Abstract
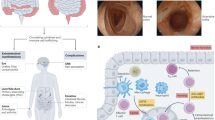
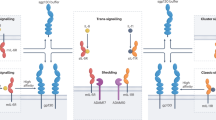
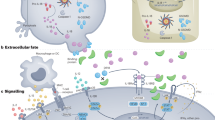
Interleukin-12 (IL-12) and interleukin-23 (IL-23), which belong to the IL-12 family of cytokines, have a key role in intestinal homeostasis and inflammation and are implicated in the pathogenesis of inflammatory bowel disease. Upon their secretion by antigen-presenting cells, they exert both pro-inflammatory and anti-inflammatory receptor-mediated effects. An increased understanding of these biological effects, particularly the pro-inflammatory effects mediated by IL-12 and IL-23, has led to the development of monoclonal antibodies that target a subunit common to IL-12 and IL-23 (p40; targeted by ustekinumab and briakinumab), or the IL-23-specific subunit (p19; targeted by risankizumab, guselkumab, brazikumab and mirikizumab). This Review provides a summary of the biology of the IL-12 family cytokines IL-12 and IL-23, discusses the role of these cytokines in intestinal homeostasis and inflammation, and highlights IL-12- and IL-23-directed drug development for the treatment of Crohn’s disease and ulcerative colitis.
Key points
-
IL-12 and IL-23, which are members of the IL-12 family of cytokines, have a key role in intestinal homeostasis and inflammation, including in inflammatory bowel disease.
-
Multiple IL-12- and/or IL-23-neutralizing antibodies have been tested in immune-mediated diseases, including Crohn’s disease and ulcerative colitis.
-
In addition to demonstrated efficacy for clinical, endoscopic and histological outcomes, targeting IL-12 and/or IL-23 is a safe treatment strategy.
-
The exact positioning of such antibodies in current treatment algorithms will be influenced by ongoing head-to-head trials and evaluation of predictive molecular markers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664 (2020).
Baumgart, D. C. & Le Berre, C. Newer biologic and small-molecule therapies for inflammatory bowel disease. N. Engl. J. Med. 385, 1302–1315 (2021).
Roda, G., Jharap, B., Neeraj, N. & Colombel, J. F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol. 7, e135 (2016).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Verstockt, B., Van Assche, G., Vermeire, S. & Ferrante, M. Biological therapy targeting the IL-23/IL-17 axis in inflammatory bowel disease. Expert. Opin. Biol. Ther. 17, 31–47 (2017).
Kobayashi, M. et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170, 827–845 (1989).
Hsieh, C. S. et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260, 547–549 (1993).
Manetti, R. et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 177, 1199–1204 (1993).
Seder, R. A., Gazzinelli, R., Sher, A. & Paul, W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl Acad. Sci. USA 90, 10188–10192 (1993).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Brombacher, F. et al. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int. Immunol. 11, 325–332 (1999).
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Tesmer, L. A., Lundy, S. K., Sarkar, S. & Fox, D. A. Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008).
Pirhonen, J., Matikainen, S. & Julkunen, I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J. Immunol. 169, 5673–5678 (2002).
Verreck, F. A. et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl Acad. Sci. USA 101, 4560–4565 (2004).
Cella, M. et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J. Exp. Med. 184, 747–752 (1996).
Wesa, A. & Galy, A. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 3, 14 (2002).
Ma, X. et al. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 183, 147–157 (1996).
Luque-Martin, R. et al. IFN-gamma drives human monocyte differentiation into highly proinflammatory macrophages that resemble a phenotype relevant to psoriasis. J. Immunol. 207, 555–568 (2021).
Shi, Q. et al. PGE2 elevates IL-23 production in human dendritic cells via a cAMP dependent pathway. Mediat. Inflamm. 2015, 984690 (2015).
Geyer, C. E. et al. C-reactive protein controls IL-23 production by human monocytes. Int. J. Mol. Sci. 22, 11638 (2021).
Lim, K. S. et al. Inflammatory and mitogenic signals drive interleukin 23 subunit alpha (IL23A) secretion independent of IL12B in intestinal epithelial cells. J. Biol. Chem. 295, 6387–6400 (2020).
Macho-Fernandez, E. et al. Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells. Mucosal Immunol. 8, 403–413 (2015).
