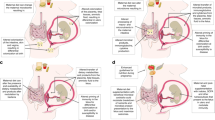
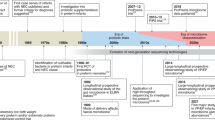
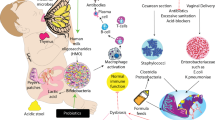
Abstract
Environmental enteric dysfunction (EED) is a subclinical syndrome of intestinal inflammation, malabsorption and barrier disruption that is highly prevalent in low- and middle-income countries in which poverty, food insecurity and frequent exposure to enteric pathogens impair growth, immunity and neurodevelopment in children. In this Review, we discuss advances in our understanding of EED, intestinal adaptation and the gut microbiome over the ‘first 1,000 days’ of life, spanning pregnancy and early childhood. Data on maternal EED are emerging, and they mirror earlier findings of increased risks for preterm birth and fetal growth restriction in mothers with either active inflammatory bowel disease or coeliac disease. The intense metabolic demands of pregnancy and lactation drive gut adaptation, including dramatic changes in the composition, function and mother-to-child transmission of the gut microbiota. We urgently need to elucidate the mechanisms by which EED undermines these critical processes so that we can improve global strategies to prevent and reverse intergenerational cycles of undernutrition.
Key points
-
Maternal and neonatal anthropometry are key predictors of childhood stunting, highlighting the intergenerational nature of undernutrition and pinpointing the first 1,000 days of life as a critical window for development.
-
Pregnancy and lactation are metabolically demanding, requiring an expansion of small intestinal absorptive capacity; enteropathies adversely affect perinatal outcomes.
-
Environmental enteric dysfunction (EED) is characterized by inflammation, increased barrier permeability, and reduced absorptive capacity. Its prevalence and consequences in mothers in low and middle-income countries warrant urgent investigation.
-
Gut microbial communities are disrupted during EED and undernutrition in humans, and confer aspects of these phenotypes to gnotobiotic mice; nutrient processing, absorption and regulation of immunity are potential mechanisms.
-
Infants inherit a substantial portion of their microbiome from their mothers. Maternal microorganisms, breast milk and epigenetics are implicated in intergenerational undernutrition.
-
Gut microbial communities in early life shape host immunity, with potential consequences for survival, growth and cognitive development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Prendergast, A. J. & Humphrey, J. H. The stunting syndrome in developing countries. Paediatr. Int. Child. Health 34, 250–265 (2014).
Gomes, C. F., Sousa, M., Lourenço, I., Martins, D. & Torres, J. Gastrointestinal diseases during pregnancy: what does the gastroenterologist need to know? Ann. Gastroenterol. 31, 385–394 (2018).
Guerrant, R. L., DeBoer, M. D., Moore, S. R., Scharf, R. J. & Lima, A. A. M. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 10, 220–229 (2013).
Syed, S., Ali, A. & Duggan, C. Environmental enteric dysfunction in children. J. Pediatr. Gastroenterol. Nutr. 63, 6–14 (2016).
Watanabe, K. & Petri, W. A. Environmental enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine 10, 25–32 (2016).
Fagundes-Neto, U., Viaro, T., Wehba, J., Patrício, F. R. & Machado, N. L. Tropical enteropathy (environmental enteropathy) in early childhood: a syndrome caused by contaminated environment. J. Trop. Pediatr. 30, 204–209 (1984).
Campbell, R. K. et al. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. J. Pediatr. Gastroenterol. Nutr. 65, 40–46 (2017).
Kosek, M. et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg. 88, 390–396 (2013).
McCormick, B. J. J. et al. Dynamics and trends in fecal biomarkers of gut function in children from 1–24 months in the MAL-ED study. Am. J. Trop. Med. Hyg. 96, 465–472 (2017).
Tickell, K. D., Atlas, H. E. & Walson, J. L. Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 17, 181 (2019).
Kosek, M. N. & MAL-ED Network Investigators. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine 18, 109–117 (2017).
Marie, C., Ali, A., Chandwe, K., Petri, W. A. & Kelly, P. Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy. Mucosal Immunol. 11, 1290–1298 (2018).
Levine, M. M. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 8, 129 (2010).
