Abstract
Necrotizing enterocolitis (NEC) remains among the most common and devastating diseases in neonates. Despite advances in neonatal clinical care, specific treatment strategies and diagnostic modalities remain lacking. As a result, morbidity and mortality remain high. Improved understanding of the pathogenesis of NEC has the potential for improved therapeutics. Some of the areas of research leading to promising discoveries include inhibition of Toll-like receptor signaling, modulation of vascular endothelial growth factor signal pathways, defining metabolomic alterations in NEC to discover potential biomarkers, probing for genetic predispositions to NEC susceptibility, determining mechanistic relations between anemia and NEC, and microflora modulation through the use of probiotics. All of these areas may represent novel promising approaches to the prevention and treatment of NEC. This review will focus on these current and possible therapeutic perspectives.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is one of the deadliest diseases affecting preterm neonates. Despite over six decades of research, there is still no known prevention or cure, with mortality rates remaining as high as 15–35%.1 Risk factors for NEC include prematurity, enteral formula feeding, and altered gut microflora.2,3,4 Currently, there are no widely accepted preventative methods other than breast milk feeding, and treatment strategies following the development of NEC include withholding feedings, intestinal decompression, broad-spectrum antibiotic therapy, and surgical intervention if the disease progresses. Improved understanding of the pathogenesis of NEC will allow for development and application of more effective preventative and therapeutic strategies. This review will provide an overview of some of the recent studies focusing on clinical or experimental NEC, highlight advances made toward a better understanding of its pathogenesis, and will describe potential preventive approaches and therapies for this disease. We will close with a special perspective from a parent of a child who was afflicted with NEC.
Association with anemia and impaired vasculature
Anemia and vascular insufficiency have been identified as risk factors for NEC due to their role in predisposing the intestine to ischemia.5,6 Lack of sufficient intestinal blood flow is thought to cause hypoxia and ischemia–reperfusion injury to the intestine, which can lead to intestinal injury secondary to reactive oxygen species (ROS) production.1,7 Recently, anemia has been shown to result in intestinal inflammation and gut barrier disruption through proinflammatory cytokine production.8 Additionally, the underdeveloped intestinal microvasculature of the premature infant may play a role in the development of NEC. This microvascular deficiency has been shown to be unable to support the metabolic demands of the growing neonate, resulting in intestinal ischemia and necrosis in animal models of NEC.5,9,10
Other recent data demonstrate an association between anemia and immune alterations that increase an infant’s susceptibility to developing NEC. Specifically, a recent animal study by Stowell and co-workers6 demonstrate that with increasing anemia, there was an increase in an important proinflammatory cytokine, interferon-γ. Furthermore, induced anemia led to significantly increased hypoxia, resulting in an increase in proinflammatory cytokines. Further supporting this idea of anemia playing a role in the pathogenesis of NEC, Patel et al.8 in a multicenter prospective human study showed that very low birth weight infants with severe anemia were more likely to develop NEC compared to infants who did not have severe anemia (adjusted hazard ratio 5.99, 95% confidence interval (CI), 2.00–18, p = 0.001). Lastly, in a recent work, Maheshwari and co-workers11 used a novel mouse model of transfusion-associated NEC to show a mechanistic association between severe anemia, followed by transfusion and subsequent NEC-like injury in the immature intestine. In this model, anemia induced a dose-dependent macrophage infiltration into the intestine. These macrophages were then activated by blood transfusions to induce inflammation and injury. These data are the first to propose a mechanistic link between anemia, transfusions, and subsequent intestinal injury.
One of the focuses of the De Plaen Laboratory has been the discovery of ways to improve intestinal microvasculature integrity and to prevent ischemic injury.10 In a neonatal mouse model of NEC, they showed that dimethyloxalylglycine (DMOG), a hydroxylase inhibitor, decreased the incidence and mortality of severe NEC by increasing the expression of intestinal vascular endothelial growth factor (VEGF). In their animal model, after injection of DMOG prior to induction of NEC, there was a 2.16-fold decrease in mortality (p < 0.005) compared to vehicle control injection.10 Furthermore, they demonstrated improved proliferation of intestinal epithelial cells, and protection against intestinal injury.10 These results are significant because the number of cells expressing VEGF has been shown to be decreased in human infants with NEC.12 If inadequate VEGF signaling can predispose to the hypoxia and vascular insufficiency that are associated with NEC, this can be an important target for possible therapeutics.
