Abstract
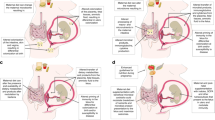
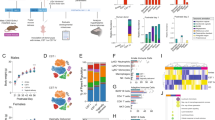
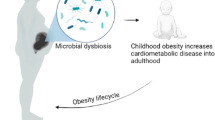
The gut microbiome has important roles in host metabolism and immunity, and microbial dysbiosis affects human physiology and health. Maternal immunity and microbial metabolites during pregnancy, microbial transfer during birth, and transfer of immune factors, microorganisms and metabolites via breastfeeding provide critical sources of early-life microbial and immune training, with important consequences for human health. Only a few studies have directly examined the interactions between the gut microbiome and the immune system during pregnancy, and the subsequent effect on offspring development. In this Review, we aim to describe how the maternal microbiome shapes overall pregnancy-associated maternal, fetal and early neonatal immune systems, focusing on the existing evidence and highlighting current gaps to promote further research.
Key points
-
Microbiota–host interactions during pregnancy are key for maternal and neonatal health outcomes and healthy offspring development.
-
Little is known regarding how the maternal microbiota and metabolites shape the maternal–fetal immune system during pregnancy.
-
Limited information is available on the factors that contribute to maternal microbiota–immune system feedback during pregnancy and early lactation.
-
Prenatal and postnatal immune system–microbiome interactions are relevant for human health.
-
The role of breast milk compounds in neonatal immune system development and microbial assembly warrants further studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nuriel-Ohayon, M., Neuman, H. & Koren, O. Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 7, 1031 (2016).
Gomez de Aguero, M. et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016).
Li, Y. et al. In utero human intestine harbors unique metabolome, including bacterial metabolites. JCI Insight 5, e138751 (2020).
Vuong, H. E. et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586, 281–286 (2020).
Calatayud, M., Koren, O. & Collado, M. C. Maternal microbiome and metabolic health program microbiome development and health of the offspring. Trends Endocrinol. Metab. 30, 735–744 (2019).
Davenport, E. R. et al. The human microbiome in evolution. BMC Biol. 15, 127 (2017).
Moeller, A. H. et al. Rapid changes in the gut microbiome during human evolution. Proc. Natl Acad. Sci. USA 111, 16431–16435 (2014).
Koren, O. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012).
Wang, X. et al. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 12, 1–13 (2020).
Stensballe, L. G., Simonsen, J., Jensen, S. M., Bonnelykke, K. & Bisgaard, H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J. Pediatr. 162, 832–838.e3 (2013).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 (2018).
Nakajima, A. et al. Impact of maternal dietary gut microbial metabolites on an offspring’s systemic immune response in mouse models. Biosci. Microbiota Food Health 39, 33–38 (2020).
Thomas, J. R. et al. Human HLFneg placental erythro-myeloid progenitors give rise to HLA class IIneg Hofbauer cells. Preprint at bioRxiv https://doi.org/10.1101/2022.02.26.482080 (2022).
Thomas, J. R. et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 218, 20200891 (2021).
Suryawanshi, H. et al. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 4, eaau4788 (2018).
Toothaker, J. M. et al. Immune landscape of human placental villi using single-cell analysis. Development 149, dev200013 (2022).
Pavlicev, M. et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. 27, 349–361 (2017).
Schumacher, A., Sharkey, D. J., Robertson, S. A. & Zenclussen, A. C. Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. J. Immunol. 201, 325–334 (2018).
Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 31, 387–411 (2013).
Reyes, L. & Golos, T. G. Hofbauer cells: their role in healthy and complicated pregnancy. Front. Immunol. 9, 2628 (2018).
Hoo, R., Nakimuli, A. & Vento-Tormo, R. Innate immune mechanisms to protect against infection at the human decidual-placental interface. Front. Immunol. 11, 2070 (2020).
Thomas, J. R. et al. Primitive haematopoiesis in the human placenta gives rise to macrophages with epigenetically silenced HLA-DR. Nat. Commun. 14, 1764 (2023).
