Abstract
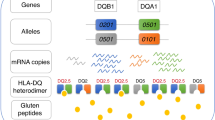
Coeliac disease is an autoinflammatory condition caused by immune reactions to cereal gluten proteins. Currently, the only available treatment for the condition is a lifelong avoidance of gluten proteins in the diet. There is an unmet need for alternative therapies. Coeliac disease has a strong association with certain HLA−DQ allotypes (DQ2.5, DQ2.2 and DQ8), and these disease-associated HLA-DQ molecules present deamidated gluten peptides to gluten-specific CD4+ T cells. The gluten-specific CD4+ T cells are the drivers of the immune reactions leading to coeliac disease. Once established, the clonotypes of gluten-specific CD4+ T cells persist for decades, explaining why patients must adhere to a gluten-free diet for life. Given the key pathogenic role of gluten-specific CD4+ T cells, tolerance-inducing therapies that target these T cells are attractive for treatment of the disorder. Lessons learned from coeliac disease might provide clues for treatment of other HLA-associated diseases for which the disease-driving antigens are unknown. Thus, intensive efforts have been and are currently implemented to bring an effective tolerance-inducing therapy for coeliac disease. This Review discusses mechanisms of the various approaches taken, summarizing the progress made, and highlights future directions in this field.
Key points
-
Coeliac disease is an autoinflammatory condition caused by hypersensitivity to cereal gluten proteins; gluten-specific CD4+ T cells drive immune reactions leading to an enteropathy and formation of autoantibodies.
-
Currently, the only available treatment is a lifelong gluten-free diet; there is an unmet need for treatment alternatives to this dietary intervention.
-
An antigen-specific therapy aiming to anergize, suppress or delete gluten-specific CD4+ T cells could be a way to treat the disease, such a tolerance-inducing therapy has the potential to become standalone therapy.
-
Various approaches for tolerance-inducing therapies have been designed and are pursued. These are currently in preclinical, phase I or phase II clinical development.
-
The molecular mechanisms involved in the tolerance-inducing therapies are not fully worked out.
-
How to best measure effectiveness of a coeliac disease therapy is debated; end points for phase III trials might not be the same as those used for phase II trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Levescot, A., Malamut, G. & Cerf-Bensussan, N. Immunopathogenesis and environmental triggers in coeliac disease. Gut 71, 2337–2349 (2022).
Iversen, R. & Sollid, L. M. The immunobiology and pathogenesis of celiac disease. Annu. Rev. Pathol. 18, 47–70 (2023).
Makharia, G. K., Chauhan, A., Singh, P. & Ahuja, V. Review article: epidemiology of coeliac disease. Aliment. Pharmacol. Ther. 56, S3–S17 (2022).
Lebwohl, B., Sanders, D. S. & Green, P. H. R. Coeliac disease. Lancet 391, 70–81 (2018).
Silvester, J. A., Weiten, D., Graff, L. A., Walker, J. R. & Duerksen, D. R. Living gluten-free: adherence, knowledge, lifestyle adaptations and feelings towards a gluten-free diet. J. Hum. Nutr. Diet. 29, 374–382 (2016).
Silvester, J. A. et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 52, 1469–1479 (2020).
Lundin, K. E. et al. Understanding celiac disease monitoring patterns and outcomes after diagnosis: a multinational, retrospective chart review study. World J. Gastroenterol. 27, 2603–2614 (2021).
Patel, N. et al. Clinical data do not reliably predict duodenal histology at follow-up in celiac disease: a 13 center correlative study. Am. J. Surg. Pathol. https://doi.org/10.1097/PAS.0000000000002150 (2023).
De Leon Morilla, D. et al. Patients’ risk tolerance for non-dietary therapies in celiac disease. Clin. Gastroenterol. Hepatol. 20, 2647–2649 (2022).
Pinto-Sanchez, M. I. et al. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18, 875–884 (2021).
Anderson, R. P. Emergence of an adaptive immune paradigm to explain celiac disease: a perspective on new evidence and implications for future interventions and diagnosis. Expert Rev. Clin. Immunol. 18, 75–91 (2022).
Kivelä, L. et al. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18, 181–195 (2021).
Rubin, C. E., Brandborg, L. L., Phelps, P. C. & Taylor, H. C. Studies of celiac disease. I. Apparent identical and specific nature of the duodenal and proximal jejunal lesion in celiac disease and idiopathic sprue. Gastroenterology 38, 28–49 (1960).