New understanding of neurologic outcomes after NEC
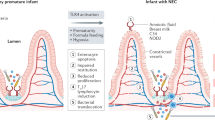
Neurodevelopmental impairment is increasingly recognized as a complication in survivors of NEC.13,14,15,16 It is thought that this cognitive impairment is the result of white matter injury by intestine-derived molecules that cross the premature blood–brain barrier.15 This NEC-associated brain injury is often characterized by demyelination and decreases in brain volume.17 Work mainly from the Hackam Laboratory has shown that Toll-like receptor 4 (TLR4), a known lipopolysaccharide receptor, likely plays a significant role in the pathogenesis of NEC by causing mucosal inflammation.18,19,20 Recently, there has been a focus on how TLR4 may contribute to neurological impairments after NEC. In a recent animal study of NEC, Hackam’s group hypothesized that activation of intestinal TLR4 leads to activation of brain microglial cells through a gut–brain signaling axis and increased oxidative stress. The activation of microglia promotes cognitive impairment.17 They confirmed that NEC-related intestinal injury led to activation of TLR4 in the brain, resulting in increased ROS, demyelination, and cognitive dysfunction. Impaired myelination was observed in different areas of the mouse brain. These findings were similar to the pattern of impaired myelination observed in human infants with NEC.21,22 Furthermore, when they evaluated older mice exposed to NEC, these mice demonstrated severe cognitive defects in the form of impaired spatial working memory and novel object recognition (p ≤ 0.001).17 Notably, these observations resemble reported findings of impaired learning and memory in human infant survivors of NEC.23 They went a step further by evaluating a possible therapeutic to mitigate the neurological injury. They accomplished this by administration of an oral antioxidant therapeutic—a dendrimer nanoparticle coupled with N-acetyl-l-cysteine. This treatment, given 48 h after initiation of NEC, resulted in significantly decreased accumulation of ROS as well as reduced microglial activation (p < 0.001). Importantly, this treatment resulted in a reduction in the observed cognitive impairments,17 and when given prophylactically there was no cognitive impairment.
Importantly, these results were mirrored in a recently published animal study by Biouss et al.24 In their murine model of NEC, they also demonstrated similar findings of smaller brain size, increased apoptosis, reduction in the number of neurons, and increased proinflammatory cytokines and activated microglia in pups exposed to NEC compared to controls (p < 0.0001). Although more work needs to be done, these findings help to elucidate the pathogenesis of NEC-associated neurologic complications and could help identify possible targets to help reduce the developmental delays seen in many NEC surviviors.