WHO. Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: key findings of the 2021 edition. WHO https://www.who.int/publications-detail-redirect/9789240025257 (2021).
Olofin, I. et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS ONE 8, e64636 (2013).
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013).
Moore, S. R., Lima, A. A. M. & Guerrant, R. L. Preventing 5 million child deaths from diarrhea in the next 5 years. Nat. Rev. Gastroenterol. Hepatol. 8, 363–364 (2011).
United Nations. Transforming our world: the 2030 agenda for sustainable development. United Nations Department of Economic and Social Affairs https://sdgs.un.org/2030agenda (2015).
Matonhodze, C. R. Leaving no one behind: impact of COVID-19 on the Sustainable Development Goals (SDGs). United Nations Development Programme https://www.undp.org/publications/leaving-no-one-behind-impact-covid-19-sustainable-development-goals-sdgs (2021).
de Onis, M. & Branca, F. Childhood stunting: a global perspective. Matern. Child. Nutr. 12, 12–26 (2016).
Chen, R. Y. et al. A microbiota-directed food intervention for undernourished children. N. Engl. J. Med. 384, 1517–1528 (2021).
Crookston, B. T. et al. Postinfancy growth, schooling, and cognitive achievement: young lives. Am. J. Clin. Nutr. 98, 1555–1563 (2013).
Prentice, A. M. et al. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr. 97, 911–918 (2013).
Lima, A. A. M. et al. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J. Pediatr. Gastroenterol. Nutr. 40, 28–35 (2005).
Chen, R. Y. et al. Duodenal microbiota in stunted undernourished children with enteropathy. N. Engl. J. Med. 383, 321–333 (2020).
Haberman, Y. et al. Mucosal genomics implicate lymphocyte activation and lipid metabolism in refractory environmental enteric dysfunction. Gastroenterology 160, 2055–2071.e0 (2021).
Hodges, P., Tembo, M. & Kelly, P. Intestinal biopsies for the evaluation of environmental enteropathy and environmental enteric dysfunction. J. Infect. Dis. 224, S856–S863 (2021).
Vonaesch, P. et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl Acad. Sci. 115, E8489–E8498 (2018).
Ordiz, M. I. et al. Environmental enteric dysfunction is associated with poor linear growth and can be identified by host fecal mRNAs. J. Pediatr. Gastroenterol. Nutr. 63, 453–459 (2016).
DeBoer, M. D. et al. Effect of scheduled antimicrobial and nicotinamide treatment on linear growth in children in rural Tanzania: a factorial randomized, double-blind, placebo-controlled trial. PLoS Med. 18, e1003617 (2021).
Tickell, K. D. & Walson, J. L. Nutritional enteric failure: neglected tropical diseases and childhood stunting. PLoS Negl. Trop. Dis. 10, e0004523 (2016).
Richard, S. A. et al. Enteric dysfunction and other factors associated with attained size at 5 years: MAL-ED birth cohort study findings. Am. J. Clin. Nutr. https://doi.org/10.1093/ajcn/nqz004 (2019).
Arndt, M. B. et al. Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. Am. J. Trop. Med. Hyg. 95, 694–701 (2016).
WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr. Suppl. 450, 76–85 (2006).
Grantz, K. L. et al. Unified standard for fetal growth: the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies. Am. J. Obstet. Gynecol. 226, 576–587.e2 (2022).
Papageorghiou, A. T. et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am. J. Obstet. Gynecol. 218, S630–S640 (2018).
Kiserud, T. et al. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 14, e1002220 (2017).
Wells, J. C. K. The new ‘obstetrical dilemma’: stunting, obesity and the risk of obstructed labour. Anat. Rec. 300, 716–731 (2017).
Dunsworth, H. M., Warrener, A. G., Deacon, T., Ellison, P. T. & Pontzer, H. Metabolic hypothesis for human altriciality. Proc. Natl Acad. Sci. USA 109, 15212–15216 (2012).
Schwarzenberg, S. J., Georgieff, M. K. & Committee on Nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 141, e20173716 (2018).
Lauer, J. M. et al. Biomarkers of maternal environmental enteric dysfunction are associated with shorter gestation and reduced length in newborn infants in Uganda. Am. J. Clin. Nutr. 108, 889–896 (2018).