Moschen, A. R., Tilg, H. & Raine, T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 16, 185–196 (2019).
Schwarz, E. & Carson, W. E. III Analysis of potential biomarkers of response to IL-12 therapy. J. Leukoc. Biol. 112, 557–567 (2022).
Grohmann, U. et al. Positive regulatory role of IL-12 in macrophages and modulation by IFN-gamma. J. Immunol. 167, 221–227 (2001).
Parham, C. et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168, 5699–5708 (2002).
Awasthi, A. et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182, 5904–5908 (2009).
Frucht, D. M. IL-23: a cytokine that acts on memory T cells. Sci. STKE 2002, pe1 (2002).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Glassman, C. R. et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 184, 983–999.e924 (2021).
Thierfelder, W. E. et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382, 171–174 (1996).
Floss, D. M. et al. Identification of canonical tyrosine-dependent and non-canonical tyrosine-independent STAT3 activation sites in the intracellular domain of the interleukin 23 receptor. J. Biol. Chem. 288, 19386–19400 (2013).
Sun, R., Hedl, M. & Abraham, C. IL23 induces IL23R recycling and amplifies innate receptor-induced signalling and cytokines in human macrophages, and the IBD-protective IL23R R381Q variant modulates these outcomes. Gut 69, 264–273 (2020).
Becker, C. et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 112, 693–706 (2003).
Fuss, I. J. et al. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm. Bowel Dis. 12, 9–15 (2006).
Kullberg, M. C. et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 (2006).
Grohmann, U. et al. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity 9, 315–323 (1998).
Yang, R. et al. IL-12 + IL-18 cosignaling in human macrophages and lung epithelial cells activates cathelicidin and autophagy, inhibiting intracellular mycobacterial growth. J. Immunol. 200, 2405–2417 (2018).
Xing, Z., Zganiacz, A. & Santosuosso, M. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-α and nitric oxide from macrophages via IFN-γ induction. J. Leukoc. Biol. 68, 897–902 (2000).
Sun, R. & Abraham, C. IL23 promotes antimicrobial pathways in human macrophages, which are reduced with the IBD-protective IL23R R381Q variant. Cell Mol. Gastroenterol. Hepatol. 10, 673–697 (2020).
Bastos, K. R. et al. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes Infect. 6, 630–636 (2004).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Monteleone, I., Sarra, M., Pallone, F. & Monteleone, G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr. Mol. Med. 12, 592–597 (2012).
Punkenburg, E. et al. Batf-dependent Th17 cells critically regulate IL-23 driven colitis-associated colon cancer. Gut 65, 1139–1150 (2016).
Huber, S. et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3– and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34, 554–565 (2011).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Maxwell, J. R. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750 (2015).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009).
Bauche, D. et al. IL-23 and IL-2 activation of STAT5 is required for optimal IL-22 production in ILC3s during colitis. Sci. Immunol. 5, eaav1080 (2020).
Aden, K. et al. Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Rep. 16, 2208–2218 (2016).
Powell, N. et al. Interleukin 6 increases production of cytokines by colonic innate lymphoid cells in mice and patients with chronic intestinal inflammation. Gastroenterology 149, 456–467.e15 (2015).
Peng, V., Jaeger, N. & Colonna, M. Innate lymphoid cells and inflammatory bowel disease. Adv. Exp. Med. Biol. 1365, 97–112 (2022).
Bauche, D. et al. LAG3+ regulatory T cells restrain interleukin-23-producing CX3CR1+ gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity 49, 342–352 (2018).
Rankin, L. C. & Arpaia, N. Treg cells: a LAGging hand holds the double-edged sword of the IL-23 axis. Immunity 49, 201–203 (2018).
Powell, N. et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut 69, 578–590 (2020).
Simmons, C. P. et al. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching–effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 168, 1804–1812 (2002).
Zundler, S. & Neurath, M. F. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 26, 559–568 (2015).
Sarin, R., Wu, X. & Abraham, C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl Acad. Sci. USA 108, 9560–9565 (2011).
Pidasheva, S. et al. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS ONE 6, e25038 (2011).
Di Meglio, P. et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS ONE 6, e17160 (2011).
