Abstract
Necrotizing enterocolitis (NEC) is the leading cause of death and disability from gastrointestinal disease in premature infants. Recent discoveries have shed light on a unifying theorem to explain the pathogenesis of NEC, suggesting that specific treatments might finally be forthcoming. A variety of experiments have highlighted how the interaction between bacterial signalling receptors on the premature intestine and an abnormal gut microbiota incites a pro-inflammatory response in the intestinal mucosa and its underlying endothelium that leads to NEC. Central amongst the bacterial signalling receptors implicated in NEC development is the lipopolysaccharide receptor Toll-like receptor 4 (TLR4), which is expressed at higher levels in the premature gut than in the full-term gut. The high prenatal intestinal expression of TLR4 reflects the role of TLR4 in the regulation of normal gut development, and supports additional studies indicating that NEC develops in response to signalling events that occur in utero. This Review provides new evidence explaining the pathogenesis of NEC, explores new findings indicating that NEC development has origins before birth, and discusses future questions and opportunities for discovery in this field.
Key points
-
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants and is characterized by the acute onset of patchy necrosis throughout the intestine, leading to systemic sepsis.
-
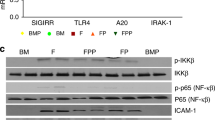
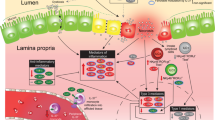
NEC induction requires the activation of Toll-like receptor 4 (TLR4) on the intestinal epithelium by the intestinal microbiota of the premature host, leading to enterocyte death, mucosal injury and translocation of bacteria into the circulation.
-
TLR4 is expressed at higher levels in the premature than in the full-term intestine due to its role in the regulation of normal gut development, and is inhibited by breast milk and amniotic fluid in vitro and in vivo.
-
Studies in animals and human NEC tissue have shown that activation of the aryl hydrocarbon receptor in utero can modulate the risk of NEC through effects on TLR4, potentially offering an opportunity during pregnancy for NEC prevention.
-
Additional studies have identified causative roles for epigenetic modulation of key signalling molecules and the inflammasome in NEC pathogenesis, and have shed light on the effects of NEC on the developing brain and lung.
-
New prevention and therapeutic approaches for NEC are designed to interfere with the abnormal host–microorganism signalling that occurs during the prenatal and early postnatal periods that lead to NEC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alsaied, A., Islam, N. & Thalib, L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. https://doi.org/10.21203/rs.3.rs-17868/v2 (2020).
Patel, R. M., Ferguson, J., McElroy, S. J., Khashu, M. & Caplan, M. S. Defining necrotizing enterocolitis: current difficulties and future opportunities. Pediatr. Res. 88, 10–15 (2020).
Garg, P. M. et al. Hematological predictors of mortality in neonates with fulminant necrotizing enterocolitis. J. Perinatol. 41, 1110–1121 (2021).
Hall, N. J., Eaton, S. & Pierro, A. Necrotizing enterocolitis: prevention, treatment, and outcome. J. Pediatr. Surg. 48, 2359–2367 (2013).
Nolan, L. S., Goree, M. & Good, M. in Necrotizing Enterocolitits: Pathogenesis, Diagnosis and Treatment Ch. 4 (ed. Hackam, D. J.) (CRC Press, 2021).
Hackam, D. J. in Necrotizing Enterocolitits: Pathogenesis, Diagnosis and Treatment Ch. 20 (ed. Hackam, D. J.) (CRC Press, 2021).
Ganapathy, V., Hay, J. W., Kim, J. H., Lee, M. L. & Rechtman, D. J. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr. 13, 127 (2013).
Garg, P. M. et al. Brain injury in preterm infants with surgical necrotizing enterocolitis: clinical and bowel pathological correlates. Pediatr. Res., https://doi.org/10.1038/s41390-021-01614-3 (2021).
Leaphart, C. L. et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Jilling, T. et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Sodhi, C. P. et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143, 708–718.e5 (2012).
Sodhi, C. P. et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 (2010).
Shaw, A. G. et al. Premature neonatal gut microbial community patterns supporting an epithelial TLR-mediated pathway for necrotizing enterocolitis. BMC Microbiol. 21, 225 (2021).
Lu, P. et al. Maternal aryl hydrocarbon receptor activation protects newborns against necrotizing enterocolitis. Nat. Commun. 12, 1042 (2021).
Zhou, Q. et al. Necrotizing enterocolitis induces T lymphocyte-mediated injury in the developing mammalian brain. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay6621 (2021).
