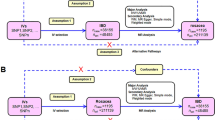
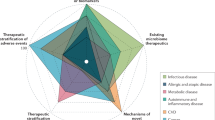
Abstract
Owing to advances in genomics that enable differentiation of molecular aetiologies, patients with monogenic inflammatory bowel disease (mIBD) potentially have access to genotype-guided precision medicine. In this Expert Recommendation, we review the therapeutic research landscape of mIBD, the reported response to therapies, the medication-related risks and systematic bias in reporting. The mIBD field is characterized by the absence of randomized controlled trials and is dominated by retrospective observational data based on case series and case reports. More than 25 off-label therapeutics (including small-molecule inhibitors and biologics) as well as cellular therapies (including haematopoietic stem cell transplantation and gene therapy) have been reported. Heterogeneous reporting of outcomes impedes the generation of robust therapeutic evidence as the basis for clinical decision making in mIBD. We discuss therapeutic goals in mIBD and recommend standardized reporting (mIBD REPORT (monogenic Inflammatory Bowel Disease Report Extended Phenotype and Outcome of Treatments) standards) to stratify patients according to a genetic diagnosis and phenotype, to assess treatment effects and to record safety signals. Implementation of these pragmatic standards should help clinicians to assess the therapy responses of individual patients in clinical practice and improve comparability between observational retrospective studies and controlled prospective trials, supporting future meta-analysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Uhlig, H. H. et al. Clinical genomics for the diagnosis of monogenic forms of inflammatory bowel disease: a position paper from the Paediatric IBD Porto group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 72, 456–473 (2021).
Bolton, C. et al. An integrated taxonomy for monogenic inflammatory bowel disease. Gastroenterology 122, 859–876 (2022).
Uhlig, H. H. & Powrie, F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu. Rev. Immunol. 36, 755–781 (2018).
Charbit-Henrion, F., Parlato, M., Malamut, G., Ruemmele, F. & Cerf-Bensussan, N. Intestinal immunoregulation: lessons from human mendelian diseases. Mucosal Immunol. 14, 1017–1037 (2021).
Sullivan, K. E., Conrad, M. & Kelsen, J. R. Very early-onset inflammatory bowel disease: an integrated approach. Curr. Opin. Allergy Clin. Immunol. 18, 459–469 (2018).
Ouahed, J. et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm. Bowel Dis. 26, 820–842 (2020).
Kammermeier, J. et al. Genomic diagnosis and care co-ordination for monogenic inflammatory bowel disease in children and adults: consensus guideline on behalf of the British Society of Gastroenterology and British Society of Paediatric Gastroenterology, Hepatology and Nutrition. Lancet Gasteroenterol. Hepatol. 8, 271–286 (2023).
Crowley, E. et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology 158, 2208–2220 (2020).
Nambu, R. et al. A systematic review of monogenic inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 20, e653–e663 (2022).
Crowley, E. et al. Impact of drug approval pathways for paediatric inflammatory bowel disease. J. Crohns Colitis 23, 331–335 (2021).
Both, T. et al. Inflammatory bowel disease in primary immunodeficiency disorders is a heterogeneous clinical entity requiring an individualized treatment strategy: a systematic review. Autoimmun. Rev. 20, 102872 (2021).
Boztug, K. et al. Stem-cell gene therapy for the Wiskott–Aldrich syndrome. N. Engl. J. Med. 363, 1918–1927 (2010).
Kawai, T. et al. A gene therapy clinical study of a patient with X-linked chronic granulomatous disease. Mol. Ther. 24, S87–S88 (2016).
Kohn, D. B. et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat. Med. 26, 200–206 (2020).
Glocker, E.-O. et al. Infant colitis – it’s in the genes. Lancet 376, 1272 (2010).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Forbes, L. R. et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J. Allergy Clin. Immunol. 142, 1665–1669 (2018).
Rudra, S. et al. Ruxolitinib: targeted approach for treatment of autoinflammatory very early onset inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 20, 1408–1410 (2022).