Kirby, M. A. et al. Biomarkers of environmental enteric dysfunction and adverse birth outcomes: an observational study among pregnant women living with HIV in Tanzania. eBioMedicine 84, 104257 (2022).
Tersigni, C. et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Update 20, 582–593 (2014).
Cornish, J. et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 56, 830–837 (2007).
Alstead, E. M. & Nelson-Piercy, C. Inflammatory bowel disease in pregnancy. Gut 52, 159–161 (2003).
Leonard, M. M. et al. Value of IgA tTG in predicting mucosal recovery in children with celiac disease on a gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 64, 286–291 (2017).
Liu, T.-C. et al. A novel histological index for evaluation of environmental enteric dysfunction identifies geographic-specific features of enteropathy among children with suboptimal growth. PLoS Negl. Trop. Dis. 14, e0007975 (2020).
Thurber, C. et al. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci. Adv. 5, eaaw0341 (2019).
Hammond, K. A., Konarzewski, M., Torres, R. M. & Diamond, J. Metabolic ceilings under a combination of peak energy demands. Physiological Zool. 67, 1479–1506 (1994).
Hammond, K. A. & Diamond, J. Maximal sustained energy budgets in humans and animals. Nature 386, 457–462 (1997).
Hammond, K. A. Adaptation of the maternal intestine during lactation. J. Mammary Gland. Biol. Neoplasia 2, 243–252 (1997).
Butte, N. F., Wong, W. W., Treuth, M. S., Ellis, K. J. & O’Brian Smith, E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr. 79, 1078–1087 (2004).
Kominiarek, M. A. & Rajan, P. Nutrition recommendations in pregnancy and lactation. Med. Clin. North Am. 100, 1199–1215 (2016).
Butte, N. F. & King, J. C. Energy requirements during pregnancy and lactation. Public Health Nutr. 8, 1010–1027 (2005).
Nakada, D. et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505, 555–558 (2014).
Sabet Sarvestani, F., Rahmanifar, F. & Tamadon, A. Histomorphometric changes of small intestine in pregnant rat. Vet. Res. Forum 6, 69–73 (2015).
Prieto, R. M., Ferrer, M., Fe, J. M., Rayó, J. M. & Tur, J. A. Morphological adaptive changes of small intestinal tract regions due to pregnancy and lactation in rats. Ann. Nutr. Metab. 38, 295–300 (1994).
Boyne, R., Fell, B. F. & Robb, I. The surface area of the intestinal mucosa in the lactating rat. J. Physiol. 183, 570–575 (1966).
Casirola, D. M. & Ferraris, R. P. Role of the small intestine in postpartum weight retention in mice. Am. J. Clin. Nutr. 78, 1178–1187 (2003).
Şensoy, E. & Öznurlu, Y. Determination of the changes on the small intestine of pregnant mice by histological, enzyme histochemical, and immunohistochemical methods. Turk. J. Gastroenterol. 30, 917–924 (2019).
Zhou, Z. et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci. Rep. 9, 8367 (2019).
Drucker, D. J. The discovery of GLP-2 and development of teduglutide for short bowel syndrome. ACS Pharmacol. Transl. Sci. 2, 134–142 (2019).
Guan, X. et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130, 150–164 (2006).
Kahr, M. K. et al. SERUM GLP-2 is increased in association with excess gestational weight gain. Am. J. Perinatol. https://doi.org/10.1055/s-0041-1728828 (2021).
Garella, R., Squecco, R. & Baccari, M. C. Site-related effects of relaxin in the gastrointestinal tract through nitric oxide signalling: an updated report. Curr. Protein Pept. Sci. 18, 1254–1262 (2017).
Lemmens, K., Doggen, K. & De Keulenaer, G. W. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am. J. Physiol. Heart Circ. Physiol. 300, H931–H942 (2011).
Kilik, U. et al. Maturation of human intestinal epithelium from pluripotency in vitro. Preprint at bioRxiv https://doi.org/10.1101/2021.09.24.460132 (2021).
Collado, M. C., Isolauri, E., Laitinen, K. & Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899 (2008).
Koren, O. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012).
