Abstract
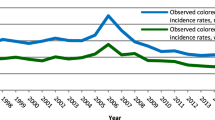
Colorectal cancer (CRC) incidence rates decreased by up to 50% in older age groups in the USA in the era of the widespread uptake of screening colonoscopy, despite adverse trends in CRC risk factors and increasing CRC incidence at younger ages. However, reported first results from a randomized trial, the NordICC study, suggested rather modest effects of screening colonoscopy. As outlined in this Perspective, the apparent discrepancy between real-world and trial evidence could be explained by strong attenuation of effect estimates from screening endoscopy trials by several factors, including limited screening adherence, widespread uptake of colonoscopy outside the screening offers and the inclusion of prevalent, non-preventable CRC cases in reported numbers of incident cases. Alternative interpretations of screening endoscopy trial results accounting for prevalence bias are in line with trends in CRC incidence reduction in countries offering CRC screening, and should encourage more widespread implementation and uptake of effective CRC screening.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data presented in this paper were extracted from published original articles or publicly accessible online data sources (including GLOBOCAN and the German Centre for Cancer Registry Data (ZfKD)) and can be retrieved from the references provided in the figure legends.
References
Siegel, R. L. et al. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Winawer, S. J. et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 329, 1977–1781 (1993).
Bretthauer, M. et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 387, 1547–1556 (2022).
Siegel, R. L. et al. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 67, 177–193 (2017).
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73, 233–254 (2023).
Cardoso, R. et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 22, 1002–1013 (2021).
Sinicrope, F. A. Increasing incidence of early-onset colorectal cancer. N. Engl. J. Med. 386, 1547–1558 (2022).
Patel, S. G. et al. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 7, 262–274 (2022).
Shah, R. R. et al. Trends in the incidence of early-onset colorectal cancer in all 50 United States from 2001 through 2017. Cancer 128, 299–310 (2022).
Ben-Aharon, I. et al. Early-onset cancer in the gastrointestinal tract is on the rise–evidence and implications. Cancer Discov. 13, 538–551 (2023).
Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72, 338–344 (2023).
Brenner, H., Hoffmeister, M., Arndt, V. & Haug, U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br. J. Cancer 96, 828–831 (2007).
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020).
Cho, K. R. & Vogelstein, B. Genetic alterations in the adenoma–carcinoma sequence. Cancer 70, 1727–1731 (1992).
Launoy, G., Smith, T. C., Duffy, S. W. & Bouvier, V. Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int. J. Cancer 73, 220–224 (1997).
Prevost, T. C., Launoy, G., Duffy, S. W. & Chen, H. H. Estimating sensitivity and sojourn time in screening for colorectal cancer: a comparison of statistical approaches. Am. J. Epidemiol. 148, 609–619 (1998).
Brenner, H., Altenhofen, L., Katalinic, A., Lansdorp-Vogelaar, I. & Hoffmeister, M. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German National Screening Colonoscopy Database. Am. J. Epidemiol. 174, 1140–1146 (2011).
Brenner, H., Altenhofen, L., Stock, C. & Hoffmeister, M. Natural history of colorectal adenomas: birth cohort analysis among 3.6 million participants of screening colonoscopy. Cancer Epidemiol. Biomark. Prev. 22, 1043–1051 (2013).
Shaukat, A. & Levin, T. R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 19, 521–531 (2022).
Hewitson, P., Glasziou, P., Watson, E., Towler, B. & Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am. J. Gastroenterol. 103, 1541–1549 (2008).
Shaukat, A. et al. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 369, 1106–1114 (2013).
Brenner, H. & Tao, S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur. J. Cancer 49, 3049–3054 (2013).
Grobbee, E. J. et al. Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals. Cochrane Database Syst. Rev. 6, CD009276 (2022).
Hoffman, R. M. et al. Colorectal cancer screening adherence is higher with fecal immunochemical tests than guaiac-based fecal occult blood tests: a randomized, controlled trial. Prev. Med. 50, 297–299 (2010).
Schreuders, E. H., Grobbee, E. J., Spaander, M. C. & Kuipers, E. J. Advances in fecal tests for colorectal cancer screening. Curr. Treat. Options Gastroenterol. 14, 152–162 (2016).