Jia, H. et al. Toll like receptor 4 mediated lymphocyte imbalance induces Nec-induced lung injury. Shock 52, 215–223 (2019).
Nino, D. F. et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aan0237 (2018).
Jia, H. et al. Pulmonary epithelial TLR4 activation leads to lung injury in neonatal necrotizing enterocolitis. J. Immunol. 197, 859–871 (2016).
Neu, J., Chen, M. & Beierle, E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 14, 137–144 (2005).
Israel, E. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr. 83, 27–32 (1994).
Maynard, A. A. et al. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G614–G622 (2010).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Bowker, R. M., Yan, X. & De Plaen, I. G. Intestinal microcirculation and necrotizing enterocolitis: the vascular endothelial growth factor system. Semin. Fetal Neonatal Med. 23, 411–415 (2018).
Sullivan, B. A. & Fairchild, K. D. Predictive monitoring for sepsis and necrotizing enterocolitis to prevent shock. Semin. Fetal Neonatal Med. 20, 255–261 (2015).
Waard, M. et al. Time to full enteral feeding for very low-birth-weight infants varies markedly among hospitals worldwide but may not be associated with incidence of necrotizing enterocolitis: the NEOMUNE-NeoNutriNet cohort study. J. Parenter. Enter. Nutr. 43, 658–667 (2019).
Sisk, P. M., Lovelady, C. A., Dillard, R. G., Gruber, K. J. & O’Shea, T. M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 27, 428–433 (2007).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatr 129, e298–e304 (2012).
Sankaran, K. et al. Gastrointestinal polyposis in pediatric patients. J. Pediatr. Gastroenterol. Nutr. 39, 366–372 (2004).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Battersby, C., Santhalingam, T., Costeloe, K. & Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 103, F182–F189 (2018).
Rolnitsky, A. et al. A quality improvement intervention to reduce necrotizing enterocolitis in premature infants with probiotic supplementation. Pediatr. Qual. Saf. 4, e201 (2019).
Zozaya, C. et al. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: a multicenter cohort study. Front. Pediatr. 8, 188 (2020).
Ballance, W. A., Dahms, B. B., Shenker, N. & Kliegman, R. M. Evaluation and treatment of congenital syphilis. J. Pediatr. 117, 843–852 (1990).
Remon, J. I. et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J. Perinatol. 35, 755–762 (2015).
Egan, C. E. et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J. Clin. Invest. 126, 495–508 (2016).
Ballance, W. A., Dahms, B. B., Shenker, N. & Kliegman, R. M. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J. Pediatr. 117, S6–S13 (1990).
Hackam, D. J. Necrotizing Enterocolitis: Pathogenesis, Diagnosis and Treatment 302 (CRC Press, 2021).
Okuyama, H. et al. A comparison of the clinical presentation and outcome of focal intestinal perforation and necrotizing enterocolitis in very-low-birth-weight neonates. Pediatr. Surg. Int. 18, 704–706 (2002).
Humberg, A. et al. Surgical necrotizing enterocolitis but not spontaneous intestinal perforation is associated with adverse neurological outcome at school age. Sci. Rep. 10, 2373 (2020).
Shah, T. A. et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J. Perinatol. 32, 552–558 (2012).
Altit, G., Bhombal, S., Hopper, R. K., Tacy, T. A. & Feinstein, J. Death or resolution: the “natural history” of pulmonary hypertension in bronchopulmonary dysplasia. J. Perinatol. 39, 415–425 (2019).
Seeman, S. M., Mehal, J. M., Haberling, D. L., Holman, R. C. & Stoll, B. J. Infant and maternal risk factors related to necrotising enterocolitis-associated infant death in the United States. Acta Paediatr. 105, e240–e246 (2016).
Zhao, M. & Burisch, J. Impact of genes and the environment on the pathogenesis and disease course of inflammatory bowel disease. Dig. Dis. Sci. 64, 1759–1769 (2019).
Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 89, S13–S20 (2013).
Silber, G. H. Hematochezia in infants less than 6 months of age. Arch. Pediatr. Adolesc. Med. 140, 1097 (1986).
Fisher, J. G. et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J. Pediatr. Surg. 49, 1215–1219 (2014).
Shin, S. H. et al. Surgical necrotizing enterocolitis versus spontaneous intestinal perforation in white matter injury on brain magnetic resonance imaging. Neonatology 110, 148–154 (2016).