Genetic associations
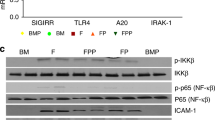
The role of genetic predisposition to NEC is an exciting new area of research. Advances in genomic research, as well as epidemiologic data demonstrating differences among populations in the prevalence of NEC, have paved the way to discovering genetics associations in NEC.25,26 Candidate gene or pathway approaches, as well as genome-wide and exosome sequencing, have led to the discovery of potential genetic variants associated with NEC,27 allowing for future targeted care for infants who possess these genes. The current body of literature evaluating genetic factors in NEC has focused on genes that regulate intestinal immunity and inflammation, as well as genes that regulate oxidative stress, apoptosis, and cell repair.27,28 One particular gene of interest is single immunoglobulin interleukin-1-related receptor (SIGIRR), which has genetic variants present in 5–6% of the population.29 SIGGIR is a known gene whose products inhibit/regulate TLR signaling, which has been shown to be exaggerated in NEC, leading to severe and widespread inflammation.30,31 Sampath et al.29 were the first to report a pilot study identifying a disease phenotype associated with SIGIRR in humans. In this study, they reported that genetic variants resulting in loss of SIGIRR function led to an exaggerated inflammatory response to lipopolysaccharide. Several gene variations were noted in 10 of 17 premature infants with stage II + NEC.29 To further the understanding of SIGIRR’s role in NEC pathogenesis, they recently used SIGIRR−/− mice to demonstrate that in the absence of SIGIRR, mice exposed to experimental NEC had increased intestinal inflammation and apoptosis, and increased NEC severity.31
In addition to the advances in animal studies of genetic associations, there has also been some progress in human studies. Autophagy has been shown to play a role in inflammatory bowel disease32,33,34,35 and potentially also in NEC,36,37 which share many of the same inflammatory pathways. The Sampath Laboratory recently published a newly discovered association between variation in the autophagy gene, ATG16L1 (autophagy-related 16-like 1), and NEC in human neonates.38 In this multicenter study, genotyping was performed on over 1000 patients <35 weeks gestation. Their study demonstrated that a significant number of infants who developed NEC also had an ATG16L1 gene variant (AA genotype) (p = 0.009). Those with the AA genotype of this variant had a statistically significant 2.5-fold increase in NEC (odds ratio (OR) = 2.5, 95% CI = 1.2–5.6, p = 0.01).38 While these results are exciting, genetic association studies in NEC continue to face many challenges, particularly lack of validation. While further studies will be needed to verify the validity and utility of these findings, they represent a way to both screen for susceptibility to NEC and open new pathways for potential therapeutic targets.
Metabolic associations
Diagnosis and early detection of NEC remain difficult, with diagnosis often occurring after the disease has significantly progressed. Biomarkers of disease are objective and quantifiable chemical molecules that mark biological processes, allowing for early detection and diagnosis. Biomarker research has become well accepted,39 with promise for targeted treatments. However, no biomarker exists for NEC. A biomarker for NEC would have tremendous potential in improving our management of at-risk patients, as well as achieving a better understanding of NEC pathogenesis. In light of the often rapid progression of the disease, biomarkers that would identify NEC early, even before clinical signs occur, would be highly beneficial.
The Sylvester Laboratory has made progress in biomarker discovery for NEC. Their focus has been on investigating and identifying molecular characteristics in premature infants that contribute to the development of NEC. Using metabolomic profiling via an already established newborn screening obtained at birth, they have been able to associate 14 acylcarnitine levels with an increased risk of developing NEC.40 In a retrospective cohort study evaluating neonatal intensive care unit admission at a single institution, they were able to associate an increased risk of developing NEC with 14 different acylcarnitines and their levels (OR 1.78, 95% CI: 1.53–2.02, and OR 1.76, 95% CI: 1.51–2.06).40 The key finding of this study is the ability to demonstrate a possible biomarker that is present at birth before clinical changes occur. In addition to metabolomic studies, there have also been proteomic studies in NEC pathogenesis. By evaluating urinary protein biomarkers, Sylvester et al.41 were able to identify several proteins that might assist in differentiating NEC from other causes of sepsis. More recently, they are evaluating the possible utility of fecal calprotectin as a marker of developing NEC. Calprotectin is a protein found in neutrophils and secreted during high leukocyte turnover, such as in inflammation,42 and has been previously examined as a biomarker for NEC.43,44 To date, many studies looking at calprotectin have been inconclusive due to the variable range of levels demonstrated in the neonates studied. Additionally, calprotectin is nonspecific for NEC, as it is elevated in other clinical conditions.
The Burrin and Sanglid Laboratories have performed extensive research on the intestinal microbiome, and how dietary carbohydrate metabolism and antibiotic administration affects gut microbiome and metabolism, in an attempt to determine the risk of developing intestinal injury in piglet models of NEC. One metabolite of particular interest in their studies is bile acids, which are primary components of lipid and carbohydrate metabolism.45,46 In their studies, elevated levels of cholic acid and glycochenodeoxycholic acid were found in untreated piglets exposed to NEC. These same findings have been demonstrated in patients with inflammatory bowel disease.45 It is thought that the presence of these metabolites may represent increased inflammation. These intriguing discoveries again have the potential to improve our ability to diagnose and to monitor NEC.