Marsh, M. N. in Coeliac Disease (ed. Marsh, M. N.) 136–191 (Blackwell Scientific Publications, 1992).
Ferguson, A. & Murray, D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut 12, 988–994 (1971).
Mayassi, T. & Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol. 11, 1281–1289 (2018).
Jabri, B. et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology 118, 867–879 (2000).
Hüe, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Baklien, K., Brandtzaeg, P. & Fausa, O. Immunoglobulins in jejunal mucosa and serum from patients with adult coeliac disease. Scand. J. Gastroenterol. 12, 149–159 (1977).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 102, 330–354 (1992).
Spencer, J. & Sollid, L. M. The human intestinal B-cell response. Mucosal Immunol. 9, 1113–1124 (2016).
Fenton, T. M. et al. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity 52, 557–570.e6 (2020).
Bartolome-Casado, R. et al. Resident memory CD8 T cells persist for years in human small intestine. J. Exp. Med. 216, 2412–2426 (2019).
Bartolome-Casado, R. et al. CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile. Mucosal Immunol. 14, 402–410 (2021).
Landsverk, O. J. et al. Antibody-secreting plasma cells persist for decades in human intestine. J. Exp. Med. 214, 309–317 (2017).
Nistico, L. et al. Concordance, disease progression, and heritability of coeliac disease in Italian twins. Gut 55, 803–808 (2006).
Trynka, G. et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat. Genet. 43, 1193–1201 (2011).
Sollid, L. M. The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics 69, 605–616 (2017).
Matzaraki, V., Kumar, V., Wijmenga, C. & Zhernakova, A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol. 18, 76 (2017).
Lundin, K. E. A. et al. Gliadin-specific, HLA-DQ(α1*0501,β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178, 187–196 (1993).
van de Wal, Y. et al. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc. Natl Acad. Sci. USA 95, 10050–10054 (1998).
Lundin, K. E. A., Scott, H., Fausa, O., Thorsby, E. & Sollid, L. M. T cells from the small intestinal mucosa of a DR4, DQ7/DR4, DQ8 celiac disease patient preferentially recognize gliadin when presented by DQ8. Hum. Immunol. 41, 285–291 (1994).
Sjöström, H. et al. Identification of a gliadin T-cell epitope in coeliac disease: general importance of gliadin deamidation for intestinal T-cell recognition. Scand. J. Immunol. 48, 111–115 (1998).
Sollid, L. M. et al. Update 2020: nomenclature and listing of celiac disease-relevant gluten epitopes recognized by CD4+ T cells. Immunogenetics 72, 85–88 (2020).
Ráki, M. et al. Similar responses of intestinal T cells from untreated children and adults with celiac disease to deamidated gluten epitopes. Gastroenterology 153, 787–798.e4 (2017).
Qiao, S. W., Dahal-Koirala, S., Eggesbo, L. M., Lundin, K. E. A. & Sollid, L. M. Frequency of gluten-reactive T cells in active celiac lesions estimated by direct cell cloning. Front. Immunol. 12, 646163 (2021).
Christophersen, A. et al. Tetramer-visualized gluten-specific CD4+ T cells in blood as a potential diagnostic marker for coeliac disease without oral gluten challenge. United European Gastroenterol. J. 2, 268–278 (2014).
Christophersen, A. et al. Distinct phenotype of CD4+ T cells driving celiac disease identified in multiple autoimmune conditions. Nat. Med. 25, 734–737 (2019).
Bodd, M. et al. Direct cloning and tetramer staining to measure the frequency of intestinal gluten-reactive T cells in celiac disease. Eur. J. Immunol. 43, 2605–2612 (2013).
Anderson, R. P. et al. Whole blood interleukin-2 release test to detect and characterize rare circulating gluten-specific T cell responses in coeliac disease. Clin. Exp. Immunol. 204, 321–334 (2021).
Dieterich, W. et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3, 797–801 (1997).
Molberg, Ø. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 (1998).
van de Wal, Y. et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 (1998).
Sollid, L. M., Molberg, Ø., McAdam, S. & Lundin, K. E. Autoantibodies in coeliac disease: tissue transglutaminase — guilt by association? Gut 41, 851–852 (1997).
