Abstract
Pancreatic ductal adenocarcinoma (PDAC) has a rising incidence and is one of the most lethal human malignancies. Much is known regarding the biology and pathophysiology of PDAC, but translating this knowledge to the clinic to improve patient outcomes has been challenging. In this Review, we discuss advances and practice-changing trials for PDAC. We briefly review therapeutic failures as well as ongoing research to refine the standard of care, including novel biomarkers and clinical trial designs. In addition, we highlight contemporary areas of research, including poly(ADP-ribose) polymerase inhibitors, KRAS-targeted therapies and immunotherapies. Finally, we discuss the future of pancreatic cancer research and areas for improvement in the next decade.
Key points
-
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy, with cytotoxic chemotherapy remaining the mainstay of treatment for most patients.
-
Ongoing trials are evaluating ‘classical’ versus ‘basal-like’ expression signatures to inform the therapeutic selection of standard cytotoxic regimens.
-
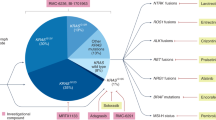
Small subsets of patients, including those with mutations in BRCA1, BRCA2, PALB2 and KRASG12C, rare fusions (NRG1, NTRK), and mismatch repair deficiency, benefit from targeted therapies.
-
KRAS is a critical target for therapeutic evaluation in PDAC and proof-of-principle approaches have validated targeting in KRASG12C; ongoing trials are evaluating allele-specific inhibitors and pan-(K)RAS inhibitors against the more common allele variants G12D, G12V and G12R.
-
Immune-checkpoint inhibitor blockade and other immune therapies have not had utility for most patients with PDAC; however, select rare individuals beyond mismatch repair deficiency have seen major benefit, leading to optimism that these results can be expanded to a broader patient population.
-
Clinical trial design strategies are changing and PDAC is a prototypic disease to investigate novel drug development paradigms; innovation in clinical trial design has led to the integration of platform-type studies that incorporate novel statistical design, leading to expediencies in drug development, timelines, cost and other resources. Platform studies enable parallel investigation of multiple novel agents, with early signal adjudication of success or failure to allow efficient signal seeking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Mahaseth, H. et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 42, 1311–1315 (2013).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Wainberg, Z. A. et al. NAPOLI-3: a randomized, open-label phase 3 study of liposomal irinotecan + 5-fluorouracil/leucovorin + oxaliplatin (NALIRIFOX) versus nab-paclitaxel + gemcitabine in treatment-naïve patients with metastatic pancreatic ductal adenocarcinoma (mPDAC). J. Clin. Oncol. 41, LBA661 (2023).
Conroy, T. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406 (2018).
Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Uesaka, K. et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388, 248–257 (2016).
Springfeld, C. et al. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/s41571-023-00746-1 (2023).
Springfeld, C. & Neoptolemos, J. P. The role of neoadjuvant therapy for resectable pancreatic cancer remains uncertain. Nat. Rev. Clin. Oncol. 19, 285–286 (2022).
Schwarz, L. et al. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy: a multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). J. Clin. Oncol. 40, 4134 (2022).
Labori, K. J. et al. Short-course neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer: a multicenter randomized phase-II trial (NORPACT-1). J. Clin. Oncol. 41, LBA4005 (2023).
Katz, M. H. et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 151, e161137 (2016).
Ghaneh, P. et al. ESPAC-5F: four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J. Clin. Oncol. 38, 4505 (2020).
Versteijne, E. et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J. Clin. Oncol. 40, 1220–1230 (2022).
Katz, M. H. G. et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 phase 2 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.2319 (2022).
Ghaneh, P. et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 157–168 (2023).
Pishvaian, M. J. et al. Molecular profiling of patients with pancreatic cancer: initial results from the know your tumor initiative. Clin. Cancer Res. 24, 5018–5027 (2018).
Golan, T. et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 381, 317–327 (2019).
Hu, Z. I. et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin. Cancer Res. 24, 1326–1336 (2018).
Muir, P. et al. The real cost of sequencing: scaling computation to keep pace with data generation. Genome Biol. 17, 53 (2016).
Aguirre, A. J. et al. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. 8, 1096–1111 (2018).
Lowery, M. A. et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: potential actionability and correlation with clinical phenotype. Clin. Cancer Res. 23, 6094–6100 (2017).