García-Gómez, E., González-Pedrajo, B. & Camacho-Arroyo, I. Role of sex steroid hormones in bacterial–host interactions. BioMed. Res. Int. 2013, e928290 (2012).
Gohir, W. et al. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 6, 310–320 (2015).
Nuriel-Ohayon, M. et al. Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 27, 730–736.e3 (2019).
DiGiulio, D. B. et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl Acad. Sci. USA 112, 11060–11065 (2015).
Bisanz, J. E. et al. Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of moringa-supplemented probiotic yogurt. Appl. Environ. Microbiol. 81, 4965–4975 (2015).
Gough, E. K. et al. Maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe. EBioMedicine 68, 103421 (2021).
Lammert, C. R. et al. Cutting edge: critical roles for microbiota-mediated regulation of the immune system in a prenatal immune activation model of autism. J. Immunol. https://doi.org/10.4049/jimmunol.1701755 (2018).
Kim, S. et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 (2017).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Vuong, H. E. et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature https://doi.org/10.1038/s41586-020-2745-3 (2020).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Ahmed, T., Hossain, M. & Sanin, K. I. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann. Nutr. Metab. 61, 8–17 (2012).
McGuire, S. WHO guideline: vitamin A supplementation in pregnant women. Geneva: WHO, 2011; WHO guideline: vitamin A supplementation in postpartum women. Geneva: WHO, 2011. Adv. Nutr. 3, 215–216 (2012).
Grizotte-Lake, M. et al. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 49, 1103–1115.e6 (2018).
Jijon, H. B. et al. Intestinal epithelial cell-specific RARα depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol. 11, 703–715 (2018).
Kheirouri, S. & Alizadeh, M. Decreased serum and mucosa immunoglobulin A levels in vitamin A and zinc-deficient mice. Cent. Eur. J. Immunol. 39, 165–169 (2014).
Hibberd, M. C. et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci. Transl. Med. 9, eaal4069 (2017).
Alves da Silva, A. V. et al. Murine methyl donor deficiency impairs early growth in association with dysmorphic small intestinal crypts and reduced gut microbial community diversity. Curr. Dev. Nutr. 3, nzy070 (2019).
Forgie, A. J. et al. The impact of maternal and early life malnutrition on health: a diet-microbe perspective. BMC Med. 18, 135 (2020).
Mayneris-Perxachs, J. et al. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am. J. Clin. Nutr. 104, 1253–1262 (2016).
Preidis, G. A. et al. Composition and function of the undernourished neonatal mouse intestinal microbiome. J. Nutr. Biochem. 26, 1050–1057 (2015).
Marwarha, G., Claycombe-Larson, K., Schommer, J. & Ghribi, O. Maternal low protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring. J. Nutr. Biochem. 45, 54–66 (2017).
Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–481 (2012).
Martinez-Guryn, K. et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 23, 458–469.e5 (2018).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Semova, I. et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12, 277–288 (2012).
Makki, K., Deehan, E. C., Walter, J. & Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018).
Priyadarshini, M. et al. Maternal short-chain fatty acids are associated with metabolic parameters in mothers and newborns. Transl. Res. 164, 153–157 (2014).
Nakajima, A. et al. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J. Immunol. 199, 3516–3524 (2017).
Thorburn, A. N. et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 6, 7320 (2015).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Yu, Y., Raka, F. & Adeli, K. The role of the gut microbiota in lipid and lipoprotein metabolism. J. Clin. Med. 8, 2227 (2019).
de Onis, M., Blössner, M. & Villar, J. Levels and patterns of intrauterine growth retardation in developing countries. Eur. J. Clin. Nutr. 52, S5–S15 (1998).
Christian, P. et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol. 42, 1340–1355 (2013).
Arifeen, S. E. et al. Infant growth patterns in the slums of Dhaka in relation to birth weight, intrauterine growth retardation, and prematurity. Am. J. Clin. Nutr. 72, 1010–1017 (2000).
Sato, Y. et al. Maternal gut microbiota is associated with newborn anthropometrics in a sex-specific manner. J. Dev. Orig. Health Dis. https://doi.org/10.1017/S2040174419000138 (2019).
Shiozaki, A. et al. Intestinal microbiota is different in women with preterm birth: results from terminal restriction fragment length polymorphism analysis. PLoS ONE 9, e111374 (2014).