Schliefsteiner, C., Ibesich, S. & Wadsack, C. Placental Hofbauer cell polarization resists inflammatory cues in vitro. Int. J. Mol. Sci. 21, 736 (2020).
Carosella, E. D., Rouas-Freiss, N., Tronik-Le Roux, D., Moreau, P. & LeMaoult, J. HLA-G: an immune checkpoint molecule. Adv. Immunol. 127, 33–144 (2015).
Richardson, C., King, W. & Vashisht, K. CD28 expression in human CD163+ placental Hofbauer cells. Placenta 89, 8–9 (2020).
Estrada-Capetillo, L. et al. CD28 is expressed by macrophages with anti-inflammatory potential and limits their T-cell activating capacity. Eur. J. Immunol. 51, 824–834 (2021).
Stras, S. F. et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev. Cell 51, 357–373.e5 (2019).
Schreurs, R. et al. Human fetal TNF-α-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50, 462–476.e8 (2019).
Li, N. et al. Memory CD4+ T cells are generated in the human fetal intestine. Nat. Immunol. 20, 301–312 (2019).
Pique-Regi, R. et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 8, e52004 (2019).
Angelo, L. S., Bimler, L. H., Nikzad, R., Aviles-Padilla, K. & Paust, S. CXCR6+ NK cells in human fetal liver and spleen possess unique phenotypic and functional capabilities. Front. Immunol. 10, 469 (2019).
Halkias, J. et al. CD161 contributes to prenatal immune suppression of IFNγ-producing PLZF+ T cells. J. Clin. Invest. 129, 3562–3577 (2019).
Mishra, A. et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409.e20 (2021).
Toothaker, J. M. et al. Immune cells in the placental villi contribute to intra-amniotic inflammation. Front. Immunol. 11, 866 (2020).
Odorizzi, P. M. et al. In utero priming of highly functional effector T cell responses to human malaria. Sci. Transl. Med. 10, eaat6176 (2018).
Frascoli, M. et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-γ and TNF-α. Sci. Transl. Med. 10, eaan2263 (2018).
Zhang, X. et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci. Transl. Med. 6, 238ra72 (2014).
Rackaityte, E. & Halkias, J. Mechanisms of fetal T cell tolerance and immune regulation. Front. Immunol. 11, 588 (2020).
Hossain, Z., Reza, A., Qasem, W. A., Friel, J. K. & Omri, A. Development of the immune system in the human embryo. Pediatr. Res. 92, 951–955 (2022).
Brodin, P. Immune-microbe interactions early in life: a determinant of health and disease long term. Science 376, 945–950 (2022).
Grossman, Z. & Paul, W. E. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc. Natl Acad. Sci. USA 89, 10365–10369 (1992).
Gao, Y. et al. Maternal gut microbiota during pregnancy and the composition of immune cells in infancy. Front. Immunol. 13, 986340 (2022).
Hu, M. et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat. Commun. 10, 3031 (2019).
Miller, D., Gershater, M., Slutsky, R., Romero, R. & Gomez-Lopez, N. Maternal and fetal T cells in term pregnancy and preterm labor. Cell Mol. Immunol. 17, 693–704 (2020).
Silverstein, R. B. & Mysorekar, I. U. Group therapy on in utero colonization: seeking common truths and a way forward. Microbiome 9, 7 (2021).
McGovern, N. et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546, 662–666 (2017).
Rackaityte, E. et al. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 26, 599–607 (2020).
Lewis, R. M. et al. 3D visualization of trans-syncytial nanopores provides a pathway for paracellular diffusion across the human placental syncytiotrophoblast. iScience 25, 105453 (2022).
Darby, M. G. et al. Pre-conception maternal helminth infection transfers via nursing long-lasting cellular immunity against helminths to offspring. Sci. Adv. 5, eaav3058 (2019).
Theis, K. R. et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am. J. Obstet. Gynecol. 220, 267.e1–267.e39 (2019).
Kennedy, K. M. et al. Over-celling fetal microbial exposure. Cell 184, 5839–5841 (2021).
Leiby, J. S. et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6, 196 (2018).