Sohal, D. P. S. et al. Metastatic pancreatic cancer: ASCO guideline update. J. Clin. Oncol. https://doi.org/10.1200/JCO.20.01364 (2020).
Tempero, M. A. et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 19, 439–457 (2021).
Biankin, A. V. et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491, 399–405 (2012).
Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32, 185–203.e13 (2017).
Philip, P. A. et al. Molecular characterization of KRAS wild type tumors in patients with pancreatic adenocarcinoma. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-21-3581 (2022).
Pishvaian, M. J. et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 21, 508–518 (2020).
Collisson, E. A. et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503 (2011).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Moffitt, R. A. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 47, 1168–1178 (2015).
Chan-Seng-Yue, M. et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 52, 231–240 (2020).
Puleo, F. et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology 155, 1999–2013.e3 (2018).
Connor, A. A. & Gallinger, S. Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat. Rev. Cancer 22, 131–142 (2022).
Bailey, P. et al. Refining the treatment of pancreatic cancer from big data to improved individual survival. Function 4, zqad011 (2023).
Hayashi, A. et al. A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat. Cancer 1, 59–74 (2020).
Rashid, N. U. et al. Purity independent subtyping of tumors (PurIST), a clinically robust, single-sample classifier for tumor subtyping in pancreatic cancer. Clin. Cancer Res. 26, 82–92 (2020).
Kalimuthu, S. N. et al. Morphological classification of pancreatic ductal adenocarcinoma that predicts molecular subtypes and correlates with clinical outcome. Gut 69, 317–328 (2020).
O’Kane, G. M. et al. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin. Cancer Res. 26, 4901–4910 (2020).
Aung, K. L. et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin. Cancer Res. 24, 1344–1354 (2018).
Fuentes Antrás, J. et al. Molecular characterization of long-term and short-term survivors of advanced pancreatic ductal adenocarcinoma. J. Clin. Oncol. 40, 4024 (2022).
Suurmeijer, J. A. et al. Impact of classical and basal-like molecular subtypes on overall survival in resected pancreatic cancer in the SPACIOUS-2 multicentre study. Br. J. Surg. https://doi.org/10.1093/bjs/znac272 (2022).
Williams, H. L. et al. Spatially resolved single-cell assessment of pancreatic cancer expression subtypes reveals co-expressor phenotypes and extensive intratumoral heterogeneity. Cancer Res. 83, 441–455 (2023).
Adams, C. R. et al. Transcriptional control of subtype switching ensures adaptation and growth of pancreatic cancer. eLife 8, e45313 (2019).
Knox, J. J. et al. PASS-01: pancreatic adenocarcinoma signature stratification for treatment–01. J. Clin. Oncol. 40, TPS635 (2022).
Nicolle, R. et al. A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma. Ann. Oncol. 32, 250–260 (2021).
Nicolle, R. et al. Adjuvant gemcitabine is as efficient as mFOLFIRINOX in patients with GemPred + tumor signature and resected pancreatic adenocarcinoma (PDAC): an ancillary study of the PRODIGE-24 clinical trial. ESMO Congress 2022, Abstract 1297P (2022).
Reyngold, M. et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 7, 735–738 (2021).
Hassanzadeh, C. et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv. Radiat. Oncol. 6, 100506 (2021).
Chuong, M. D. et al. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract. Radiat. Oncol. 11, 134–147 (2021).
Hoffe, S. E. et al. GRECO-2: a randomized, phase 2 study of stereotactic body radiation therapy (SBRT) in combination with GC4711 in the treatment of unresectable or borderline resectable nonmetastatic pancreatic cancer (PC). J. Clin. Oncol. 39, TPS4175 (2021).
Tuli, R. et al. Abstract B58: a phase I/II study of durvalumab and stereotactic radiotherapy in locally advanced pancreatic cancer. Cancer Res. https://doi.org/10.1158/1538-7445.PANCA19-B58 (2019).
Bagley, A. F. et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: report of first patient experience. Clin. Transl. Radiat. Oncol. 33, 66–69 (2022).
Chatterjee, N. & Walker, G. C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 58, 235–263 (2017).
Heeke, A. L. et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis. Oncol. 2018, PO.17.00286 (2018).
Park, W. et al. Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selection. Clin. Cancer Res. 26, 3239–3247 (2020).
Lowery, M. A. et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 16, 1397–1402 (2011).
Golan, T. et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 111, 1132–1138 (2014).