Hanna, M., Dey, N. & Grady, W. M. Emerging tests for noninvasive colorectal cancer screening. Clin. Gastroenterol. Hepatol. 21, 604–616 (2023).
Imperiale, T. F. et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 370, 1287–1297 (2014).
Rex, D. K. et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology 153, 307–323 (2017).
Brenner, H., Werner, S. & Chen, H. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 371, 184–185 (2014).
Peterse, E. F. P. et al. Comparing the cost-effectiveness of innovative colorectal cancer screening tests. J. Natl Cancer Inst. 113, 154–161 (2021).
Lauby-Secretan, B., Vilahur, N., Bianchini, F., Guha, N. & Straif, K., International Agency for Research on Cancer Handbook Working Group. The IARC perspective on colorectal cancer screening. N. Engl. J. Med. 378, 1734–1740 (2018).
Paszat, L. F., Sutradhar, R., Luo, J., Rabeneck, L. & Tinmouth, J. Perforation and post-polypectomy bleeding complicating colonoscopy in a population-based screening program. Endosc. Int. Open. 9, E637–E645 (2021).
Lin, J. S., Perdue, L. A., Henrikson, N. B., Bean, S. I. & Blasi, P. R. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 325, 1978–1998 (2021).
Ran, T. et al. Cost-effectiveness of colorectal cancer screening strategies – a systematic review. Clin. Gastroenterol. Hepatol. 17, 1969–1981.e15 (2019).
Cardoso, R., Niedermaier, T., Chen, C., Hoffmeister, M. & Brenner, H. Colonoscopy and sigmoidoscopy use among the average-risk population for colorectal cancer: a systematic review and trend analysis. Cancer Prev. Res. 12, 617–630 (2019).
Cardoso, R., Guo, F., Heisser, T., Hoffmeister, M. & Brenner, H. Utilisation of colorectal cancer screening tests in European countries by type of screening offer: results from the European Health Interview Survey. Cancers 12, 1409 (2020).
Niedermaier, T., Weigl, K., Hoffmeister, M. & Brenner, H. Diagnostic performance of flexible sigmoidoscopy combined with fecal immunochemical test in colorectal cancer screening: meta-analysis and modeling. Eur. J. Epidemiol. 32, 481–493 (2017).
Brenner, H., Stock, C. & Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 348, g2467 (2014).
Doubeni, C. A. et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut 67, 291–298 (2018).
Zhang, J. et al. Colonoscopic screening is associated with reduced colorectal cancer incidence and mortality: a systematic review and meta-analysis. J. Cancer 11, 5953–5970 (2020).
Guo, F. et al. Strong reduction of colorectal cancer incidence and mortality after screening colonoscopy: prospective cohort study from Germany. Am. J. Gastroenterol. 116, 967–975 (2021).
Jaacks, L. M. et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 7, 231–240 (2019).
Cardoso, R. et al. Proportion and stage distribution of screen-detected and non-screen-detected colorectal cancer in nine European countries: an international, population-based study. Lancet Gastroenterol. Hepatol. 7, 711–723 (2022).
Australian Institute of Health and Welfare. National Bowel Cancer Screening Program monitoring report 2022. AIHW https://www.aihw.gov.au/reports/cancer-screening/nbcsp-monitoring-2022/summary (2022).
Holme, Ø. et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 312, 606–615 (2014).
Atkin, W. et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 389, 1299–1311 (2017).
Miller, E. A., Pinsky, P. F., Schoen, R. E., Prorok, P. C. & Church, T. R. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol. Hepatol. 4, 101–110 (2019).
Senore, C. et al. Long-term follow-up of the Italian flexible sigmoidoscopy screening trial. Ann. Intern. Med. 175, 36–45 (2022).
Juul, F. E. et al. 15-year benefits of sigmoidoscopy screening on colorectal cancer incidence and mortality: a pooled analysis of randomized trials. Ann. Intern. Med. 176, eL230025 (2022).
Buskermolen, M. et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a microsimulation modelling study. BMJ 367, l5383 (2019).
Heisser, T., Hoffmeister, M. & Brenner, H. Effects of screening for colorectal cancer: development, documentation and validation of a multistate Markov model. Int. J. Cancer 148, 1973–1981 (2021).