Awolaran, O. & Sheth, J. Management strategies of functional intestinal obstruction of prematurity. J. Neonatal Surg. https://doi.org/10.47338/jns.v10.926 (2021).
Travadi, J. N., Patole, S. K. & Gardiner, K. Pneumatosis coli, a benign form of necrotising enterocolitis. Indian. Pediatr. 40, 349–351 (2003).
Niño, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Hackam, D. & Caplan, M. Necrotizing enterocolitis: pathophysiology from a historical context. Semin. Pediatr. Surg. 27, 11–18 (2018).
Jilling, T. et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Yu, W. et al. SIGIRR mutation identified in human necrotizing enterocolitis (NEC) disrupts STAT3-dependent microRNA expression in neonatal gut. Cell Mol. Gastroenterol. Hepatol. https://doi.org/10.1016/j.jcmgh.2021.09.009 (2021).
Sampath, V. et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatr 135, e1530–e1534 (2015).
Liu, Y., Fatheree, N. Y., Mangalat, N. & Rhoads, J. M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G608–G617 (2012).
Jilling, T., Lu, J., Jackson, M. & Caplan, M. S. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr. Res. 55, 622–629 (2004).
Afrazi, A. et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J. Biol. Chem. 289, 9584–9599 (2014).
Neal, M. D. et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 190, 3541–3551 (2013).
Yu, Y. et al. Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS ONE 8, e69620 (2013).
Werts, A. D. et al. A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis. Cell. Mol. Gastroenterol. Hepatol. 9, 403–423 (2020).
Cetin, S. et al. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J. Biol. Chem. 279, 24592–24600 (2004).
Qureshi, F. G. et al. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology 128, 1012–1022 (2005).
Neal, M. D. et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 upregulated modulator of apoptosis. J. Biol. Chem. 287, 37296–37308 (2012).
Richardson, W. M. et al. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology 139, 904–917 (2010).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606 (2001).
Neal, M. D. et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 176, 3070–3079 (2006).
Yazji, I. et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc. Natl Acad. Sci. USA 110, 9451–9456 (2013).
Kovler, M. L. et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci. Transl. Med. 13, eabg3459 (2021).
Lu, J., Jilling, T., Li, D. & Caplan, M. S. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 61, 427–432 (2007).
Chan, K. L., Wong, K. F. & Luk, J. M. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: pathogenesis and therapeutic implications. World J. Gastroenterol. 15, 4745–4752 (2009).
Liu, Y. et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G442–G450 (2009).
Le Mandat Schultz, A. et al. Expression of TLR-2, TLR-4, NOD2 and pNF-κB in a neonatal rat model of necrotizing enterocolitis. PloS ONE 2, e1102 (2007).
Sun, Q. et al. Sodium butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediat. Inflamm. 2021, (2021).
Yan, X. et al. Supplementary bovine colostrum feedings to formula-fed preterm pigs improve gut function and reduce necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 73, e39–e46 (2021).
Gribar, S. C. et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 (2009).
Klerk, D. H. et al. DNA methylation of TLR4, VEGFA, and DEFA5 is associated with necrotizing enterocolitis in preterm Infants. Front. Pediatr. https://doi.org/10.3389/fped.2021.630817 (2021).
Cho, S. X. et al. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat. Commun. 11, 5794 (2020).
Gomart, A., Vallée, A. & Lecarpentier, Y. Necrotizing enterocolitis: LPS/TLR4-induced crosstalk between canonical TGF-β/Wnt/β-catenin pathways and PPARγ. Front. Pediatr. 9, 713344 (2021).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Hackam, D. J., Good, M. & Sodhi, C. P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin. Pediatr. Surg. 22, 76–82 (2013).
Szebeni, B. et al. Genetic polymorphisms of CD14, Toll-like receptor 4, and caspase-recruitment domain 15 are not associated with necrotizing enterocolitis in very low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 42, 27–31 (2006).
White, J. R., Gong, H., Pope, B., Schlievert, P. & McElroy, S. J. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Dis. Model Mech. 10, 727–736 (2017).
Østergaard, M. V. et al. Preterm birth reduces nutrient absorption with limited effect on immune gene expression and gut colonization in pigs. J. Pediatr. Gastroenterol. Nutr. 61, 481–490 (2015).
Willems, R. et al. Introducing enteral feeding induces intestinal subclinical inflammation and respective chromatin changes in preterm pigs. Epigenomics 7, 553–565 (2015).