Kennedy, K. M. et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 613, 639–649 (2023).
Theis, K. R., Romero, R., Winters, A. D., Jobe, A. H. & Gomez-Lopez, N. Lack of evidence for microbiota in the placental and fetal tissues of rhesus macaques. mSphere 5, e00210–e00220 (2020).
Theis, K. R. et al. No consistent evidence for microbiota in murine placental and fetal tissues. mSphere 5, e00933–e01019 (2020).
Walker, W. A. & Iyengar, R. S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 77, 220–228 (2015).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Quinn, R. A. et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579, 123–129 (2020).
De Angelis, M. et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS ONE 9, e99006 (2014).
Petersen, C. et al. A rich meconium metabolome in human infants is associated with early-life gut microbiota composition and reduced allergic sensitization. Cell Rep. Med. 2, 100260 (2021).
Pessa-Morikawa, T. et al. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 22, 46 (2022).
Ferretti, P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018).
Turjeman, S., Collado, M. C. & Koren, O. The gut microbiome in pregnancy and pregnancy complications. Curr. Opin. Endocr. Metab. Res. 18, 133–138 (2021).
Souza, R. T. et al. Metabolomics applied to maternal and perinatal health: a review of new frontiers with a translation potential. Clinics 74, e894 (2019).
Lenicek, M., Olde Damink, S. W. M. & Schaap, F. G. Gut microbes take it to the next level? first insights into farnesoid X receptor agonists of microbial origin. Hepatology 72, 1483–1485 (2020).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Tenenbaum-Gavish, K. et al. First trimester biomarkers for prediction of gestational diabetes mellitus. Placenta 101, 80–89 (2020).
Vladu, I. M. et al. Maternal and fetal metabolites in gestational diabetes mellitus: a narrative review. Metabolites 12, 383 (2022).
Wang, S., Liu, Y., Qin, S. & Yang, H. Composition of maternal circulating short-chain fatty acids in gestational diabetes mellitus and their associations with placental metabolism. Nutrients 14, 3727 (2022).
Huang, X. et al. Decreased monocyte count is associated with gestational diabetes mellitus development, macrosomia, and inflammation. J. Clin. Endocrinol. Metab. 107, 192–204 (2022).
McElwain, C. J., McCarthy, F. P. & McCarthy, C. M. Gestational diabetes mellitus and maternal immune dysregulation: what we know so far. Int. J. Mol. Sci. 22, 4261 (2021).
Pinto, Y. et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 72, 918–928 (2023).
Chen, T. et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med. 19, 120 (2021).
Lv, L.-J. et al. Deep metagenomic characterization of gut microbial community and function in preeclampsia. Front. Cell Infect. Microbiol. 12, 933523 (2022).
Wang, J., Gu, X., Yang, J., Wei, Y. & Zhao, Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front. Cell Infect. Microbiol. 9, 409 (2019).
Chang, Y. et al. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 134, 289–302 (2020).
Ishimwe, J. A. Maternal microbiome in preeclampsia pathophysiology and implications on offspring health. Physiol. Rep. 9, e14875 (2021).
Patel, R. M. Short and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 33, 318–328 (2016).
Melville, J. M. & Moss, T. J. M. The immune consequences of preterm birth. Front. Neurosci. 7, 79 (2013).
Yang, R. et al. Prematurely delivering mothers show reductions of Lachnospiraceae in their gut microbiomes. BMC Microbiol. 23, 169 (2023).
Hiltunen, H. et al. Preterm infant meconium microbiota transplant induces growth failure, inflammatory activation, and metabolic disturbances in germ-free mice. Cell Rep. Med. 2, 100447 (2021).
Underwood, M. A. & Sohn, K. The microbiota of the extremely preterm infant. Clin. Perinatol. 44, 407–427 (2017).
Drell, T. et al. The development of gut microbiota in critically ill extremely low birth weight infants assessed with 16S rRNA gene based sequencing. Gut Microbes 5, 304–312 (2014).