Sonnenblick, A. et al. Complete remission, in BRCA2 mutation carrier with metastatic pancreatic adenocarcinoma, treated with cisplatin based therapy. Cancer Biol. Ther. 12, 165–168 (2011).
O’Reilly, E. M. et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J. Clin. Oncol. 38, 1378–1388 (2020).
Dasari, S. & Tchounwou, P. B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378 (2014).
Pommier, Y., O’Connor, M. J. & de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 8, 362ps17 (2016).
Lord, C. J. & Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 16, 110–120 (2016).
Park, J. H. et al. BRCA 1/2 germline mutation predicts the treatment response of FOLFIRINOX with pancreatic ductal adenocarcinoma in Korean patients. Cancers 14, 236 (2022).
Golan, T. et al. Increased rate of complete pathologic response after neoadjuvant FOLFIRINOX for BRCA mutation carriers with borderline resectable pancreatic cancer. Ann. Surg. Oncol. 27, 3963–3970 (2020).
Reiss, K. A. et al. Retrospective survival analysis of patients with advanced pancreatic ductal adenocarcinoma and germline BRCA or PALB2 mutations. JCO Precis. Oncol. https://doi.org/10.1200/po.17.00152 (2018).
Lowery, M. A. et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur. J. Cancer 89, 19–26 (2018).
Wattenberg, M. M. et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br. J. Cancer 122, 333–339 (2020).
Kindler, H. L. et al. Overall survival results from the POLO trial: a phase III study of active maintenance olaparib versus placebo for germline BRCA-mutated metastatic pancreatic cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.21.01604 (2022).
Reiss, K. A. et al. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol. 39, 2497–2505 (2021).
Brown, T. J. et al. The clinical implications of reversions in patients with advanced pancreatic cancer and pathogenic variants in BRCA1, BRCA2, or PALB2 after progression on rucaparib. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-23-1467 (2023).
Reiss, K. A. et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(22)00369-2 (2022).
Keane, F., Park, W. & O’Reilly, E. M. Homologous recombination deficiency in pancreatic cancer: poly (ADP-ribose) polymerase inhibition, checkpoint inhibition, or a combination of both? JCO Precis. Oncol. https://doi.org/10.1200/po.22.00141 (2022).
Park, W. et al. Clinico-genomic characterization of ATM and HRD in pancreas cancer: application for practice. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-22-1483 (2022).
Park, W. et al. Phase 2 trial of pembrolizumab and olaparib (POLAR) maintenance for patients (pts) with metastatic pancreatic cancer (mPDAC): two cohorts B non-core homologous recombination deficiency (HRD) and C exceptional response to platinum-therapy. J. Clin. Oncol. 41, 4140 (2023).
Li, H. et al. PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol. Cancer 19, 107 (2020).
Evers, B. et al. A high-throughput pharmaceutical screen identifies compounds with specific toxicity against BRCA2-deficient tumors. Clin. Cancer Res. 16, 99–108 (2010).
Wood, L. D. & Hruban, R. H. Pathology and molecular genetics of pancreatic neoplasms. Cancer J. 18, 492–501 (2012).
Kamisawa, T., Wood, L. D., Itoi, T. & Takaori, K. Pancreatic cancer. Lancet 388, 73–85 (2016).
Smit, V. T. et al. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 16, 7773–7782 (1988).
Almoguera, C. et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53, 549–554 (1988).
Joneson, T. & Bar-Sagi, D. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol. Cell Biol. 19, 5892–5901 (1999).
Bonni, A. et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 (1999).
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 (1990).
Huang, L., Guo, Z., Wang, F. & Fu, L. KRAS mutation: from undruggable to druggable in cancer. Signal. Transduct. Target. Ther. 6, 386 (2021).
Waters, A. M. & Der, C. J. KRAS: The critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 8, a031435 (2018).
Gibbs, J. B., Sigal, I. S., Poe, M. & Scolnick, E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc. Natl Acad. Sci. USA 81, 5704–5708 (1984).
Tong, L. A., de Vos, A. M., Milburn, M. V. & Kim, S. H. Crystal structures at 2.2 A resolution of the catalytic domains of normal ras protein and an oncogenic mutant complexed with GDP. J. Mol. Biol. 217, 503–516 (1991).