Dahl, C. et al. Gut microbiome of mothers delivering prematurely shows reduced diversity and lower relative abundance of Bifidobacterium and Streptococcus. PLoS ONE 12, e0184336 (2017).
Kimura, I. et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 367, eaaw8429 (2020).
Machado, C. J., Whitaker, A. M., Smith, S. E. P., Patterson, P. H. & Bauman, M. D. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol. Psychiat. 77, 823–832 (2015).
Bauman, M. D. et al. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol. Psychiat. 75, 332–341 (2014).
Nyangahu, D. D. et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 6, 124 (2018).
Miyoshi, J. et al. Peripartum exposure to antibiotics promotes persistent gut dysbiosis, immune imbalance, and inflammatory bowel disease in genetically prone offspring. Cell Rep. 20, 491–504 (2017).
Agüero, M. Gde et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016).
Leiby, J. S. et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6, 196 (2018).
Lauder, A. P. et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 29 (2016).
Mishra, A. et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409.e20 (2021).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra65 (2014).
Asnicar, F. et al. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems 2, e00164-16 (2017).
Yassour, M. et al. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host Microbe 24, 146–154.e4 (2018).
Ferretti, P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018).
Martín, R. et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Env. Microbiol. 75, 965–969 (2009).
Collado, M. C., Delgado, S., Maldonado, A. & Rodríguez, J. M. Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett. Appl. Microbiol. 48, 523–528 (2009).
Perez, P. F. et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119, e724–e732 (2007).
Grölund, M.-M., Lehtonen, O.-P., Eerola, E. & Kero, P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr. 28, 19–25 (1999).
Penders, J. et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006).
Biasucci, G., Benenati, B., Morelli, L., Bessi, E. & Boehm, G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J. Nutr. 138, 1796S–1800S (2008).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Shao, Y. et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121 (2019).
Chu, D. M. et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 23, 314–326 (2017).
Dominguez-Bello, M. G. et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 22, 250–253 (2016).
Korpela, K. et al. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell 183, 324–334.e5 (2020).
Mårild, K., Stephansson, O., Montgomery, S., Murray, J. A. & Ludvigsson, J. F. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology 142, 39–45.e3 (2012).
Keag, O. E., Norman, J. E. & Stock, S. J. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 15, e1002494 (2018).
Myklestad, K., Vatten, L. J., Magnussen, E. B., Salvesen, K. Å. & Romundstad, P. R. Do parental heights influence pregnancy length?: a population-based prospective study, HUNT 2. BMC Pregnancy Childbirth 13, 33 (2013).
Zhang, G. et al. Assessing the causal relationship of maternal height on birth size and gestational age at birth: a Mendelian randomization analysis. PLoS Med. 12, e1001865 (2015).
Özaltin, E., Hill, K. & Subramanian, S. V. Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA 303, 1507–1516 (2010).
Addo, O. Y. et al. Maternal height and child growth patterns. J. Pediatr. 163, 549–554.e1 (2013).
Yu, D.-H. et al. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol. 16, 211 (2015).
Zivkovic, A. M., German, J. B., Lebrilla, C. B. & Mills, D. A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl Acad. Sci. 108, 4653–4658 (2011).
Seferovic, M. D. et al. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 10, 22092 (2020).
Charbonneau, M. R. et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164, 859–871 (2016).
Cowardin, C. A. et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc. Natl Acad. Sci. 116, 11988–11996 (2019).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021).
Goldman, A. S. & Goldblum, R. M. Transfer of maternal leukocytes to the infant by human milk. In Reproductive Immunology (ed. Olding, L. B.) 205–213 (Springer, 1997).
Baban, B., Malik, A., Bhatia, J. & Yu, J. C. Presence and profile of innate lymphoid cells in human breast milk. JAMA Pediatr. 172, 594–596 (2018).
Cabinian, A. et al. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoS ONE 11, e0156762 (2016).
Ghosh, M. K., Nguyen, V., Muller, H. K. & Walker, A. M. Maternal milk T cells drive development of transgenerational Th1 immunity in offspring thymus. J. Immunol. 197, 2290–2296 (2016).