Geerlinks, A. V., Dvorak, A. M. & XIAP Deficiency Treatment Consortium. A case of XIAP deficiency successfully managed with tadekinig alfa (rhIL-18BP). J. Clin. Immunol. 42, 901–903 (2022).
Egg, D. et al. Therapeutic options for CTLA-4 insufficiency. J. Allergy Clin. Immunol. 149, 736–746 (2022).
Kammermeier, J. et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J. Crohns Colitis 11, 60–69 (2017).
Torres, J. et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J. Crohns Colitis 14, 4–22 (2020).
Raine, T. et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J. Crohns Colitis 16, 2–17 (2022).
Passerini, L. et al. Treatment with rapamycin can restore regulatory T-cell function in IPEX patients. J. Allergy Clin. Immunol. 145, 1262–1271.e13 (2020).
Azizi, G. et al. New therapeutic approach by sirolimus for enteropathy treatment in patients with LRBA deficiency. Eur. Ann. Allergy Clin. Immunol. 49, 235–239 (2017).
Maccari, M. E. et al. Disease evolution and response to rapamycin in activated phosphoinositide 3-kinase δ syndrome: the European Society for Immunodeficiencies–Activated Phosphoinositide 3-Kinase δ Syndrome Registry. Front. Immunol. 9, 543 (2018).
Gruber, C. N. et al. Complex autoinflammatory syndrome unveils fundamental principles of JAK1 kinase transcriptional and biochemical function. Immunity 53, 672 (2020).
Talbot, J. W. et al. Management of enteritis associated with tricohepatoenteric syndrome due to SKIV2L mutation using the combination of JAK1/2 inhibition and azathioprine. JPGN Rep. 3, e264 (2022).
Ye, Z. et al. Clinical and genetic spectrum of children with congenital diarrhea and enteropathy in China. Genet. Med. 21, 2224–2230 (2019).
Noel, N. et al. Efficacy and safety of thalidomide in patients with inflammatory manifestations of chronic granulomatous disease: a retrospective case series. J. Allergy Clin. Immunol. 132, 997–1000.e4 (2013).
Dai, R. et al. Altered functions of neutrophils in two Chinese patients with severe congenital neutropenia type 4 caused by G6PC3 mutations. Front. Immunol. 12, 699743 (2021).
Sokol, H. et al. Thalidomide as a treatment for refractory CGD colitis. Am. J. Gastroenterol. 104, 1069 (2009).
Kuloglu, Z. et al. An infant with severe refractory Crohn’s disease and homozygous MEFV mutation who dramatically responded to colchicine. Rheumatol. Int. 32, 783–785 (2012).
Veenbergen, S. et al. IL-10 signaling in dendritic cells controls IL-1β-mediated IFNγ secretion by human CD4+ T cells: relevance to inflammatory bowel disease. Mucosal Immunol. 12, 1201–1211 (2019).
Egg, D. et al. Increased risk for malignancies in 131 affected CTLA4 mutation carriers. Front. Immunol. 9, 2012 (2018).
Uhlig, H. H. Mendelian diseases and inflammatory bowel disease – data mining for genetic risk and disease-associated confounders. Inflamm. Bowel Dis. 24, 467–470 (2018).
Schmitt, M. M. et al. Mycophenolate-induced colitis in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. JPGN Rep. 2, e131 (2021).
Naviglio, S. et al. Severe inflammatory bowel disease associated with congenital alteration of transforming growth factor beta signaling. J. Crohns Colitis 8, 770–774 (2014).
van Andel, M. M. et al. Long-term clinical outcomes of losartan in patients with Marfan syndrome: follow-up of the multicentre randomized controlled COMPARE trial. Eur. Heart J. 41, 4181–4187 (2020).
Asgharzadeh, F. et al. The beneficial effect of combination therapy with sulfasalazine and valsartan in the treatment of ulcerative colitis. EXCLI J. 20, 236–247 (2021).