Yu, R. Y., Brazaitis, J. & Gallagher, G. The human IL-23 receptor rs11209026 A allele promotes the expression of a soluble IL-23R-encoding mRNA species. J. Immunol. 194, 1062–1068 (2015).
Sivanesan, D. et al. IL23R (interleukin 23 receptor) variants protective against inflammatory bowel diseases (IBD) display loss of function due to impaired protein stability and intracellular trafficking. J. Biol. Chem. 291, 8673–8685 (2016).
Beaudoin, M. et al. Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. PLoS Genet. 9, e1003723 (2013).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Zwiers, A. et al. Cutting edge: a variant of the IL-23R gene associated with inflammatory bowel disease induces loss of microRNA regulation and enhanced protein production. J. Immunol. 188, 1573–1577 (2012).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Brant, S. R. et al. Genome-wide association study identifies African-specific susceptibility loci in African Americans with inflammatory bowel disease. Gastroenterology 152, 206–217 (2016).
Huang, C. et al. Characterization of genetic loci that affect susceptibility to inflammatory bowel diseases in African Americans. Gastroenterology 149, 1575–1586 (2015).
Barrett, J. C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 40, 955–962 (2008).
Ben-Selma, W. & Boukadida, J. IL23R(Arg381Gln) functional polymorphism is associated with active pulmonary tuberculosis severity. Clin. Vaccin. Immunol. 19, 1188–1192 (2012).
Zakrzewski, M. et al. IL23R-protective coding variant promotes beneficial bacteria and diversity in the ileal microbiome in healthy individuals without inflammatory bowel disease. J. Crohns Colitis 13, 451–461 (2019).
Neurath, M. F., Fuss, I., Kelsall, B. L., Stuber, E. & Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182, 1281–1290 (1995).
Davidson, N. J. et al. IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J. Immunol. 161, 3143–3149 (1998).
Hue, S. et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 (2006).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Monteleone, G. et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 112, 1169–1178 (1997).
Monteleone, G., Parrello, T., Luzza, F. & Pallone, F. Response of human intestinal lamina propria T lymphocytes to interleukin 12: additive effects of interleukin 15 and 7. Gut 43, 620–628 (1998).
Mannon, P. J. et al. Anti-interleukin-12 antibody for active Crohn’s disease. N. Engl. J. Med. 351, 2069–2079 (2004).
Globig, A. M. et al. Ustekinumab inhibits T follicular helper cell differentiation in patients with Crohn’s disease. Cell Mol. Gastroenterol. Hepatol. 11, 1–12 (2021).
Wiekowski, M. T. et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166, 7563–7570 (2001).
Yen, D. et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Elson, C. O. et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology 132, 2359–2370 (2007).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Izcue, A. et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28, 559–570 (2008).
Chen, L. et al. Diet modifies colonic microbiota and CD4+ T-cell repertoire to induce flares of colitis in mice with myeloid-cell expression of interleukin 23. Gastroenterology 155, 1177–1191 (2018).
He, Z. et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 33, 1358–1371 (2021).
Eftychi, C. et al. Temporally distinct functions of the cytokines IL-12 and IL-23 drive chronic colon inflammation in response to intestinal barrier impairment. Immunity 51, 367–380 (2019).
Cox, J. H. et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 5, 99–109 (2012).
Becker, C. et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 177, 2760–2764 (2006).
Aychek, T. et al. IL-23-mediated mononuclear phagocyte crosstalk protects mice from Citrobacter rodentium-induced colon immunopathology. Nat. Commun. 6, 6525 (2015).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Rutgeerts, P. et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 155, 1045–1058 (2018).
Li, K. et al. Effects of ustekinumab on histologic disease activity in patients with Crohn’s disease. Gastroenterology 157, 1019–1031 (2019).
Sands, B. E. et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 399, 2200–2211 (2022).
Danese, S. et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): an open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol. Hepatol. 7, 294–306 (2022).
D’Haens, G. et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 399, 2015–2030 (2022).
Ferrante, M. et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 399, 2031–2046 (2022).
Ferrante, M. et al. OP25 Patients with moderate to severe Crohn’s disease with and without prior biologic failure demonstrate improved endoscopic outcomes with risankizumab: results from phase 3 induction and maintenance trials. J. Crohns Colitis 16, i027–i028 (2022).