Probiotics
An important area of interest in the prevention of NEC is the use of probiotics. One of the key mechanisms of probiotics is the potential for altering the intestinal microbiome. In some parts of the world, the use of probiotics has become standard of care since alterations in the intestinal microbiome are well recognized as playing a role in the pathogenesis of NEC.1,25 Many studies in recent decades, both in animal models and human clinical trials, have evaluated the efficacy of probiotics. These studies have used a variety of probiotics, with some using combinations of probiotic bacteria. Despite some advances in animal models, there have been inconsistent results with the use of probiotics in human clinical trials. These inconsistencies have, in part, been due to variable endpoints, dosages of administration, type of probiotic administered, timing of administration, feeding differences, and evaluation of different age groups of infants. Despite these issues, there is convincing evidence of benefit when probiotics are administered, particularly in conjunction with human milk.47 Worldwide, there have been over 30 human trials of probiotics for the prevention and treatment of NEC. A recent metanalysis, including 10,520 infants, concluded that probiotic use decreased NEC incidence and resultant mortality (relative risk: 0.53; 95% CI: 0.42–0.66).48 Despite some encouraging results from both human and animal studies, there remains a significant lack of data on the use of probiotics. Important questions have yet to be answered, including which probiotic strains are best, what dosages should be used, when probiotics should be initiated and stopped, and if they should be given with or without feeds. In addition, there are currently no US Food and Drug Administration approved or US government-regulated products available to give to premature infants. Until some of these critical key questions are answered, it remains necessary to continue research on this possible advancement in treating and preventing NEC.
Recently, the Underwood Laboratory published a review arguing for widespread use of probiotics to prevent NEC.47,49 Previously, Underwood et al.50 demonstrated that the administration of Bifidobacterium longum subsp. infantis (B. infantis) in a murine model of NEC significantly reduced the incidence and severity of NEC from 80% incidence to 40% (p < 0.01). In addition, in that same study, they demonstrated attenuation of cellular apoptosis, decreased intestinal inflammation, and decreased intestinal permeability.50
To date, all probiotic clinical trials have administered probiotics in their planktonic (free-living) state, and require multiple doses of administration to see a beneficial effect. In multiple animal studies, Besner and co-workers51,52 have demonstrated that delivery of the probiotic Lactobacillus reuteri (Lr) in its biofilm state, induced by adherence of the bacteria to maltose-loaded microspheres, leads to a significant decrease in the incidence and severity of NEC compared to delivery of free-living Lr (15% vs. 60%, p < 0.001), with decreased intestinal permeability and inflammation, and beneficial alterations in the intestinal microbiome. Notably, these beneficial effects occurred after administration of only one single dose of the probiotic. These findings support the potential for an improved next-generation microbiome therapy for NEC.
Why this matters to parents of infants with NEC (insights from Ryan Raab)
Sarah was born at 23 weeks, 6 days. Being so early and weighing only 690 g, we were terrified for her chances of survival. As the weeks went by, Sarah’s growth and development exceeded all expectations. Watching her thrive, we began to breathe easier and allowed ourselves to imagine a future with Sarah. At 59 days old, we received a call from the NICU that she was having a rough night. When we arrived at the hospital, it was clear that she had NEC. Bedside surgery determined that it was NEC totalis, and no treatment could save her life. We removed all life support and she died in our arms, within 12 h of her diagnosis.
NEC is a disease unusual in its cruelty. So often, it strikes suddenly, to babies who are otherwise flourishing. Those who survive face developmental challenges and life-long complications. The problem of NEC is not simply an academic one. My baby died of NEC, and someone else’s baby will die tomorrow. Given the slow pace of regulatory change and the great cost associated with clinical trials, how can we unite as a community of clinicians, scientists, and parents to develop creative solutions and to implement these therapies to save our babies’ lives?