Wengrower, D. et al. Losartan reduces trinitrobenzene sulphonic acid-induced colorectal fibrosis in rats. Can. J. Gastroenterol. 26, 33–39 (2012).
Liu, T. J., Shi, Y. Y., Wang, E. B., Zhu, T. & Zhao, Q. AT1R blocker losartan attenuates intestinal epithelial cell apoptosis in a mouse model of Crohn’s disease. Mol. Med. Rep. 13, 1156–1162 (2016).
Bolton, C. et al. Remission of inflammatory bowel disease in glucose-6-phosphatase 3 deficiency by allogeneic haematopoietic stem cell transplantation. J. Crohns Colitis 14, 142–147 (2020).
Dieckgraefe, B. K., Korzenik, J. R., Husain, A. & Dieruf, L. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur. J. Pediatr. 161, S88–S92 (2002).
Veiga-da-Cunha, M. et al. Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency. Proc. Natl Acad. Sci. USA 116, 1241–1250 (2019).
Wortmann, S. B. et al. Treating neutropenia and neutrophil dysfunction in glycogen storage disease type Ib with an SGLT2 inhibitor. Blood 136, 1033–1043 (2020).
Grunert, S. C. et al. Improved inflammatory bowel disease, wound healing and normal oxidative burst under treatment with empagliflozin in glycogen storage disease type Ib. Orphanet J. Rare Dis. 15, 218 (2020).
Williams, I. et al. Anti-TNF therapy for inflammatory bowel disease in patients with neurodegenerative Niemann–Pick disease type C. Wellcome Open Res. 7, 11 (2022).
O’Brien, K. J. et al. Inflammatory bowel disease in HermanskyPudlak syndrome: a retrospective single-centre cohort study. J. Intern. Med. 290, 129–140 (2021).
Mao, L. et al. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn’s disease. J. Clin. Invest. 128, 1793–1806 (2018).
Levy, M. et al. Severe early-onset colitis revealing mevalonate kinase deficiency. Pediatrics 132, e779–e783 (2013).
Shouval, D. S. et al. Interleukin 1β mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterology 151, 1100–1104 (2016).
Li, J., Shouval, D. S., Doty, A. L., Snapper, S. B. & Glover, S. C. Increased mucosal IL-22 production of an IL-10RA mutation patient following anakin treatment suggests further mechanism for mucosal healing. J. Clin. Immunol. 37, 104–107 (2017).
Canna, S. W. et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 139, 1698–1701 (2017).
Wada, T. et al. Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine 65, 74–78 (2014).
Lo, B. et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 349, 436–440 (2015).
Tesch, V. K. et al. Long-term outcome of LRBA deficiency in 76 patients after various treatment modalities as evaluated by the immune deficiency and dysregulation activity (IDDA) score. J. Allergy Clin. Immunol. 145, 1452–1463 (2020).
Ozen, A. et al. Broadly effective metabolic and immune recovery with C5 inhibition in CHAPLE disease. Nat. Immunol. 22, 128–139 (2021).
Danese, S., Solitano, V., Jairath, V. & Peyrin-Biroulet, L. The future of drug development for inflammatory bowel disease: the need to ACT (advanced combination treatment). Gut 71, 2380–2387 (2022).
Hudson, A. A., Almeida, P. & Huynh, H. Dual biologic therapy in a patient with Niemann–Pick type C and Crohn disease: a case report and literature review. JPGN Rep. 3, e225 (2022).
Goenka, A. et al. Neutrophil dysfunction triggers inflammatory bowel disease in G6PC3 deficiency. J. Leukoc. Biol. 109, 1147–1154 (2021).
Barmettler, S. et al. Gastrointestinal manifestations in X-linked agammaglobulinemia. J. Clin. Immunol. 37, 287–294 (2017).
Aydemir, S. et al. Inflammatory bowel disease and Guillain Barre syndrome in FCHO1 deficiency. J. Clin. Immunol. 41, 1406–1410 (2021).