Heisser, T., Hoffmeister, M. & Brenner, H. Model based evaluation of long-term efficacy of existing and alternative colorectal cancer screening offers: a case study for Germany. Int. J. Cancer 150, 1471–1480 (2022).
Brenner, H., Stock, C. & Hoffmeister, M. In the era of widespread endoscopy use, randomized trials may strongly underestimate the effects of colorectal cancer screening. J. Clin. Epidemiol. 66, 1144–1150 (2013).
Eurostat. Self-reported last colonoscopy by sex, age and educational level. Eurostat https://ec.europa.eu/eurostat/databrowser/view/HLTH_EHIS_PA6E__custom_3573783/default/table?lang=en (2021).
Shapiro, J. A. et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol. Biomark. Prev. 21, 895–904 (2012).
Shapiro, J. A. et al. Screening for colorectal cancer in the United States: correlates and time trends by type of test. Cancer Epidemiol. Biomark. Prev. 30, 1554–1565 (2021).
Hoffmeister, M., Cardoso, R. & Brenner, H. Colonoscopy screening and colorectal cancer incidence and mortality. N. Engl. J. Med. 388, 377–378 (2023).
Bretthauer, M., Løberg, M. & Kaminski, M. F. Colonoscopy screening and colorectal cancer incidence and mortality. Reply. N. Engl. J. Med. 388, 378–379 (2023).
Brenner, H., Altenhofen, L., Stock, C. & Hoffmeister, M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin. Gastroenterol. Hepatol. 13, 717–723 (2015).
Heisser, T., Hoffmeister, M. & Brenner, H. Significant underestimation of preventive effects in colorectal cancer screening trial. Gut, https://doi.org/10.1136/gutjnl-2022-329165 (2023).
NCD Risk Factor Collaboration (NCD-RisC)Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387, 1377–1396 (2016).
Ladabaum, U., Dominitz, J. A., Kahi, C. & Schoen, R. E. Strategies for colorectal cancer screening. Gastroenterology 158, 418–432 (2020).
Gini, A. et al. Development and validation of three regional microsimulation models for predicting colorectal cancer screening benefits in Europe. MDM Policy Pract. 6, 2381468320984974 (2021).
van den Puttelaar, R. et al. Risk-stratified screening for colorectal cancer using genetic and environmental risk factors: a cost-effectiveness analysis based on real-world data. Clin. Gastroenterol. Hepatol., https://doi.org/10.1016/j.cgh.2023.03.003 (2023).
Chen, C., Stock, C., Hoffmeister, M. & Brenner, H. Optimal age for screening colonoscopy: a modeling study. Gastrointest. Endosc. 89, 1017–1025.e12 (2019).
Yeoh, A., Mannalithara, A. & Ladabaum, U. Cost-effectiveness of earlier or more intensive colorectal cancer screening in overweight and obese patients. Clin. Gastroenterol. Hepatol. 21, 507–519 (2023).
Heisser, T., Peng, L., Weigl, K., Hoffmeister, M. & Brenner, H. Outcomes at follow-up of negative colonoscopy in average risk population: systematic review and meta-analysis. BMJ 367, l6109 (2019).
Heisser, T. et al. Prevalence of colorectal neoplasia ten or more years after a negative screening colonoscopy: registry-based study based on 120,000 repeat screening colonoscopies. JAMA Intern. Med. 183, 183–190 (2023).
Acknowledgements
This work was supported in part by grants from the German Federal Ministry of Education and Research (grant no. 01KD2104A) and the German Cancer Aid (grant no. 70114735).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to discussion of content and reviewed/edited the manuscript before submission. H.B. researched data for and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Theodore Levin, Guy Launoy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
German Centre for Cancer Registry Data (ZfKD), Robert Koch Institute: www.krebsdaten.de/database
GLOBOCAN: https://gco.iarc.fr
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brenner, H., Heisser, T., Cardoso, R. et al. Reduction in colorectal cancer incidence by screening endoscopy. Nat Rev Gastroenterol Hepatol 21, 125–133 (2024). https://doi.org/10.1038/s41575-023-00847-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00847-3
This article is cited by
-
The underestimated preventive effects of flexible sigmoidoscopy screening: re-analysis and meta-analysis of randomized trials
European Journal of Epidemiology (2024)