Iversen, R. et al. Evidence that pathogenic transglutaminase 2 in celiac disease derives from enterocytes. Gastroenterology 159, 788–790 (2020).
Rostom, A. et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology 128, S38–S46 (2005).
Sulkanen, S. et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 115, 1322–1328 (1998).
Cataldo, F., Marino, V., Ventura, A., Bottaro, G. & Corazza, G. R. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut 42, 362–365 (1998).
Sollid, L. M., Pos, W. & Wucherpfennig, K. W. Molecular mechanisms for contribution of MHC molecules to autoimmune diseases. Curr. Opin. Immunol. 31, 24–30 (2014).
Cook, L. et al. Circulating gluten-specific FOXP3+CD39+ regulatory T cells have impaired suppressive function in patients with celiac disease. J. Allergy Clin. Immunol. 140, 1592–1603.e8 (2017).
Chauhan, S. K. et al. Human small intestine contains 2 functionally distinct regulatory T-cell subsets. J. Allergy Clin. Immunol. 152, 278–289.e6 (2023).
Marietta, E. et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J. Clin. Invest. 114, 1090–1097 (2004).
de Kauwe, A. L. et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J. Immunol. 182, 7440–7450 (2009).
Freitag, T. L. et al. Gliadin-primed CD4+CD45RBlowCD25− T cells drive gluten-dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut 58, 1597–1605 (2009).
Galipeau, H. J. et al. Sensitization to gliadin induces moderate enteropathy and insulitis in nonobese diabetic-DQ8 mice. J. Immunol. 187, 4338–4346 (2011).
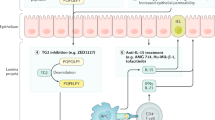
Abadie, V. et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature 578, 600–604 (2020).
Muller, U. et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J. Allergy Clin. Immunol. 101, 747–754 (1998).
Patel, D. et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J. Allergy Clin. Immunol. 131, 103–109.e7 (2013).
Palisade Group of Clinical Investigators. AR101 oral immunotherapy for peanut allergy. N. Engl. J. Med. 379, 1991–2001 (2018).
Noon, L. Prophylactic inoculation against hay fever. Lancet 177, 1572–1573 (1911).
Durham, S. R. & Shamji, M. H. Allergen immunotherapy: past, present and future. Nat. Rev. Immunol. 23, 317–328 (2023).
Richardson, N. & Wraith, D. C. Advancement of antigen-specific immunotherapy: knowledge transfer between allergy and autoimmunity. Immunother. Adv. 1, ltab009 (2021).
Kendal, A. R. & Waldmann, H. Infectious tolerance: therapeutic potential. Curr. Opin. Immunol. 22, 560–565 (2010).
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Gimmi, C. D., Freeman, G. J., Gribben, J. G., Gray, G. & Nadler, L. M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc. Natl Acad. Sci. USA 90, 6586–6590 (1993).
Anderson, P. O. et al. Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance. Eur. J. Immunol. 36, 1374–1385 (2006).
Burton, B. R. et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat. Commun. 5, 4741 (2014).
Gabryšová, L. et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J. Exp. Med. 206, 1755–1767 (2009).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016).
Sundstedt, A., O’Neill, E. J., Nicolson, K. S. & Wraith, D. C. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 170, 1240–1248 (2003).
Korneychuk, N. et al. Interleukin 15 and CD4+ T cells cooperate to promote small intestinal enteropathy in response to dietary antigen. Gastroenterology 146, 1017–1027 (2014).
Christophersen, A., Risnes, L. F., Dahal-Koirala, S. & Sollid, L. M. Therapeutic and diagnostic implications of T cell scarring in celiac disease and beyond. Trends Mol. Med. 25, 836–852 (2019).
Zheng, L., Li, J. & Lenardo, M. Restimulation-induced cell death: new medical and research perspectives. Immunol. Rev. 277, 44–60 (2017).
Klotz, L. et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl. Med. 11, eaao5563 (2019).
Thomson, A. W. & Knolle, P. A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 10, 753–766 (2010).
Zheng, M. & Tian, Z. Liver-mediated adaptive immune tolerance. Front. Immunol. 10, 2525 (2019).
Wilson, D. S. et al. Synthetically glycosylated antigens induce antigen-specific tolerance and prevent the onset of diabetes. Nat. Biomed. Eng. 3, 817–829 (2019).