Marsh, R. A. et al. Chronic granulomatous disease-associated IBD resolves and does not adversely impact survival following allogeneic HCT. J. Clin. Immunol. 39, 653–667 (2019).
Peng, K. et al. Umbilical cord blood transplantation corrects very early-onset inflammatory bowel disease in Chinese patients with IL10RA-associated immune deficiency. Inflamm. Bowel Dis. 24, 1416–1427 (2018).
Ashton, J. J., Gavin, J. & Beattie, R. M. Exclusive enteral nutrition in Crohn’s disease: evidence and practicalities. Clin. Nutr. 38, 80–89 (2019).
Murugan, D. et al. Very early onset inflammatory bowel disease associated with aberrant trafficking of IL-10R1 and cure by T cell replete haploidentical bone marrow transplantation. J. Clin. Immunol. 34, 331–339 (2014).
Freudenberg, F. et al. Therapeutic strategy in p47-phox deficient chronic granulomatous disease presenting as inflammatory bowel disease. J. Allergy Clin. Immunol. 125, 943–946.e1 (2010).
Norsa, L. et al. Inflammatory bowel disease in patients with congenital chloride diarrhoea. J. Crohns Colitis 15, 1679–1685 (2021).
Janecke, A. R. et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 24, 6614–6623 (2015).
Fiskerstrand, T. et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N. Engl. J. Med. 366, 1586–1595 (2012).
Sokol, H. et al. Intestinal dysbiosis in inflammatory bowel disease associated with primary immunodeficiency. J. Allergy Clin. Immunol. 143, 775–778.e6 (2019).
Xue, A. J. et al. Intestinal dysbiosis in pediatric Crohn’s disease patients with IL10RA mutations. World J. Gastroenterol. 26, 3098–3109 (2020).
Wang, L. et al. Gain-of-function variants in SYK cause immune dysregulation and systemic inflammation in humans and mice. Nat. Genet. 53, 500–510 (2021).
Ono, S. et al. Hematopoietic cell transplantation rescues inflammatory bowel disease and dysbiosis of gut microbiota in XIAP deficiency. J. Allergy Clin. Immunol. Pract. 9, 3767–3780 (2021).
Huang, Z. et al. Mutations in interleukin-10 receptor and clinical phenotypes in patients with very early onset inflammatory bowel disease: a Chinese VEO-IBD Collaboration Group survey. Inflamm. Bowel Dis. 23, 578–590 (2017).
Quaranta, M. et al. Consequences of identifying XIAP deficiency in an adult patient with inflammatory bowel disease. Gastroenterology 155, 231–234 (2018).
Arnold, D. E. et al. Reduced-intensity/reduced-toxicity conditioning approaches are tolerated in XIAP deficiency but patients fare poorly with acute GVHD. J. Clin. Immunol. 42, 36–45 (2022).
Ruemmele, F. M. et al. Outcome measures for clinical trials in paediatric IBD: an evidence-based, expert-driven practical statement paper of the paediatric ECCO committee. Gut 64, 438–446 (2015).
Turner, D. et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 160, 1570–1583 (2021).
Opréa, A. C.-F. et al. Therapeutic approach of very early-onset inflammatory bowel disease in a Loeys–Dietz syndrome child. JPGN Rep. 3, e139 (2022).
Attauabi, M. et al. Comparative onset of effect of biologics and small molecules in moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. EClinicalMedicine 57, 101866 (2023).
Dike, C. R., Bernat, J., Bishop, W. & DeGeeter, C. Niemann–Pick disease type C presenting as very early onset inflammatory bowel disease. BMJ Case Rep. 12, e229780 (2019).
Uzel, G. et al. Complications of tumor necrosis factor-α blockade in chronic granulomatous disease-related colitis. Clin. Infect. Dis. 51, 1429–1434 (2010).
Conrad, A. et al. Infections in patients with chronic granulomatous disease treated with tumor necrosis factor alpha blockers for inflammatory complications. J. Clin. Immunol. 41, 185–193 (2021).