Ansari, A., Bose, S., You, Y., Park, S. & Kim, Y. Molecular mechanism of microbiota metabolites in preterm birth: pathological and therapeutic insights. Int. J. Mol. Sci. 22, 8145 (2021).
Tabilas, C. et al. Early microbial exposure shapes adult immunity by altering CD8+ T cell development. Proc. Natl Acad. Sci. USA 119, e2212548119 (2022).
Gensollen, T., Iyer, S. S., Kasper, D. L. & Blumberg, R. S. How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016).
Sprockett, D., Fukami, T. & Relman, D. A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 15, 197–205 (2018).
Wesemann, D. R. & Nagler, C. R. The microbiome, timing, and barrier function in the context of allergic disease. Immunity 44, 728–738 (2016).
Grigg, J. B. & Sonnenberg, G. F. Host-microbiota interactions shape local and systemic inflammatory diseases. J. Immunol. 198, 564–571 (2017).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021).
Olin, A. et al. Stereotypic immune system development in newborn children. Cell 174, 1277–1292.e14 (2018).
Knoop, K. A. et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2, eaa01314 (2017).
Al Nabhani, Z. et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 50, 1276–1288.e5 (2019).
Sefik, E. et al. Mucosal immunology. individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Riskin, A. et al. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr. Res. 71, 220–225 (2012).
van den Elsen, L. W. J., Kollmann, T. R. & Verhasselt, V. Microbial antigen in human milk: a natural vaccine? Mucosal Immunol. 15, 1058–1059 (2022).
Donaldson, G. P. et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 360, 795–800 (2018).
Yokanovich, L. T., Newberry, R. D. & Knoop, K. A. Regulation of oral antigen delivery early in life: implications for oral tolerance and food allergy. Clin. Exp. Allergy 51, 518–526 (2021).
Yang, S., Fujikado, N., Kolodin, D., Benoist, C. & Mathis, D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594 (2015).
Aaltonen, J., Bjorses, P., Sandkuijl, L., Perheentupa, J. & Peltonen, L. An autosomal locus causing autoimmune disease: autoimmune polyglandular disease type I assigned to chromosome 21. Nat. Genet. 8, 83–87 (1994).
Zegarra-Ruiz, D. F. et al. Thymic development of gut-microbiota-specific T cells. Nature 594, 413–417 (2021).
Redwine, L. S., Altemus, M., Leong, Y. M. & Carter, C. S. Lymphocyte responses to stress in postpartum women: relationship to vagal tone. Psychoneuroendocrinology 26, 241–251 (2001).
Lovelady, C. A., Fuller, C. J., Geigerman, C. M., Hunter, C. P. & Kinsella, T. C. Immune status of physically active women during lactation. Med. Sci. Sports Exerc. 36, 1001–1007 (2004).
Zimmer, J. P., Garza, C., Heller, M. E., Butte, N. & Goldman, A. S. Postpartum maternal blood helper T (CD3+CD4+) and cytotoxic T (CD3+CD8+) cells: correlations with iron status, parity, supplement use, and lactation status. Am. J. Clin. Nutr. 67, 897–904 (1998).
Hu, J. et al. Immune cell profiling of preeclamptic pregnant and postpartum women by single-cell RNA sequencing. Int. Rev. Immunol. 11, 1–12 (2022).
Coley, E. J. L. & Hsiao, E. Y. Malnutrition and the microbiome as modifiers of early neurodevelopment. Trends Neurosci. 44, 753–764 (2021).
Williams, J. E. et al. Strong multivariate relations exist among milk, oral, and fecal microbiomes in mother-infant dyads during the first six months postpartum. J. Nutr. 149, 902–914 (2019).
Carrothers, J. M. et al. Fecal microbial community structure is stable over time and related to variation in macronutrient and micronutrient intakes in lactating women. J. Nutr. 145, 2379–2388 (2015).
Nunn, K. L. et al. Changes in the vaginal microbiome during the pregnancy to postpartum transition. Reprod. Sci. 28, 1996–2005 (2021).
Stinson, L. F. & Geddes, D. T. Microbial metabolites: the next frontier in human milk. Trends Microbiol. 30, 408–410 (2022).