Bozward, A. G., Ronca, V., Osei-Bordom, D. & Oo, Y. H. Gut-liver immune traffic: deciphering immune-pathogenesis to underpin translational therapy. Front. Immunol. 12, 711217 (2021).
Oberhuber, G. Histopathology of celiac disease. Biomed. Pharmacother. 54, 368–372 (2000).
Syage, J. A. et al. A composite morphometric duodenal biopsy mucosal scale for celiac disease encompassing both morphology and inflammation. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2023.10.031 (2023).
Leffler, D. et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 62, 996–1004 (2013).
Sarna, V. K. et al. HLA-DQ:gluten tetramer test in blood gives better detection of coeliac patients than biopsy after 14-day gluten challenge. Gut 67, 1606–1613 (2018).
Murray, J. A. et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology 152, 787–798.e2 (2017).
Schuppan, D. et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N. Engl. J. Med. 385, 35–45 (2021).
Taavela, J. et al. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS ONE 8, e76163 (2013).
Werkstetter, K. J. et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology 153, 924–935 (2017).
Gruver, A. M. et al. Pathologist-trained machine learning classifiers developed to quantitate celiac disease features differentiate endoscopic biopsies according to modified marsh score and dietary intervention response. Diagn. Pathol. 18, 122 (2023).
Stamnaes, J. et al. In well-treated celiac patients low-level mucosal inflammation predicts response to 14-day gluten challenge. Adv. Sci. 8, 2003526 (2021).
Petroff, D. et al. Antibody concentrations decrease 14-fold in children with celiac disease on a gluten-free diet but remain high at 3 months. Clin. Gastroenterol. Hepatol. 16, 1442–1449 (2018).
Goel, G. et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv. 5, eaaw7756 (2019).
Tye-Din, J. A. et al. Elevated serum interleukin-2 after gluten correlates with symptoms and is a potential diagnostic biomarker for coeliac disease. Aliment. Pharmacol. Ther. 50, 901–910 (2019).
Anderson, R. P., Degano, P., Godkin, A. J., Jewell, D. P. & Hill, A. V. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6, 337–342 (2000).
Anderson, R. P. et al. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 54, 1217–1223 (2005).
Ráki, M. et al. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc. Natl Acad. Sci. USA 104, 2831–2836 (2007).
Han, A. et al. Dietary gluten triggers concomitant activation of CD4+ and CD8+ αβ T cells and γδ T cells in celiac disease. Proc. Natl Acad. Sci. USA 110, 13073–13078 (2013).
Zühlke, S. et al. CD38 expression on gluten-specific T cells is a robust marker of gluten re-exposure in coeliac disease. United European Gastroenterol. J. 7, 1337–1344 (2019).
Leonard, M. M. et al. Evaluating responses to gluten challenge: a randomized, double-blind, 2-dose gluten challenge trial. Gastroenterology 160, 720–733.e8 (2021).
Hardy, M. Y. et al. A sensitive whole blood assay detects antigen-stimulated cytokine release from CD4+ T cells and facilitates immunomonitoring in a phase 2 clinical trial of Nexvax2 in coeliac disease. Front. Immunol. 12, 661622 (2021).
Adelman, D. C. Patient reported outcomes: instrument development and selection issues. US Patent application US 2015/0223747A1 (2015).
Leffler, D. A. et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 148, 1311–1319.e6 (2015).
Canestaro, W. J., Edwards, T. C. & Patrick, D. L. Systematic review: patient-reported outcome measures in coeliac disease for regulatory submissions. Aliment. Pharmacol. Ther. 44, 313–331 (2016).
Daveson, A. J. M. et al. Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment. Pharmacol. Ther. 51, 244–252 (2020).
Cartee, A. K. et al. Plasma IL-2 and symptoms response after acute gluten exposure in subjects with celiac disease or nonceliac gluten sensitivity. Am. J. Gastroenterol. 117, 319–326 (2022).
Ahonen, I. et al. Prevalence of vomiting and nausea and associated factors after chronic and acute gluten exposure in celiac disease. BMC Gastroenterol. 23, 301 (2023).
FDA. Celiac disease: developing drugs for adjunctive treatment to a gluten-free diet. FDA-2021-D-1238. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/celiac-disease-developing-drugs-adjunctive-treatment-gluten-free-diet (2023).