Braun, C. J. et al. Gene therapy for Wiskott-Aldrich syndrome – long-term efficacy and genotoxicity. Sci. Transl Med. 6, 227–233 (2014).
Vogelin, M. et al. The impact of azathioprine-associated lymphopenia on the onset of opportunistic infections in patients with inflammatory bowel disease. PLoS ONE 11, e0155218 (2016).
Nori, M. ‘Negative’ clinical trials in rare diseases and beyond: reclassification and potential solutions. Future Rare Dis. 1, 10.2217/frd-2020-0005 (2021).
de Luca, A. et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl Acad. Sci. USA 111, 3526–3531 (2014).
Hahn, K. J. et al. Treatment with anakinra, a recombinant IL-1 receptor antagonist, unlikely to induce lasting remission in patients with CGD colitis. Am. J. Gastroenterol. 110, 938–939 (2015).
Aschenbrenner, D. et al. Pathogenic interleukin-10 receptor alpha variants in humans – balancing natural selection and clinical implications. J. Clin. Immunol. 43, 495–511 (2023).
Marsh, R. A. et al. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood 121, 877–883 (2013).
Kuehn, H. S. et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345, 1623–1627 (2014).
Schubert, D. et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 20, 1410–1416 (2014).
Gagne, J. J., Thompson, L., O’Keefe, K. & Kesselheim, A. S. Innovative research methods for studying treatments for rare diseases: methodological review. BMJ 349, g6802 (2014).
Whicher, D., Philbin, S. & Aronson, N. An overview of the impact of rare disease characteristics on research methodology. Orphanet J. Rare Dis. 13, 14 (2018).
Santoro, M. et al. Rare disease registries classification and characterization: a data mining approach. Public Health Genomics 18, 113–122 (2015).
European Commission. Set of common data elements for rare diseases registration. EU RD Platform https://eu-rd-platform.jrc.ec.europa.eu/set-of-common-data-elements_en (2023).
Salter, C. G. et al. Biallelic PI4KA variants cause neurological, intestinal and immunological disease. Brain 144, 3597–3610 (2021).
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research (US Department of Health and Human Services, 1979).
Borysowski, J., Ehni, H. J. & Gorski, A. Ethics review in compassionate use. BMC Med. 15, 136 (2017).
Vohra, S. et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 Statement. J. Clin. Epideminol 76, 9–17 (2016).
Turner, D. et al. Designing clinical trials in paediatric inflammatory bowel diseases: a PIBDnet commentary. Gut 69, 32–41 (2020).
Faingelernt, Y. et al. Correlation between the Nancy Histopathology Index and markers of disease activity in pediatric ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 76, 782–785 (2023).
Ricciuto, A. et al. Prospective evaluation of endoscopic and histologic indices in pediatric ulcerative colitis using centralized review. Am. J. Gastroenterol. 116, 2052–2059 (2021).
Walsh, A. J., Bryant, R. V. & Travis, S. P. Current best practice for disease activity assessment in IBD. Nat. Rev. Gastroenterol. Hepatol. 13, 567–579 (2016).
Tran, F. et al. Patient reported outcomes in chronic inflammatory diseases: current state, limitations and perspectives. Front. Immunol. 12, 614653 (2021).
Grassly, N. C. et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double-blind randomised placebo-controlled trial in seronegative Indian infants. Lancet Infect. Dis. 16, 905–914 (2016).
Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 29, 49–58 (2023).
Raine, T. et al. Establishment of a validated central reading system for ileocolonoscopy in an academic setting. Gut 71, 661–664 (2022).
Byrne, M. F. et al. Application of deep learning models to improve ulcerative colitis endoscopic disease activity scoring under multiple scoring systems. J. Crohns Colitis 19, 463–471 (2023).
Sutton, R. T., Zai Ane, O. R., Goebel, R. & Baumgart, D. C. Artificial intelligence enabled automated diagnosis and grading of ulcerative colitis endoscopy images. Sci. Rep. 12, 2748 (2022).