Ghosh, M. K., Muller, H. K. & Walker, A. M. Lactation-based maternal educational immunity crosses MHC class I barriers and can impart Th1 immunity to Th2-biased recipients. J. Immunol. 199, 1729–1736 (2017).
Dutta, P. & Burlingham, W. J. Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism 1, 2–10 (2010).
Kinder, J. M., Stelzer, I. A., Arck, P. C. & Way, S. S. Immunological implications of pregnancy-induced microchimerism. Nat. Rev. Immunol. 17, 483–494 (2017).
Tuboly, S., Bernáth, S., Glávits, R., Kovács, A. & Megyeri, Z. Intestinal absorption of colostral lymphocytes in newborn lambs and their role in the development of immune status. Acta Vet. Hung. 43, 105–115 (1995).
Tuboly, S., Bernáth, S., Glávits, R. & Medveczky, I. Intestinal absorption of colostral lymphoid cells in newborn piglets. Vet. Immunol. Immunopathol. 20, 75–85 (1988).
Lemke, H., Coutinho, A. & Lange, H. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol. 25, 180–186 (2004).
Martín, R. et al. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediat. 143, 754–758 (2003).
Khodayar-Pardo, P., Mira-Pascual, L., Collado, M. C. & Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 34, 599–605 (2014).
Wilson, E. & Butcher, E. C. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J. Exp. Med. 200, 805–809 (2004).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Bokulich, N. A. et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 8, 343ra82–343ra82 (2016).
Vatanen, T. et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594 (2018).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Subramanian, S. et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014).
Hsiao, A. et al. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 515, 423–426 (2014).
Guerrant, D. I. et al. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 61, 707–713 (1999).
Rogawski, E. T. et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob. Health 6, e1319–e1328 (2018).
Brown, E. M. et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat. Commun. 6, 7806 (2015).
Bolick, D. T. et al. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. J. Med. Microbiol. 62, 896–905 (2013).
Roche, J. K., Cabel, A., Sevilleja, J., Nataro, J. & Guerrant, R. L. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J. Infect. Dis. 202, 506–514 (2010).
Giallourou, N. et al. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog. 14, e1007083 (2018).
Rhoades, N. S. et al. Growth faltering regardless of chronic diarrhea is associated with mucosal immune dysfunction and microbial dysbiosis in the gut lumen. Mucosal Immunol. https://doi.org/10.1038/s41385-021-00418-2 (2021).
Schwarzer, M. et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351, 854–857 (2016).
Yan, J. et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl Acad. Sci. 113, E7554–E7563 (2016).
Jiang, N. M. et al. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am. J. Trop. Med. Hyg. 97, 974–979 (2017).
Bland, S. T. et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav. Immun. 24, 329–338 (2010).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Knoop, K. A. et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2, eaao1314 (2017).
Al Nabhani, Z. et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity https://doi.org/10.1016/j.immuni.2019.02.014 (2019).
Ramanan, D. et al. An immunologic mode of multigenerational transmission governs a gut treg setpoint. Cell 181, 1276–1290.e13 (2020).
Huus, K. E. et al. Immunoglobulin recognition of fecal bacteria in stunted and non-stunted children: findings from the Afribiota study. Microbiome 8, 113 (2020).
Kau, A. L. et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med. 7, 276ra24–276ra24 (2015).
Bhattacharjee, A. et al. Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy. Immunity 54, 1745–1757.e7 (2021).
Miyazaki, A. et al. Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine 36, 6270–6281 (2018).
Vlasova, A. N. et al. Protein malnutrition modifies innate immunity and gene expression by intestinal epithelial cells and human rotavirus infection in neonatal gnotobiotic pigs. mSphere 2, e00046-17 (2017).
Twitchell, E. L. et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 8, 51 (2016).
Molès, J.-P. et al. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 29, 133–143 (2018).
Weström, B., Arévalo Sureda, E., Pierzynowska, K., Pierzynowski, S. G. & Pérez-Cano, F.-J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 11, 1153 (2020).
Catassi, C., Bonucci, A., Coppa, G. V., Carlucci, A. & Giorgi, P. L. Intestinal permeability. Changes during the first month: effect of natural versus artificial feeding. J. Pediatr. Gastroenterol. Nutr. 21, 383–386 (1995).