Berndt, N., Hamilton, A. D. & Sebti, S. M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 11, 775–791 (2011).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Lim, S. M. et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew. Chem. Int. Ed. 53, 199–204 (2014).
Prior, I. A., Lewis, P. D. & Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 72, 2457–2467 (2012).
Biernacka, A. et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 209, 195–198 (2016).
Bekaii-Saab, T. S. et al. KRYSTAL-1: updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. J. Clin. Oncol. 40, 519 (2022).
Hallin, J. et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Pant, S. et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with advanced solid tumors harboring a KRASG12C mutation. J. Clin. Oncol. 41, 425082 (2023).
Lanman, B. A. et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J. Med. Chem. 63, 52–65 (2020).
Strickler, J. H. et al. Sotorasib in KRAS p.G12C-mutated advanced pancreatic cancer. N. Engl. J. Med. 388, 33–43 (2023).
Li, J. et al. A phase I/II study of first-in-human trial of JAB-21822 (KRAS G12C inhibitor) in advanced solid tumors. J. Clin. Oncol. 40, 3089 (2022).
Bournet, B. et al. KRAS G12D mutation subtype is a prognostic factor for advanced pancreatic adenocarcinoma. Clin. Transl. Gastroenterol. 7, e157 (2016).
Li, S., Balmain, A. & Counter, C. M. A model for RAS mutation patterns in cancers: finding the sweet spot. Nat. Rev. Cancer 18, 767–777 (2018).
Wang, X. et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS(G12D) inhibitor. J. Med. Chem. 65, 3123–3133 (2022).
Kemp, S. B. et al. Efficacy of a small-molecule inhibitor of KrasG12D in immunocompetent models of pancreatic cancer. Cancer Discov. 13, 298–311 (2023).
Hallin, J. et al. Anti-tumor efficacy of a potent and selective non-covalent KRASG12D inhibitor. Nat. Med. 28, 2171–2182 (2022).
Zhang, Z. & Shokat, K. M. Bifunctional small-molecule ligands of K-Ras induce its association with immunophilin proteins. Angew. Chem. Int. Ed. 58, 16314–16319 (2019).
Gustafson, W. C. et al. Direct targeting of RAS in pancreatic ductal adenocarcinoma with RMC-6236, a first-in-class, RAS-selective, orally bioavailable, tri-complex RASMULTI(ON) inhibitor. J. Clin. Oncol. 40, 591 (2022).
Gentile, D. R. et al. Ras binder induces a modified switch-II pocket in GTP and GDP states. Cell Chem. Biol. 24, 1455–1466.e14 (2017).
McGee, J. H. et al. Exceptionally high-affinity Ras binders that remodel its effector domain. J. Biol. Chem. 293, 3265–3280 (2018).
Hillig, R. C. et al. Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS-SOS1 interaction. Proc. Natl Acad. Sci. USA 116, 2551–2560 (2019).
Hofmann, M. H. et al. BI-3406, a potent and selective SOS1-KRAS interaction inhibitor, is effective in KRAS-driven cancers through combined MEK inhibition. Cancer Discov. 11, 142–157 (2021).
Punekar, S. R., Velcheti, V., Neel, B. G. & Wong, K.-K. The current state of the art and future trends in RAS-targeted cancer therapies. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/s41571-022-00671-9 (2022).
Bery, N., Miller, A. & Rabbitts, T. A potent KRAS macromolecule degrader specifically targeting tumours with mutant KRAS. Nat. Commun. 11, 3233 (2020).
Nagashima, T. et al. ASP3082, a First-in-class novel KRAS G12D degrader, exhibits remarkable anti-tumor activity in KRAS G12D mutated cancer models. Eur. J. Cancer 174, S30 (2022).
Pant, S. et al. First-in-human phase 1 trial of ELI-002 immunotherapy as treatment for subjects with Kirsten rat sarcoma (KRAS)-mutated pancreatic ductal adenocarcinoma and other solid tumors. J. Clin. Oncol. 40, TPS2701 (2022).
O’Reilly, E. M. et al. AMPLIFY-201, a first-in-human safety and efficacy trial of adjuvant ELI-002 2P immunotherapy for patients with high-relapse risk with KRAS G12D- or G12R-mutated pancreatic and colorectal cancer. J. Clin. Oncol. 41, 2528 (2023).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Surana, R. et al. Phase I study of mesenchymal stem cell (MSC)-derived exosomes with KRASG12D siRNA in patients with metastatic pancreatic cancer harboring a KRASG12D mutation. J. Clin. Oncol. 40, TPS633 (2022).