Crowley, E., Griffiths, A. M. & Jairath, V. pIBD Clinical Trials Outcome Group Heterogeneity in efficacy and safety endpoints for pediatric clinical trials in inflammatory bowel disease: a need for harmonization. Gastroenterology 163, 1137–1144 (2022).
Cavounidis, A. et al. Hermansky-Pudlak syndrome type 1 causes impaired anti-microbial immunity and inflammation due to dysregulated immunometabolism. Mucosal Immunol. 15, 1431–1446 (2022).
Friedrich, M. et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27, 1970–1981 (2021).
Huang, C. et al. Genetic risk for inflammatory bowel disease is a determinant of Crohn’s disease development in chronic granulomatous disease. Inflamm. Bowel Dis. 22, 2794–2801 (2016).
Jardine, S. et al. Drug screen identifies leflunomide for treatment of inflammatory bowel disease caused by TTC7A deficiency. Gastroenterology 158, 1000–1015 (2020).
Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S. & Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356, 513–519 (2017).
Omarjee, O. et al. LACC1 deficiency links juvenile arthritis with autophagy and metabolism in macrophages. J. Exp. Med. 218, e20201006 (2021).
Coulter, T. I. & Cant, A. J. The treatment of activated PI3Kδ syndrome. Front. Immunol. 9, 2043 (2018).
Minshawi, F. et al. The generation of an engineered interleukin-10 protein with improved stability and biological function. Front. Immunol. 11, 1794 (2020).
Zurita-Turk, M. et al. Attenuation of intestinal inflammation in IL-10 deficient mice by a plasmid carrying Lactococcus lactis strain. BMC Biotechnol. 20, 38 (2020).
Braat, H. et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. 4, 754–759 (2006).
Bruijnen, S. T. G. et al. F8-IL10: a new potential antirheumatic drug evaluated by a PET-guided translational approach. Mol. Pharm. 16, 273–281 (2019).
Watanabe, S. et al. Transplantation of intestinal organoids into a mouse model of colitis. Nat. Protoc. 17, 649–671 (2022).
Ko, J. Z., Johnson, S. & Dave, M. Efficacy and safety of mesenchymal stem/stromal cell therapy for inflammatory bowel diseases: an up-to-date systematic review. Biomolecules 11, 82 (2021).
Sweeney, C. L. et al. Correction of X-CGD patient HSPCs by targeted CYBB cDNA insertion using CRISPR/Cas9 with 53BP1 inhibition for enhanced homology-directed repair. Gene Ther. 28, 373–390 (2021).
Madsen, K. L. et al. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology 118, 1094–1105 (2000).
Li, S. et al. Intermittent antibiotic treatment accelerated the development of colitis in IL-10 knockout mice. Biomed. Pharmacother. 146, 112486 (2021).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
de la Morena-Barrio, M. E. et al. MPI-CDG with transient hypoglycosylation and antithrombin deficiency. Haematologica 104, e79–e82 (2019).
Yang, L. et al. Phenotype, genotype, treatment, and survival outcomes in patients with X-linked inhibitor of apoptosis deficiency. J. Allergy Clin. Immunol. 150, 456–466 (2022).
Čechová, A. et al. Consensus guideline for the diagnosis and management of mannose phosphate isomerase-congenital disorder of glycosylation. J. Inherit. Metab. Dis. 43, 671–693 (2020).
