledging gender bias in research article citation. Using a custom script available on our github (https://github.com/mdmparis/Estimating_gender_bias_in_references), we estimated that among the 139 references cited in the main text, approximately 45% (63) have a female first author and approximately 15% (21) have a female last author.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
H.G. is employed by Generare Biosciences. A.B. declares no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Eugene Koonin, John van der Oost and Lennart Randau for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Georjon, H., Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat Rev Microbiol 21, 686–700 (2023). https://doi.org/10.1038/s41579-023-00934-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-023-00934-x
This article is cited by
-
Inhibitors of bacterial immune systems: discovery, mechanisms and applications
Nature Reviews Genetics (2024)
-
An OLD protein teaches us new tricks: prokaryotic antiviral defense
Nature Communications (2024)
-
Anti-phage defence through inhibition of virion assembly
Nature Communications (2024)
-
A break in mitochondrial endosymbiosis as a basis for inflammatory diseases
Nature (2024)
-
Investigation into scalable and efficient enterotoxigenic Escherichia coli bacteriophage production
Scientific Reports (2024)