Varghese, A. M. et al. Early-onset pancreas cancer: clinical descriptors, genomics, and outcomes. J. Natl Cancer Inst. 113, 1194–1202 (2021).
Heining, C. et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov. 8, 1087–1095 (2018).
Singhi, A. D. et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology 156, 2242–2253.e4 (2019).
Lee, M. S. & Pant, S. Personalizing medicine with germline and somatic sequencing in advanced pancreatic cancer: current treatments and novel opportunities. Am. Soc. Clin. Oncol. Educ. Book https://doi.org/10.1200/EDBK_321255 (2021).
Luchini, C. et al. KRAS wild-type pancreatic ductal adenocarcinoma: molecular pathology and therapeutic opportunities. J. Exp. Clin. Cancer Res. 39, 227 (2020).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 21, 271–282 (2020).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
O’Reilly, E. M. & Hechtman, J. F. Tumour response to TRK inhibition in a patient with pancreatic adenocarcinoma harbouring an NTRK gene fusion. Ann. Oncol. 30, viii36–viii40 (2019).
Pishvaian, M. J. et al. Entrectinib in TRK and ROS1 fusion-positive metastatic pancreatic cancer. JCO Precis. Oncol. https://doi.org/10.1200/PO.18.00039 (2018).
Gower, A., Golestany, B., Gong, J., Singhi, A. D. & Hendifar, A. E. Novel ALK fusion, PPFIBP1-ALK, in pancreatic ductal adenocarcinoma responsive to alectinib and lorlatinib. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00365 (2020).
Hyman, D. et al. 365O – Durability of response with larotrectinib in adult and pediatric patients with TRK fusion cancer. Ann. Oncol. https://doi.org/10.1093/annonc/mdz431.002 (2019).
Schram, A. M. et al. Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-21-1119 (2022).
Ueda, S. et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 29, e1–e8 (2004).
Bruns, C. J. et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 60, 2926–2935 (2000).
Ng, S. S., Tsao, M. S., Nicklee, T. & Hedley, D. W. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol. Cancer Ther. 1, 777–783 (2002).
Van Cutsem, E. et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 27, 2231–2237 (2009).
Philip, P. A. et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J. Clin. Oncol. 28, 3605–3610 (2010).
Moore, M. J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 25, 1960–1966 (2007).
Qin, S. et al. Nimotuzumab combined with gemcitabine versus gemcitabine in K-RAS wild-type locally advanced or metastatic pancreatic cancer: a prospective, randomized-controlled, double-blinded, multicenter, and phase III clinical trial. J. Clin. Oncol. 40, LBA4011 (2022).
Balachandran, V. P. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017).
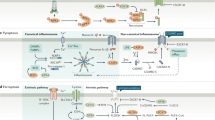
Grünwald, B. T. et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 184, 5577–5592.e18 (2021).
Clark, C. E. et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 67, 9518–9527 (2007).
Zhang, Y. et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66, 124–136 (2017).
Hegde, S. et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37, 289–307.e9 (2020).
Lin, J. H. et al. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. 217, e20190673 (2020).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv4 (2016).
O’Reilly, E. M. et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 5, 1431–1438 (2019).
Terrero, G. et al. Ipilimumab/nivolumab therapy in patients with metastatic pancreatic or biliary cancer with homologous recombination deficiency pathogenic germline variants. JAMA Oncol. 8, 1–3 (2022).
Kim, A. M. J., Nemeth, M. R. & Lim, S.-O. 4-1BB: a promising target for cancer immunotherapy. Front. Oncol. 12, 968360 (2022).
Muth, S. T. et al. CD137 agonist-based combination immunotherapy enhances activated, effector memory T cells and prolongs survival in pancreatic adenocarcinoma. Cancer Lett. 499, 99–108 (2021).
Zheng, L. et al. 812 Urelumab (anti-CD137 agonist) in combination with vaccine and nivolumab treatments is safe and associated with pathologic response as neoadjuvant and adjuvant therapy for resectable pancreatic cancer. J. Immunother. Cancer 8, A486 (2020).
Vonderheide, R. H. CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020).
Grewal, I. S. & Flavell, R. A. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 153, 85–106 (1996).
Ma, D. Y. & Clark, E. A. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 21, 265–272 (2009).