Kijner, S., Kolodny, O. & Yassour, M. Human milk oligosaccharides and the infant gut microbiome from an eco-evolutionary perspective. Curr. Opin. Microbiol. 68, 102156 (2022).
Selma-Royo, M., Calvo Lerma, J., Cortes-Macias, E. & Collado, M. C. Human milk microbiome: from actual knowledge to future perspective. Semin. Perinatol. 45, 151450 (2021).
Lycke, N. Y. & Bemark, M. The regulation of gut mucosal IgA B-cell responses: recent developments. Mucosal Immunol. 10, 1361–1374 (2017).
Rosa, F. et al. Human milk oligosaccharides impact cellular and inflammatory gene expression and immune response. Front. Immunol. 13, 907529 (2022).
Donald, K., Petersen, C., Turvey, S. E., Finlay, B. B. & Azad, M. B. Secretory IgA: linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 30, 650–659 (2022).
Gustafson, C. E. et al. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 7, 467–477 (2014).
Rogier, E. W. et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl Acad. Sci. USA 111, 3074–3079 (2014).
Gopalakrishna, K. P. et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115 (2019).
Chutipongtanate, S., Morrow, A. L. & Newburg, D. S. Human milk extracellular vesicles: a biological system with clinical implications. Cells 11, 2345 (2022).
Gleeson, J. P. et al. Profiling of mature-stage human breast milk cells identifies six unique lactocyte subpopulations. Sci. Adv. 8, eabm6865 (2022).
Dawod, B., Marshall, J. S. & Azad, M. B. Breastfeeding and the developmental origins of mucosal immunity: how human milk shapes the innate and adaptive mucosal immune systems. Curr. Opin. Gastroenterol. 37, 547–556 (2021).
Acknowledgements
The authors thank R. Michita and S. Turjeman for their valuable input. Much appreciation to C. Eke for his contributions to the original version of the Figures. O.K. is supported by the European Research Council Consolidator grant (grant agreement no. 101001355). I.U.M. is supported by NIH/NICHD grant R01 HD091218 and 3R01HD091218-04S1(RADx-UP Supplement). O.K. and M.C.C. acknowledge the support by Biostime Institute Nutrition and Care (BINC) grant. L.K. is supported by Yale University pilot funds, Binational Science Foundation (2019075), and the National Institutes of Health (R21TR002639, R21HD102565, R01DK129552 and R01AI171980). P.B. is supported by grants from Knut and Alice Wallenberg Foundation (2019.0181), Svenska sällskapet för Medicinsk forskning, SSMF (CG-22-0148-H-02), the Swedish Research council (2019-01495, 2022-01567), EU Horizon project grant UNDINE (grant agreement: 101057100) and Wellcome Trust Discovery award (226507/Z/22/Z). M.C.C. was supported by a European Research Council Starting grant (grant agreement no. 639226), the Spanish Ministry of Science and Innovation (grant ref. PID2022-139475OB-I00), PROMETEO GVA (ref. 2020/012) and also from the Horizon Europe Program (INITIALISE- 101094099 project). M.C.C. also acknowledges the Spanish government MCIN/AEI to the Center of Excellence Accreditation Severo Ochoa (CEX2021-001189-S/ MCIN/AEI / 10.13039/501100011033).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
O.K. is a co-founder of Shela Accurate Diagnosis LTD (Israel). I.U.M. serves on the scientific advisory board of Luca Biologics. P.B. serves on the scientific advisory board of Pixelgen Technologies AB (Sweden), Oxford Immune Algorithmics (UK), and Scailyte AG (Switzerland) and is an executive board member of Kancera AB (Sweden) and co-founder of Cytodelics AB (Sweden). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Nardhy Gomez-Lopez and Gérard Eberl for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koren, O., Konnikova, L., Brodin, P. et al. The maternal gut microbiome in pregnancy: implications for the developing immune system. Nat Rev Gastroenterol Hepatol 21, 35–45 (2024). https://doi.org/10.1038/s41575-023-00864-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00864-2