Chowning, R. et al. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J. Perinatol. 36, 221–224 (2016).
Hair, A. B. et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed. Med. 11, 70–74 (2016).
Miller, J. et al. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 10, 1–35 (2018).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Sodhi, C. P. et al. The human milk oligosaccharides 2′-fucosyllactose and 6′-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting Toll-like receptor 4 signaling. Pediatr. Res. 89, 91–101 (2021).
Mulvihill, S. J., Stone, M. M., Fonkalsrud, E. W. & Debas, H. T. Trophic effect of amniotic fluid on fetal gastrointestinal development. J. Surg. Res. 40, 291–296 (1986).
Hofmann, G. E. & Abramowicz, J. S. Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta Obstet. Gynecol. Scand. 69, 217–221 (1990).
Good, M. et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc. Natl Acad. Sci. USA 109, 11330–11335 (2012).
Been, J. V., Lievense, S., Zimmermann, L. J., Kramer, B. W. & Wolfs, T. G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J. Pediatr. 162, 236–242 e232 (2013).
Karatepe, H. O. et al. The effect of vascular endothelial growth factor overexpression in experimental necrotizing enterocolitis. Pediatr. Surg. Int. 30, 327–332 (2014).
Yan, X. et al. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G716–G725 (2016).
Moonen, R. M. et al. Risk of necrotizing enterocolitis associated with the single nucleotide polymorphisms VEGF C-2578A, IL-18 C-607A, and IL-4 receptor α-chain A-1902G: a validation study in a prospective multicenter cohort. Front. Pediatr. 8, 45 (2020).
Schirbel, A. et al. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology 144, 613 (2013).
Heise, T. et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin. Ther. 38, 2265–2276 (2016).
Greenwood, C. et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J. Pediatr. 165, 23–29 (2014).
La Rosa, P. S. et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl Acad. Sci. USA 111, 12522–12527 (2014).
Neu, J. Necrotizing enterocolitis: a multi-omic approach and the role of the microbiome. Dig. Dis. Sci. 65, 789–796 (2020).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Wang, Y. et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 3, 944–954 (2009).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE https://doi.org/10.1371/journal.pone.0020647 (2011).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31 (2017).
Torrazza, R. M. & Neu, J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J. Perinatol. 31, S29–S34 (2011).
Olm, M. R. et al. Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci. Adv. 5, eaax5727 (2019).
Hill, C. et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
AlFaleh, K. & Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, CD005496 (2014).
Chi, C. et al. Effects of probiotics in preterm infants: a network meta-analysis. Pediatr 147, e20200706 (2021).
Brower-Sinning, R. et al. Mucosa-associated bacterial diversity in necrotizing enterocolitis. PLoS ONE 9, e105046 (2014).
Tanner, S. M. et al. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am. J. Pathol. 185, 4–16 (2015).
Kim, C. S. & Claud, E. C. Necrotizing enterocolitis pathophysiology: how microbiome data alter our understanding. Clin. Perinatol. 46, 29–38 (2019).
Wu, S.-F., Caplan, M. & Lin, H.-C. Necrotizing enterocolitis: old problem with new hope. Pediatr. Neonatol. 53, 158–163 (2012).
Weitkamp, J. H. et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62, 73–82 (2013).
Pang, Y., Du, X., Xu, X., Wang, M. & Li, Z. Impairment of regulatory T cells in patients with neonatal necrotizing enterocolitis. Int. Immunopharmacol. 63, 19–25 (2018).
Ma, F. et al. Interleukin-6-mediated CCR9(+) interleukin-17-producing regulatory T cells polarization increases the severity of necrotizing enterocolitis. EBioMedicine 44, 71–85 (2019).
Qazi, K. R. et al. Extremely preterm infants have significant alterations in their conventional T cell compartment during the first weeks of life. J. Immunol. 204, 68–77 (2020).
Liu, Y. et al. Lactobacillus reuteri DSM 17938 feeding of healthy newborn mice regulates immune responses while modulating gut microbiota and boosting beneficial metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G824–G838 (2019).
Schwarz, J. et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin. Exp. Immunol. 191, 328–337 (2018).
He, Y. M. et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat. Med. 24, 224–231 (2018).
Liu, Y. et al. Lactoferrin-induced myeloid-derived suppressor cell therapy attenuates pathologic inflammatory conditions in newborn mice. J. Clin. Invest. 129, 4261–4275 (2019).