Moore, S. R. et al. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G831–G839 (2015).
Bein, A. et al. Nutritional deficiency in an intestine-on-a-chip recapitulates injury hallmarks associated with environmental enteric dysfunction. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00899-x (2022).
Kummerlowe, C. et al. Single-cell profiling of environmental enteropathy reveals signatures of epithelial remodeling and immune activation. Sci. Transl. Med. 14, eabi8633 (2022).
Syed, S. et al. Assessment of machine learning detection of environmental enteropathy and celiac disease in children. JAMA Netw. Open 2, e195822 (2019).
Gora, M. J. et al. Tethered capsule endomicroscopy: from bench to bedside at a primary care practice. J. Biomed. Opt. 21, 104001 (2016).
Thompson, A. J. et al. The potential role of optical biopsy in the study and diagnosis of environmental enteric dysfunction. Nat. Rev. Gastroenterol. Hepatol. 14, 727–738 (2017).
Hambidge, K. M. et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the women first trial. Am. J. Clin. Nutr. 109, 457–469 (2019).
Pickering, A. J. et al. The WASH Benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob. Health 7, e1139–e1146 (2019).
Jones, K. D. et al. Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial. BMC Med. 12, 133 (2014).
Gehrig, J. L. et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 365, eaau4732 (2019).
Ito, M. et al. Fermented foods and preterm birth risk from a prospective large cohort study: the Japan environment and children’s study. Env. Health Prev. Med. 24, 25 (2019).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell https://doi.org/10.1016/j.cell.2021.06.019 (2021).
Bobilev, D. et al. 1953. VE303, a rationally designed bacterial consortium for prevention of recurrent Clostridioides difficile (C. Difficile) infection (rCDI), stably restores the gut microbiota after vancomycin (vanco)-induced dysbiosis in adult healthy volunteers (HV). Open Forum Infect. Dis. 6, S60 (2019).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Walsh, C., Lane, J. A., van Sinderen, D. & Hickey, R. M. From lab bench to formulated ingredient: characterization, production, and commercialization of human milk oligosaccharides. J. Funct. Foods 72, 104052 (2020).
Huda, M. N. et al. Stool microbiota and vaccine responses of infants. Pediatrics 134, e362–e372 (2014).
de Rooij, S. R., Wouters, H., Yonker, J. E., Painter, R. C. & Roseboom, T. J. Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl Acad. Sci. USA 107, 16881–16886 (2010).
Acknowledgements
The authors were supported by the US National Institutes of Health (awards R01HD105729 (to C.A.C.), D43TW007585 (to S.R.M., S.S., Z.J. and S.A.A.), K23DK117061 (to S.S.), K43TW010697 (to N.I.) and U19AI116491 (to S.R.M.)); and by the Bill and Melinda Gates Foundation (grants OPP1144149 (to S.R.M.) and OPP1138727 (to S.A.A.)). The authors gratefully acknowledge insights derived from conversations with Bill and Melinda Gates Foundation programme officers R. Elliott, C. Damman, J. Yan, H. Gammill and V. Ridaura.
Author information
Authors and Affiliations
Contributions
S.R.M. and C.A.C. researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed/edited the manuscript before submission. S.S., N.I., Z.J. and J.I. researched data for the article, wrote the article, and reviewed/edited the manuscript before submission. K.S. wrote the article and reviewed/edited the manuscript before submission. S.A.A. researched data for the article, made a substantial contribution to discussion of content, and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.R.M. was a paid consultant for Takeda on paediatric short bowel syndrome in 2020. S.R.M. receives royalties from UpToDate for a chapter entitled “Persistent diarrhoea in children in resource-limited countries”. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Rashidul Haque, Pascale Vonaesch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cowardin, C.A., Syed, S., Iqbal, N. et al. Environmental enteric dysfunction: gut and microbiota adaptation in pregnancy and infancy. Nat Rev Gastroenterol Hepatol 20, 223–237 (2023). https://doi.org/10.1038/s41575-022-00714-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00714-7
This article is cited by
-
Microbial cues stimulate linear growth in undernourished mice
Nature Reviews Gastroenterology & Hepatology (2023)