Bossuyt, P. et al. OP40 Efficacy of risankizumab induction and maintenance therapy by baseline Crohn’s disease location: post hoc analysis of the phase 3 ADVANCE, MOTIVATE, and FORTIFY studies. J. Crohns Colitis 16, i048 (2022).
Sandborn, W. J. et al. The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active Crohn’s disease: week 12 interim analyses from the phase 2 GALAXI 1 study. U. Eur. Gastroenterol. 8, 64 (2020).
Sandborn, W. J. et al. Guselkumab for the treatment of Crohn’s disease: Induction results from the Phase 2 GALAXI-1 study. Gastroenterology https://doi.org/10.1053/j.gastro.2022.01.047 (2022).
Danese, S. et al. OP24 Clinical efficacy and safety of guselkumab maintenance therapy in patients with moderately to severely active Crohn’s disease: week 48 analyses from the phase 2 GALAXI 1 study. J. Crohns Colitis 16, i026–i027 (2022).
Sands, B. E. et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 153, 77–86 (2017).
Sands, B. E. et al. 1003 — Efficacy and safety of mirikizumab (LY3074828) in a phase 2 study of patients with Crohn’s disease. Gastroenterology 156, S216 (2019).
Sands, B. E. et al. Efficacy and safety of mirikizumab after 52-weeks maintenance treatment in patients with moderate-to-severe Crohn’s disease. Gastroenterology 160, S37 (2021).
Panaccione, R. et al. Briakinumab for treatment of Crohn’s disease: results of a randomized trial. Inflamm. Bowel Dis. 21, 1329–1340 (2015).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381, 1201–1214 (2019).
Leong, R. et al. DOP55 Long-term outcomes after histologic-endoscopic mucosal healing: results from the UNIFI study in ulcerative colitis. J. Crohns Colitis 16, i102–i103 (2022).
D’Haens, G. et al. OP26 Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-1 study. J. Crohns Colitis 16, i028–i029 (2022).
Dignass, A. et al. OP23 The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active ulcerative colitis: phase 2b QUASAR study results through week 12. J. Crohns Colitis 16, i025–i026 (2022).
Sands, B. E. et al. OP36 Efficacy and safety of combination induction therapy with guselkumab and golimumab in participants with moderately-to-severely active ulcerative colitis: results through week 12 of a phase 2a randomized, double-blind, active-controlled, parallel-group, multicenter, proof-of-concept study. J. Crohns Colitis 16, i042–i043 (2022).
Sandborn, W. J. et al. Safety of ustekinumab in inflammatory bowel disease: pooled safety analysis of results from phase 2/3 studies. Inflamm. Bowel Dis. 27, 994–1007 (2021).
Fiorentino, D. et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J. Am. Acad. Dermatol. 77, 845–854 (2017).
Honap, S. et al. Effectiveness and safety of ustekinumab in inflammatory bowel disease: a systematic review and meta-analysis. Dig. Dis. Sci. 67, 1018–1035 (2022).
Martinez-Barricarte, R. et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 3, eaau6759 (2018).
Teng, M. W. et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 21, 719–729 (2015).
Meeks, K. D., Sieve, A. N., Kolls, J. K., Ghilardi, N. & Berg, R. E. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J. Immunol. 183, 8026–8034 (2009).
Abraham, C. & Cho, J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm. Bowel Dis. 15, 1090–1100 (2009).
Shih, V. F. et al. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc. Natl Acad. Sci. USA 111, 13942–13947 (2014).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Patel, D. D. & Kuchroo, V. K. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity 43, 1040–1051 (2015).
Schulz, S. M. et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J. Immunol. 181, 7891–7901 (2008).
Schmitt, H. et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 68, 814–828 (2018).
Sandborn, W. et al. OP303 efficacy and safety of mirikizumab (LY3074828) in patients with moderate-to-severe ulcerative colitis in a phase 2 study. U. Eur. Gastroenterol. J. 6, A119–A120 (2018).
Doherty, M. K. et al. Fecal microbiota signatures are associated with response to ustekinumab therapy among Crohn’s disease patients. mBio 9, e02120–02117 (2018).
Sandborn, W. J. et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology 158, 537–549 (2020).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389, 1699–1709 (2017).
Gisbert, J. P. & Chaparro, M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J. Crohns Colitis 14, 694–709 (2020).