Liu, J. et al. TFF3 mediates the NF-κB/COX2 pathway to regulate PMN-MDSCs activation and protect against necrotizing enterocolitis. Eur. J. Immunol. 51, 1110–1125 (2021).
Kostlin, N. et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) in breast milk (BM); GR-MDSC accumulate in human BM and modulate T-cell and monocyte function. Front. Immunol. 9, 1098 (2018).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Vincent, D. et al. NEC is likely a NETs dependent process and markers of NETosis are predictive of NEC in mice and humans. Sci. Rep. 8, 12612 (2018).
Klinke, M. et al. Development of an improved murine model of necrotizing enterocolitis shows the importance of neutrophils in NEC pathogenesis. Sci. Rep. 10, 8049 (2020).
Namachivayam, K. et al. Targeted inhibition of thrombin attenuates murine neonatal necrotizing enterocolitis. Proc. Natl Acad. Sci. USA 117, 10958–10969 (2020).
MohanKumar, K. et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 10, 3494 (2019).
Namachivayam, K., Mohankumar, K., Garg, L., Torres, B. A. & Maheshwari, A. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr. Res. 81, 817–824 (2017).
Maheshwari, A. Role of platelets in neonatal necrotizing enterocolitis. Pediatr. Res. 89, 1087–1093 (2021).
Good, M. et al. Neonatal necrotizing enterocolitis-associated DNA methylation signatures in the colon are evident in stool samples of affected individuals. Epigenomics 13, 829–844 (2021).
Zhu, F. et al. Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice. J. Neuroinflammation https://doi.org/10.1186/s12974-021-02111-4 (2021).
Mohankumar, K. et al. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr. Res. 79, 951–961 (2016).
Zhang, H. et al. SOCS3 protects against neonatal necrotizing enterocolitis via suppressing NLRP3 and AIM2 inflammasome activation and p65 nuclear translocation. Mol. Immunol. 122, 21–27 (2020).
Fernandez, R., D’Apremont, I., Dominguez, A. & Tapia, J. L. Survival and morbidity of very low birth weight infant in a South American neonatal network. Arch. Argent. Pediatr. 112, 405–412 (2014).
Drucker, N. A., Jensen, A. R., Te Winkel, J. P., Ferkowicz, M. J. & Markel, T. A. Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis. J. Pediatr. Surg. 53, 1208–1214 (2018).
Drucker, N. A., Jensen, A. R., te Winkel, J. P., Ferkowicz, M. J. & Markel, T. A. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J. Pediatr. Surg. 53, 1208–1214 (2018).
Willis, K. A. & Ambalavanan, N. Necrotizing enterocolitis and the gut-lung axis. Semin. Perinatol. https://doi.org/10.1016/j.semperi.2021.151454 (2021).
Soraisham, A. S., Amin, H. J., Al-Hindi, M. Y., Singhal, N. & Sauve, R. S. Does necrotising enterocolitis impact the neurodevelopmental and growth outcomes in preterm infants with birthweight ≤1250 g? J. Paediatr. Child. Health 42, 499–504 (2006).
Sonntag, J. et al. Growth and neurodevelopmental outcome of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr. 89, 528–532 (2000).
Kuik, S. J. et al. Time to full enteral feeding after necrotizing enterocolitis in preterm-born children is related to neurodevelopment at 2-3 years of age. Early Hum. Dev. 147, 105091 (2020).
Hickey, M., Georgieff, M. & Ramel, S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin. Fetal Neonatal Med. 23, 426–432 (2018).
Saunders, L. et al. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr. Perinat. Epidemiol. 28, 235–244 (2014).
Parlapani, E. et al. The Mediterranean diet adherence by pregnant women delivering prematurely: association with size at birth and complications of prematurity. J. Matern. Fetal Neonatal Med. 32, 1084–1091 (2019).
Weintraub, A. S. et al. Antenatal antibiotic exposure in preterm infants with necrotizing enterocolitis. J. Perinatol. 32, 705–709 (2012).
Sood, B. G. et al. The risk of necrotizing enterocolitis after indomethacin tocolysis. Pediatr 128, e54–e62 (2011).
Roberts, D., Brown, J., Medley, N. & Dalziel, S. R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.cd004454.pub3 (2017).
Watson, S. N. & McElroy, S. J. Potential prenatal origins of necrotizing enterocolitis. Gastroenterol. Clin. North. Am. 50, 431–444 (2021).
Deshpande, G., Rao, S., Patole, S. & Bulsara, M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatr 125, 921–930 (2010).