Great leaps forward are possible in medicine. In 1960, the survival rate for childhood acute lymphoblastic leukemia was near 0%. Fifty years later, the survival rate had risen to over 90%.53 This success story shows us what is possible through concerted collaboration. Some advances in NEC prevention have already been made, such as the use of human milk instead of formula and implementation of standardized feeding protocols. While novel therapies carry great potential to save even more lives, we also must ensure that these currently accepted prevention methods are used for every baby. We must develop a standard of care for NEC prevention and treatment.
What would my Sarah be like today? Our family will forever be incomplete, and even the happiest moments in our lives will be tinged with grief. This is the price of NEC. Clinical progress on NEC has shown only modest improvements in the past 40 years. NEC does not need to remain inevitable or untreatable. Together, we can find innovative ways to accelerate progress and make NEC a medical success story too.
Conclusion
NEC remains one of the most severe diseases affecting preterm neonates. There are many new potential prevention and therapeutic approaches for NEC focused in a multitude of directions, from inflammatory signaling pathways, to probiotics, to attenuation of antioxidative stress, and the use of immunomodulatory agents. Although some of these findings in experimental and clinical NEC are promising, the approach to treatment remains challenging at best. NEC is a complex multifactorial disease that may represent a collection of different diseases ending in a final common pathway.54,55,56 This has historically made significant translations from animal models to clinical care challenging.57 More high-quality clinical trials as well as intensive mechanistic research are still needed to verify the validity and long-term outcomes of these approaches.
References
Wang, K., Tao, G. & Sylvester, K. G. Recent Advances in prevention and therapies for clinical or experimental necrotizing enterocolitis. Dig. Dis. Sci. 64, 3078–3085 (2019).
Good, M., Sodhi, C. P. & Hackam, D. J. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev. Clin. Immunol. 10, 875–884 (2014).
Elgin, T. G., Kern, S. L. & McElroy, S. J. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin. Ther. 38, 706–715 (2016).
Sankaran, K. et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J. Pediatr. Gastroenterol. Nutr. 39, 366–372 (2004).
Bowker, R. M., Yan, X. & De Plaen, I. G. Intestinal microcirculation and necrotizing enterocolitis: the vascular endothelial growth factor system. Semin. Fetal Neonatal Med. 23, 411–415 (2018).
Arthur, C. M. et al. Anemia induces gut inflammation and injury in an animal model of preterm infants. Transfusion 59, 1233–1245 (2019).
Perrone, S., Tataranno, M. L., Santacroce, A., Negro, S. & Buonocore, G. The role of oxidative stress on necrotizing enterocolitis in very low birth weight infants. Curr. Pediatr. Rev. 10, 202–207 (2014).
Patel, R. M. et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 315, 889–897 (2016).
Yan, X. et al. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G716–G725 (2016).
Bowker, R. M. et al. Dimethyloxalylglycine preserves the intestinal microvasculature and protects against intestinal injury in a neonatal mouse NEC model: role of VEGF signaling. Pediatr. Res. 83, 545–553 (2018).
MohanKumar, K. et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 10, 3494 (2019).
Sabnis, A. et al. Intestinal vascular endothelial growth factor is decreased in necrotizing enterocolitis. Neonatology 107, 191–198 (2015).
Robinson, J. R. et al. Neurodevelopmental considerations in surgical necrotizing enterocolitis. Semin. Pediatr. Surg. 27, 52–56 (2018).
Rees, C. M., Pierro, A. & Eaton, S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child Fetal Neonatal Ed. 92, F193–F198 (2007).
Hintz, S. R. et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115, 696–703 (2005).
Adams-Chapman, I. Necrotizing enterocolitis and neurodevelopmental outcome. Clin. Perinatol. 45, 453–466 (2018).
Nino, D. F. et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 10, eaan0237. https://doi.org/10.1126/scitranslmed.aan0237 (2018).