Dalal, R. S. et al. Predictors and outcomes of ustekinumab dose intensification in ulcerative colitis: a multicenter cohort study. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2021.03.028 (2021).
Dalal, R. S., Njie, C., Marcus, J., Gupta, S. & Allegretti, J. R. Predictors of ustekinumab failure in Crohn’s disease after dose intensification. Inflamm. Bowel Dis. 27, 1294–1301 (2021).
Lefevre, P. L. C., Shackelton, L. M. & Vande Casteele, N. Factors influencing drug disposition of monoclonal antibodies in inflammatory bowel disease: implications for personalized medicine. BioDrugs 33, 453–468 (2019).
Wang, Z. et al. Population pharmacokinetic-pharmacodynamic model-based exploration of alternative ustekinumab dosage regimens for patients with Crohn’s disease. Br. J. Clin. Pharmacol. 88, 323–335 (2022).
Bots, S. J. et al. Anti-drug antibody formation against biologic agents in inflammatory bowel disease: a systematic review and meta-analysis. BioDrugs 35, 715–733 (2021).
Adedokun, O. J. et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology 154, 1660–1671 (2018).
Verstockt, B. et al. Ustekinumab exposure-outcome analysis in Crohn’s disease only in part explains limited endoscopic remission rates. J. Crohns Colitis 13, 864–872 (2019).
Hanzel, J. et al. Peak concentrations of ustekinumab after intravenous induction therapy identify patients with Crohn’s disease likely to achieve endoscopic and biochemical remission. Clin. Gastroenterol. Hepatol. 19, 111–118 (2021).
Adedokun, O. J. et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 18, 2244–2255 (2020).
Alsoud, D., Vermeire, S. & Verstockt, B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: hype or hope. Curr. Opin. Pharmacol. 55, 17–30 (2020).
D’Haens, G. R. et al. 775a risankizumab induction therapy in patients with moderate-to-severe Crohn’s disease with intolerance or inadequate response to conventional and/or biologic therapy: results from the phase 3 ADVANCE study. Gastroenterology 161, e28 (2021).
Sands, B. E. et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology 158, 537–549 (2021).
Danese, S. et al. OP28 The effect of guselkumab induction therapy on early clinical outcome measures in patients with Moderately to Severely Active Crohn’s Disease: Results from the phase 2 GALAXI 1 study. J. Crohns Colitis 15, S027–S028 (2021).
Strober, B. et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J. Eur. Acad. Dermatol. Venereol. 34, 2830–2838 (2020).
Irving, P. M. et al. OP02 Ustekinumab versus adalimumab for induction and maintenance therapy in moderate-to-severe Crohn’s disease: the SEAVUE study. J. Crohns Colitis 15, S001–S002 (2021).
Singh, S. et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 6, 1002–1014 (2021).
Singh, S., Murad, M. H., Fumery, M., Dulai, P. S. & Sandborn, W. J. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin. Gastroenterol. Hepatol. 18, 2179–2191 (2020).
Burr, N. E., Gracie, D. J., Black, C. J. & Ford, A. C. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut https://doi.org/10.1136/gutjnl-2021-326390 (2021).
Lasa, J. S., Olivera, P. A., Danese, S. & Peyrin-Biroulet, L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 7, 161–170 (2022).
Guillo, L., D’Amico, F., Danese, S. & Peyrin-Biroulet, L. Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J. Crohns Colitis 15, 1236–1243 (2021).
Li, S. J., Perez-Chada, L. M. & Merola, J. F. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J. Psoriasis Psoriat. Arthritis 4, 70–80 (2019).
Puig, L., Morales-Munera, C. E., Lopez-Ferrer, A. & Geli, C. Ustekinumab treatment of TNF antagonist-induced paradoxical psoriasis flare in a patient with psoriatic arthritis: case report and review. Dermatology 225, 14–17 (2012).
Sandborn, W. J. et al. Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease: the IM-UNITI trial. Clin. Gastroenterol. Hepatol. 20, 578–590 (2022).
Garg, R. et al. Real-world effectiveness and safety of ustekinumab in elderly Crohn’s disease patients. Dig. Dis. Sci. https://doi.org/10.1007/s10620-021-07117-9 (2021).
Hayashi, M. et al. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J. Dermatol. 41, 974–980 (2014).