Byrne, K. T. & Vonderheide, R. H. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 15, 2719–2732 (2016).
Winograd, R. et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 3, 399–411 (2015).
O’Hara, M. H. et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. 22, 118–131 (2021).
Padron, L. J. et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 28, 1167–1177 (2022).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Chavez-Galan, L., Olleros, M. L., Vesin, D. & Garcia, I. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front. Immunol. 6, 263 (2015).
Yoshikawa, K. et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 103, 2012–2020 (2012).
Cannarile, M. A. et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 5, 53 (2017).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
Mitchem, J. B. et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 (2013).
Candido, J. B. et al. CSF1R+ macrophages sustain pancreatic tumor growth through T cell suppression and maintenance of key gene programs that define the squamous subtype. Cell Rep. 23, 1448–1460 (2018).
Magkouta, S. F. et al. CSF1/CSF1R axis blockade limits mesothelioma and enhances efficiency of anti-PDL1 immunotherapy. Cancers 13, 2546 (2021).
Wang-Gillam, A. et al. A randomized phase II study of cabiralizumab (cabira) + nivolumab (nivo) ± chemotherapy (chemo) in advanced pancreatic ductal adenocarcinoma (PDAC). J. Clin. Oncol. 37, TPS465 (2019).
Columbus, G. Nivolumab/Cabiralizumab Combo Misses PFS Endpoint in Pancreatic Cancer https://www.onclive.com/view/nivolumabcabiralizumab-combo-misses-pfs-endpoint-in-pancreatic-cancer (2020).
Li, B.-H., Garstka, M. A. & Li, Z.-F. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol. Immunol. 117, 201–215 (2020).
Sanford, D. E. et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 19, 3404–3415 (2013).
Nywening, T. M. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 17, 651–662 (2016).
Noel, M. et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest. New Drugs 38, 800–811 (2020).
Cherney, R. J. et al. BMS-813160: a potent CCR2 and CCR5 dual antagonist selected as a clinical candidate. ACS Med. Chem. Lett. 12, 1753–1758 (2021).
Le, D. et al. Abstract CT124: a phase Ib/II study of BMS-813160, a CC chemokine receptor (CCR) 2/5 dual antagonist, in combination with chemotherapy or nivolumab in patients (pts) with advanced pancreatic or colorectal cancer. Cancer Res. 78, CT124 (2018).
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016).
Caligiuri, G. & Tuveson, D. A. Activated fibroblasts in cancer: perspectives and challenges. Cancer Cell 41, 434–449 (2023).
Huang, H. et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell 40, 656–673.e7 (2022).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Sherman, M. H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014).
Perez, K. et al. Vitamin D receptor agonist paricalcitol plus gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer. J. Clin. Oncol. 38, TPS784 (2020).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
Haas, A. R. et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 27, 1919–1929 (2019).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
Leidner, R. et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N. Engl. J. Med. 386, 2112–2119 (2022).
Melief, C. J. M. T-cell immunotherapy against mutant KRAS for pancreatic cancer. N. Engl. J. Med. 386, 2143–2144 (2022).
Balachandran, V. P. et al. Phase I trial of adjuvant autogene cevumeran, an individualized mRNA neoantigen vaccine, for pancreatic ductal adenocarcinoma. J. Clin. Oncol. 40, 2516 (2022).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Haldar, S. D. et al. A phase I study of a mutant KRAS-targeted long peptide vaccine combined with ipilimumab/nivolumab in resected pancreatic cancer and MMR-proficient metastatic colorectal cancer. J. Clin. Oncol. 41, TPS814 (2023).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806.e12 (2019).
Tanaka, M. et al. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J. Histochem. Cytochem. 59, 942–952 (2011).
Sahin, U. et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 14, 7624–7634 (2008).
Overman, M. J. et al. A phase I, first-in-human, open-label, dose escalation and expansion study of PT886 in adult patients with advanced gastric, gastroesophageal junction, and pancreatic adenocarcinomas. J. Clin. Oncol. 41, TPS765 (2023).
Körner, M., Waser, B., Strobel, O., Büchler, M. & Reubi, J. C. Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. 5, 17 (2015).
Yin, X. et al. Evaluation of neurotensin receptor 1 as a potential imaging target in pancreatic ductal adenocarcinoma. Amino Acids 49, 1325–1335 (2017).