Acknowledgements
The authors thank patients and their families who have shared their experiences and contributed to research. The authors thank the Crohn’s in Childhood Research Association (CICRA), the XLP Research Trust and the Chronic Granulomatous Disorder Society (CGD Society) for their supportive feedback. A.M.M., H.H.U., C.K., D.K., S.B.S., J.C. and D.P.B.McG. are supported by the Leona M. and Harry B. Helmsley Charitable Trust. S.P.T. and H.H.U. are supported by the NIHR Oxford Biomedical Research Centre, University of Oxford; K.J. and C.B. are supported by the GOSH NIHR Biomedical Research Centre, London. B.G. is supported by RESIST-EXC 2155-Project (ID 390874280) and the BMBF (GAIN 01GM1910A). S.B.S is funded by the Wolpow Family Chair in IBD Treatment and Research, the Egan Family Foundation Chair, the Children’s Rare Disease Cohort Initiative, and NIDDK RC2DK122532. A.M.M. is funded by the Canada Research Chair (Tier 1) in Paediatric IBD, CIHR Foundation Grant, and NIDDK NIH (RC2DK118640 and RC2DK122532).
Author information
Authors and Affiliations
Contributions
H.H.U., S.P.T. and A.M.M. researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed/edited the manuscript before submission. C.B., J.C., M.D., B.G., J.K., D.K., C.K., B.L., D.P.B.McG., F.R., D.S.S., S.B.S. and D.C.W. made a substantial contribution to discussion of content, and reviewed/edited the manuscript before submission. A.M.G., S.H., K.J., M.J.L., L.d.R. and D.T. made a substantial contribution to discussion of content, wrote the article, and reviewed/edited the manuscript before submission. Y.H., H.K., S.K. and A.Ö. researched data for the article, made a substantial contribution to discussion of content, and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
This Expert Recommendation was not influenced or supported by any commercial entity outside the academic institutions. H.H.U. received research support or consultancy fees from Janssen, Eli Lilly, UCB Pharma, BMS/Celgene, MiroBio, OMass and Mestag. J.C. reports that Sema4 performed sequencing of BioMe biobank, RGC (Regeneron Genetics Center) supported MRGPRX2 studies, ImmuneID supported biomarker development, Renalytix supported biomarker development. M.D. received a consulting fees from AbbVie Inc., Arena Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb Company, Celgene Corporation, Eli Lilly and Company, F. Hoffmann-La Roche Ltd., Genentech Inc, Gilead, Janssen Global Services LLB, Pfizer Inc., Prometheus Biosciences, Takeda Pharmaceuticals USA Inc. and UCB SA; performed contracted research for AbbVie Inc., Janssen Global Services LLC, Pfizer Inc. and Prometheus Biosciences; has an ownership interest in Trellus Health Inc., and has received a licensing fee from Takeda Pharmaceuticals USA Inc. A.M.G. has acted as a consultant or advisory board member for Abbvie, Amgen, Bristol-Myers Squibb, Lilly, Merck, Janssen, Pfizer and Takeda; has received speaker fees from Abbvie and Janssen; and has received investigator-initiated research support from Abbvie. B.G. receives funding for his research from the following third parties: Deutsche Forschungsgemeinschaft (DFG), the E-rare program of the EU, managed by the DFG, the Netzwerke Seltener Erkrankungen of the German Ministry of Education and Research (BMBF), Merck KGaA, Takeda Pharma Vertrieb GmbH & Co. KG, Bristol-Myers Squibb GmbH & Co. KGaA, Novartis Pharma AG, and CSL Behring GmbH. During the last 3 years B.G. was an adviser to the following companies: Bristol-Myers Squibb, Adivo Associates Germany, Pharming Group NV, Epimune GmbH, GigaGen Inc., Atheneum Partners GmbH, UCB Pharma SA and Roche Pharma AG. S.H. reports consultancy fees from Takeda, Videregen and Pharming; and honoraria from Biotest and UCB. K.J. received speaker fees from Celltrion. S.K. reports grants given to the institution from Mead Johnson, Nestec Nutrition and BioGaia, and personal fees from Nestlé, Danone, Mead Johnson, Biocodex, Shire, Abbvie, Vifor, Pharmacosmos, Celgene. ThermoFisher, Janssen and Pfizer outside the submitted work. D.K. reports a sponsor research agreement with Celsius Therapeutics Inc., D.P.B.McG. is a consultant and holds stock in Prometheus Biosciences, and has consulted for Takeda, Gilead, Pfizer and Prometheus Laboratories. A.