Chen, C.-C. & Walker, W. A. Probiotics and the mechanism of necrotizing enterocolitis. Semin. Pediatr. Surg. 22, 94–100 (2013).
Good, M. et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G1021–G1032 (2014).
Neal, M. D. et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE 8, e65779 (2013).
Sodhi, C. P. et al. Intestinal epithelial TLR-4 activation is required for the development of acute lung injury after trauma/hemorrhagic shock via the release of HMGB1 from the gut. J. Immunol. 194, 4931–4939 (2015).
Härtel, C. et al. NOD2 loss-of-function mutations and risks of necrotizing enterocolitis or focal intestinal perforation in very low-birth-weight infants. Inflamm. Bowel Dis. 22, 249–256 (2016).
Feng, J., El-Assal, O. N. & Besner, G. E. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J. Pediatr. Surg. 41, 144–149 (2006).
Rager, T. M., Olson, J. K., Zhou, Y., Wang, Y. & Besner, G. E. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J. Pediatr. Surg. 51, 942–947 (2016).
Nitkin, C. R. et al. Stem cell therapy for preventing neonatal diseases in the 21st century: current understanding and challenges. Pediatr. Res. 87, 265–276 (2020).
Nolte-‘t Hoen, E. N. et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 40, 9272–9285 (2012).
Salomon, C. et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 8, e68451 (2013).
Halpern, M. D. et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-α. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G757–G764 (2006).
Gopalakrishna, K. P. et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115 (2019).
Villamor-Martinez, E., Hundscheid, T., Kramer, B. W., Hooijmans, C. R. & Villamor, E. Stem cells as therapy for necrotizing enterocolitis: a systematic review and meta-analysis of preclinical studies. Front. Pediatr. 8, 578984 (2020).
O’Connell, J. S. et al. Administration of extracellular vesicles derived from human amniotic fluid stem cells: a new treatment for necrotizing enterocolitis. Pediatr. Surg. Int. 37, 301–309 (2021).
Weis, V. G. et al. Human placental-derived stem cell therapy ameliorates experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G658–G674 (2021).
Koike, Y. et al. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat. Commun. https://doi.org/10.1038/s41467-020-18750-9 (2020).
Roset Bahmanyar, E., Out, H. J. & Van Duin, M. Women and babies are dying from inertia: a collaborative framework for obstetrical drug development is urgently needed. Am. J. Obstet. Gynecol. 225, 43–50 (2021).
Blakely, M. L. et al. Initial laparotomy versus peritoneal drainage in extremely low birthweight infants with surgical necrotizing enterocolitis or isolated intestinal perforation: a multicenter randomized clinical trial. Ann. Surg. 274, e370–e380 (2021).
Kovler, M. L., Sodhi, C. P. & Hackam, D. J. Precision-based modeling approaches for necrotizing enterocolitis. Dis. Model. Mech. https://doi.org/10.1242/dmm.044388 (2020).
Acknowledgements
The authors thank all the investigators in the field who have contributed in so many ways to the research described in this Review, and apologize to those authors whose work was omitted owing to space considerations. The authors also thank T. H. Phelps from Johns Hopkins University Department of Art as Applied to Medicine for assistance with the original illustrations. D.J.H. is supported by R01GM078238 and R01DK117186 and R35GM141956 from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
D.J.H. is principal investigator on a sponsored research grant from Abbott Nutrition, and a sponsored research grant from Noveome; neither Noveome nor Abbott had any role in the current manuscript. C.P.S. declares no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Erika Claud, Jörn-Hendrik Weitkamp and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hackam, D.J., Sodhi, C.P. Bench to bedside — new insights into the pathogenesis of necrotizing enterocolitis. Nat Rev Gastroenterol Hepatol 19, 468–479 (2022). https://doi.org/10.1038/s41575-022-00594-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00594-x
This article is cited by
-
Risk factors of necrotizing enterocolitis in twin preterm infants
BMC Pediatrics (2024)
-
Gestational age-specific hematological features in preterm infants with necrotizing enterocolitis
Pediatric Research (2024)
-
Do hematological biomarkers predict surgical necrotizing enterocolitis?
Pediatric Research (2024)
-
miR-375-3p targets YWHAB to attenuate intestine injury in neonatal necrotizing enterocolitis
Pediatric Surgery International (2024)
-
Exploration of pathogenic microorganism within the small intestine of necrotizing enterocolitis
World Journal of Pediatrics (2024)