Baum, R. P. et al. 177Lu-3BP-227 for neurotensin receptor 1-targeted therapy of metastatic pancreatic adenocarcinoma: first clinical results. J. Nucl. Med. 59, 809–814 (2018).
Dean, A. et al. Dual αV-integrin and neuropilin-1 targeting peptide CEND-1 plus nab-paclitaxel and gemcitabine for the treatment of metastatic pancreatic ductal adenocarcinoma: a first-in-human, open-label, multicentre, phase 1 study. Lancet Gastroenterol. Hepatol. 7, 943–951 (2022).
Hurtado de Mendoza, T. et al. Tumor-penetrating therapy for β5 integrin-rich pancreas cancer. Nat. Commun. 12, 1541 (2021).
Sugahara, K. N. et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328, 1031–1035 (2010).
Kasi, A. et al. Phase Ib/IIa trial of CEND‐1 in combination with neoadjuvant FOLFIRINOX-based therapies in pancreatic, colorectal, and appendiceal cancers (CENDIFOX). J. Clin. Oncol. 40, TPS4195 (2022).
Bates, S. E. Pancreatic cancer: challenge and inspiration. Clin. Cancer Res. 23, 1628 (2017).
Van Norman, G. A. Drugs, devices, and the FDA: part 1: an overview of approval processes for drugs. JACC Basic Transl. Sci. 1, 170–179 (2016).
Li, A. & Bergan, R. C. Clinical trial design: past, present, and future in the context of big data and precision medicine. Cancer 126, 4838–4846 (2020).
FDA. Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics Guidance for Industry https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and (2022).
Bogin, V. Master protocols: new directions in drug discovery. Contemp. Clin. Trials Commun. 18, 100568 (2020).
Chung, V. et al. SO-4 phase Ib/II, open-label, randomised evaluation of atezolizumab plus RO6874281 vs control in MORPHEUS–pancreatic ductal adenocarcinoma. Ann. Oncol. 31 (Suppl. 3), S218 (2020).
Pellat, A., Boutron, I. & Ravaud, P. Availability of results of trials studying pancreatic adenocarcinoma over the past 10 years. Oncologist https://doi.org/10.1093/oncolo/oyac156 (2022).
Adamska, A., Domenichini, A. & Falasca, M. Pancreatic ductal adenocarcinoma: current and evolving therapies. Int. J. Mol. Sci. 18, 1338 (2017).
Wang-Gillam, A. et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387, 545–557 (2016).
Van Cutsem, E. et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol. 38, 3185–3194 (2020).
Philip, P. A. et al. Avenger 500, a phase III open-label randomized trial of the combination of CPI-613 with modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas. J. Clin. Oncol. 37, TPS479 (2019).
Sonbol, M. B. et al. CanStem111P trial: a phase III study of napabucasin plus nab-paclitaxel with gemcitabine. Future Oncol. 15, 1295–1302 (2019).
Tempero, M. et al. Ibrutinib in combination with nab-paclitaxel and gemcitabine for first-line treatment of patients with metastatic pancreatic adenocarcinoma: phase III RESOLVE study. Ann. Oncol. 32, 600–608 (2021).
Hecht, J. R. et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J. Clin. Oncol. 39, 1108–1118 (2021).
Tempero, M. A. et al. Adjuvant nab-paclitaxel + gemcitabine in resected pancreatic ductal adenocarcinoma: results from a randomized, open-label, phase III trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.22.01134 (2022).
Acknowledgements
Cancer Center Support Grant/Core Grant: P30 CA008748. SPORE: 1P50CA257881-01A1.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
Z.I.H. declares no competing interests. E.M.O. receives institutional research funding from Genentech-Roche, Celgene–Bristol-Myers Squibb, BioNTech, AstraZeneca, Arcus, Elicio Therapeutics, Parker Institute, and Pertzye and has consulting, advisory and/or Steering Committee roles for Boehringer Ingelheim, BioNTech, Ipsen, Merck, Novartis, AstraZeneca, BioSapien, Astellas, BMS, Thetis, Autem, Novogene, Tempus, Agios, Genentech-Roche, Eisai, Merus, Genentech-Roche, Servier, Syros, and Leap Therapeutics.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks John Neoptolemos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Z.I., O’Reilly, E.M. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol 21, 7–24 (2024). https://doi.org/10.1038/s41575-023-00840-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00840-w