Ö. acts as a steering committee member for a clinical trial sponsored by Regeneron Pharmaceuticals and received research funds from this company, and also has a pending patent on C5 inhibitor treatment of CHAPLE. L.d.R. reports collaboration (including involvement in industry-sponsored studies, an investigator-initiated study and consultancy) with Abbvie, Lilly, Takeda, Janssen and Pfizer. F.R. served as scientific board member or has received speaker fees from Johnson & Johnson, Centocor, AbbVie, Nestlé Nutrition Institute, Nestlé Health Science, Takeda, Celgene, Biogen and Bristol-Meyers Squibb. D.S.S. reports a research grant from Takeda, and consulting and lecturing fees from Jansen, AbbVie and Takeda. S.B.S. is supported by grants or in-kind contributions from Pfizer, Novartis, Janssen, Merck and Regeneron, is on the scientific advisory boards of Pfizer, Janssen, IFM Therapeutics, Lycera, Inc., Celgene, Lilly, Pandion Therapeutics and Applied Molecular Transport, and has consulted for Amgen and Hoffman La-Roche. S.P.T. was employed by the University of Oxford (and, until 31 March 2022, Oxford University Hospitals NHS Trust), has received graah support from AbbVie, Buhlmann, Celgene, ECCO, Helmsley Trust, IOIBD, Janssen, Lilly, Pfizer, Takeda, UCB, UKIERI, Vifor and Norman Collisson Foundation, consulting fees from Abacus, AbbVie, Actial, ai4gi, Alcimed, Allergan, Amgen, Aptel, Arena, Asahi, Aspen, Astellas, Atlantic, AstraZeneca, Barco, Biocare, Biogen, BLPharma, Boehringer Ingelheim, Bristol-Meyers Squibb, Buhlmann, Calcico, Celgene, Cellerix, Cerimon, ChemoCentryx, Chiesi, CisBio, ComCast, Coronado, Cosmo, Ducentis, Dynavax, Elan, Enterome, Equillium, Falk, Ferring, FPRT Bio, Galapagos, Genentech/Roche, Genzyme, Gilead, Glenmark, Grunenthal, GSK, GW Pharmaceuticals, Immunocore, Immunometabolism, Indigo, Janssen, Lexicon, Lilly, Medarex, Medtrix, Merck, Merrimack, Mestag. Millenium, Neovacs, Novartis, Novo Nordisk, NPS-Nycomed, Ocera, Optima, Origin, Otsuka, Palau, Pentax, Pfizer, Pharmaventure, Phesi, Phillips, P&G, Pronota, Protagonist, Proximagen, Resolute, Robarts, Sandoz, Santarus, Satisfai, Sensyne Health, Shire, SigmoidPharma, Sorriso, Souffinez, Syndermix, Synthon, Takeda, Theravance, Tigenix, Tillotts, Topivert, Trino Therapeutics with Wellcome Trust, TxCell, UCB Pharma, Vertex, VHsquared, Vifor, Warner Chilcott and Zeria, and speaker fees from AbbVie, Amgen, Biogen, Falk, Ferring, Janssen, Pfizer, Shire, Takeda and UCB, but has no stocks or share options. D.T. has received consultation fees, a research grant, royalties or honoraria from Janssen, Pfizer, Hospital for Sick Children, Ferring, Abbvie, Takeda, Atlantic Health, Shire, Celgene, Lilly, Roche, ThermoFisher, Bristol-Meyers-Squibb and SorrisoPharma. D.C.W. has received consultancy fees, speaker fees and/or travel support from Abbvie, Nestlé Health Sciences and Roche. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Robert Beattie, Judith Kelsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov database: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uhlig, H.H., Booth, C., Cho, J. et al. Precision medicine in monogenic inflammatory bowel disease: proposed mIBD REPORT standards. Nat Rev Gastroenterol Hepatol 20, 810–828 (2023). https://doi.org/10.1038/s41575-023-00838-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00838-4