SSCD Executive Council & SSCD Writing Committte.FDA draft guidance for developing drugs for adjunctive treatment to a gluten-free diet: comments by the Society for the Study of Celiac Disease. Gastroenterology 164, 495–496 (2023).
Lebwohl, B. et al. Standardizing randomized controlled trials in celiac disease: an international multidisciplinary appropriateness study. Gastroenterology 166, 88–102 (2023).
Getts, D. R., Shea, L. D., Miller, S. D. & King, N. J. Harnessing nanoparticles for immune modulation. Trends Immunol. 36, 419–427 (2015).
Casey, L. M. et al. Mechanistic contributions of Kupffer cells and liver sinusoidal endothelial cells in nanoparticle-induced antigen-specific immune tolerance. Biomaterials 283, 121457 (2022).
Freitag, T. L. et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology 158, 1667–1681.e12 (2020).
Kelly, C. P. et al. TAK-101 nanoparticles induce gluten-specific tolerance in celiac disease: a randomized, double-blind, placebo-controlled study. Gastroenterology 161, 66–80.e8 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT04530123 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05660109 (2023).
Murray, J. A. et al. Safety and tolerability of KAN-101, a liver-targeted immune tolerance therapy, in patients with coeliac disease (ACeD): a phase 1 trial. Lancet Gastroenterol. Hepatol. 8, 735–747 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05574010 (2024).
Goel, G. et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol. Hepatol. 2, 479–493 (2017).
Truitt, K. E. et al. Randomised clinical trial: a placebo-controlled study of subcutaneous or intradermal NEXVAX2, an investigational immunomodulatory peptide therapy for coeliac disease. Aliment. Pharmacol. Ther. 50, 547–555 (2019).
Daveson, A. J. M. et al. Epitope-specific immunotherapy targeting CD4-positive T cells in celiac disease: safety, pharmacokinetics, and effects on intestinal histology and plasma cytokines with escalating dose regimens of Nexvax2 in a randomized, double-blind, placebo-controlled phase 1 study. EBioMedicine 26, 78–90 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT03644069 (2019).
Tye-Din, J. A. et al. Efficacy and safety of gluten peptide-based antigen-specific immunotherapy (Nexvax2) in adults with coeliac disease after bolus exposure to gluten (RESET CeD): an interim analysis of a terminated randomised, double-blind, placebo-controlled phase 2 study. Lancet Gastroenterol. Hepatol. 8, 446–457 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT04806737 (2021).
Huibregtse, I. L. et al. Induction of antigen-specific tolerance by oral administration of Lactococcus lactis delivered immunodominant DQ8-restricted gliadin peptide in sensitized nonobese diabetic Abo Dq8 transgenic mice. J. Immunol. 183, 2390–2396 (2009).
Thomson, A. W., Turnquist, H. R. & Raimondi, G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 9, 324–337 (2009).
Maldonado, R. A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015).
Li, J. et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376, eabi9591 (2022).
Osman, A. A. et al. B cell epitopes of gliadin. Clin. Exp. Immunol. 121, 248–254 (2000).
Zhou, C. et al. Focused B cell response to recurring gluten motif with implications for epitope spreading in celiac disease. Cell Rep. 41, 111541 (2022).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Yu, W., Freeland, D. M. H. & Nadeau, K. C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 16, 751–765 (2016).
Acknowledgements
Work in the author’s laboratory is funded by grants from Stiftelsen KG Jebsen (project SKGJ- MED-017), the University of Oslo World-leading research program on human immunology (WL-IMMUNOLOGY), the Research Council of Norway (projects 333380, 324302, 295844, 287234) and the South-Eastern Norway Regional Health Authority (projects 2016113, 2018068, 2020027 and 2023075).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
During the past 3 years, the author has been a consultant for Bristol Myers Squibb, GSK, Mozart Therapeutics, Ono Pharmaceutical, Precigen ActoBio, Sanofi, SQZ Biotech, Takeda and Topas Therapeutics. He has also previously been a consultant for ImmusanT.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Nadine Cerf-Bensussan, Bana Jabri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Celiac Disease Foundation Therapy List: https://celiac.org/about-celiac-disease/future-therapies-for-celiac-disease
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sollid, L.M. Tolerance-inducing therapies in coeliac disease — mechanisms, progress and future directions. Nat Rev Gastroenterol Hepatol 21, 335–347 (2024). https://doi.org/10.1038/s41575-024-00895-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-024-00895-3