Ferrante, M. et al. Long-term safety and efficacy of risankizumab treatment in patients with Crohn’s disease: results from the phase 2 open-label extension study. J. Crohns Colitis 15, 2001–2010 (2021).
Acknowledgements
The authors would like to thank L. M. Shackelton and S. Donegan for critical technical review and editing. B.V. is supported by the Clinical Research Fund (KOOR) at the University Hospitals Leuven and the Research Council at KU Leuven. N.V.C. is supported in part by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Author information
Authors and Affiliations
Consortia
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
Alimentiv Inc. is an academic gastrointestinal contract research organization (CRO), operating under the Alimentiv Health Trust. Alimentiv Inc. provides comprehensive clinical trial services, precision medicine offerings, and centralized imaging solutions for endoscopy, histopathology, and other imaging modalities. The beneficiaries of the Alimentiv Health Trust are the employees of the enterprises it holds. None of the authors is a beneficiary of the Alimentiv Health Trust. B.V., N.V.C., G.D’H., B.G.F., V.J., C.M., W.J.S. and A.S. are consultants to Alimentiv Inc. and have a primary academic appointment; they do not hold equity positions or shares in Alimentiv Inc. B.V. reports research support from AbbVie, Biora Therapeutics, Pfizer, Sossei Heptares and Takeda; speaker’s fees from Abbvie, Biogen, Bristol Myers Squibb, Celltrion, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, Truvion and Viatris; and consultancy fees from Abbvie, Alimentiv, Applied Strategic, Atheneum, Biora Therapeutics, Bristol Myers Squibb, Galapagos, Guidepont, Inotrem, Inotrem, Ipsos, Janssen, Mylan, Progenity, Sandoz, Sosei Heptares, Takeda Tillots Pharma and Viatris. A.S. reports research grants from Roche-Genentech, Abbvie, GSK, Scipher Medicine, Alimentiv Inc, Boehringer Ingelheim and Origo Biopharma; consulting fees from Genentech, GSK, Pfizer, HotSpot Therapeutics, Alimentiv, Origo Biopharma and Boxer Capital. B.E.S. reports research grants from Takeda, Pfizer, Theravance Biopharma R&D and Janssen; consulting fees from 4D Pharma, Abivax, Abbvie, Alimentiv, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Bacainn Therapeutics, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Capella Bioscience, Celgene, Celltrion Healthcare, ClostraBio, Enthera, F.Hoffmann-La Roche, Ferring, Galapagos, Gilead, GlaxoSmithKline, GossamerBio, Immunic, Index Pharmaceuticals, Innovation Pharmaceuticals, Ironwood Pharmaceuticals, Janssen, Kaleido, Kallyope, Lilly, MiroBio, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palatin Technologies, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Sun Pharma, Surrozen, Takeda, Target PharmaSolutions, Teva Branded Pharmaceutical Products R&D, Thelium, Theravance Biopharma R&D, TLL Pharma, USWM Enterprises, Ventyx Biosciences, Viela Bio, Vivante Health and Vivelix Pharmaceuticals; and holds stock in Vivante Health and Ventyx Biosciences. M.F.N. reports consulting fees from MSD Sharp & Dohme GmbH, PPM Services, IFM Therapeutics, Sterna Biologicals, Boehringer Ingleheim GmbH and Co. KG, Janssen Cilag GmbH, Pentax Europe GmbH, Takeda, Amgen GmbH, Pfizer Pharma, Falk Foundation e.v., Abbvie and Celgene. N.V.C. reports research grants and personal fees from R-Biopharm, Takeda and UCB; and personal fees from Alimentiv, Inc (formerly Robarts Clinical Trials, Inc), Celltrion and Prometheus. These activities were all outside the submitted work. C.A. and H.L. declare no competing interests. The competing interests of the Alimentiv Translational Research Consortium Member Authors are listed in Supplementary Box 1.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Giovanni Monteleone and Herbert Tilg for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verstockt, B., Salas, A., Sands, B.E. et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 20, 433–446 (2023). https://doi.org/10.1038/s41575-023-00768-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00768-1
This article is cited by
-
Are all the IL-23 blockers the same in inflammatory bowel disease?
Nature Reviews Gastroenterology & Hepatology (2024)