Egan, C. E. et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J. Clin. Invest. 126, 495–508 (2016).
Niño, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Good, M. et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8, 1166–1179 (2015).
Shin, S. H. et al. Surgical necrotizing enterocolitis versus spontaneous intestinal perforation in white matter injury on brain magnetic resonance imaging. Neonatology 110, 148–154 (2016).
Merhar, S. L., Ramos, Y., Meinzen-Derr, J. & Kline-Fath, B. M. Brain magnetic resonance imaging in infants with surgical necrotizing enterocolitis or spontaneous intestinal perforation versus medical necrotizing enterocolitis. J. Pediatr. 164, 410–412.e411 (2014).
Martin, C. R. et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J. Pediatr. 157, 751–756.e751 (2010).
Biouss, G. et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J. Neuroinflamm. 16, 97 (2019).
Hackam, D. J., Sodhi, C. P. & Good, M. New insights into necrotizing enterocolitis: from laboratory observation to personalized prevention and treatment. J. Pediatr. Surg. 54, 398–404 (2019).
Jilling, T. et al. Surgical necrotizing enterocolitis in extremely premature neonates is associated with genetic variations in an intergenic region of chromosome 8. Pediatr. Res. 83, 943–953 (2018).
Cuna, A. & Sampath, V. Genetic alterations in necrotizing enterocolitis. Semin. Perinatol. 41, 61–69 (2017).
Cuna, A., George, L. & Sampath, V. Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin. Fetal Neonatal Med. 23, 387–393 (2018).
Sampath, V. et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 135, e1530–e1534 (2015).
Hackam, D. J. & Sodhi, C. P. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell. Mol. Gastroenterol. Hepatol. 6, 229–238 e221 (2018).
Fawley, J. et al. Single-immunoglobulin interleukin-1-related receptor regulates vulnerability to TLR4-mediated necrotizing enterocolitis in a mouse model. Pediatr. Res. 83, 164–174 (2018).
Wang, S. L. et al. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell Death Dis. 10, 391 (2019).
Lavoie, S. et al. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife 8, e39982. https://doi.org/10.7554/eLife.39982 (2019).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Iida, T., Onodera, K. & Nakase, H. Role of autophagy in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 23, 1944–1953 (2017).
Yuan, Y. et al. TNF-alpha induces autophagy through ERK1/2 pathway to regulate apoptosis in neonatal necrotizing enterocolitis model cells IEC-6. Cell Cycle 17, 1390–1402 (2018).
Neal, M. D. et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 190, 3541–3551 (2013).
Sampath, V. et al. A functional ATG16L1 (T300A) variant is associated with necrotizing enterocolitis in premature infants. Pediatr. Res. 81, 582–588 (2017).
Strimbu, K. & Tavel, J. A. What are biomarkers? Curr. Opin. HIV AIDS 5, 463–466 (2010).
Sylvester, K. G. et al. Acylcarnitine profiles reflect metabolic vulnerability for necrotizing enterocolitis in newborns born premature. J. Pediatr. 181, 80–85.e81 (2017).
Sylvester, K. G. et al. A novel urine peptide biomarker-based algorithm for the prognosis of necrotising enterocolitis in human infants. Gut 63, 1284–1292 (2014).
Nakayuenyongsuk, W. et al. Point-of-care fecal calprotectin monitoring in preterm infants at risk for necrotizing enterocolitis. J. Pediatr. 196, 98–103.e101 (2018).
MacQueen, B. C. et al. Reference intervals for stool calprotectin in preterm neonates and their utility for the diagnosis of necrotizing enterocolitis. J. Perinatol. 38, 1379–1385 (2018).
van Zoonen, A. et al. Serial fecal calprotectin in the prediction of necrotizing enterocolitis in preterm neonates. J. Pediatr. Surg. 54, 455–459 (2019).
Jiang, P. et al. Antibiotic treatment preventing necrotising enterocolitis alters urinary and plasma metabolomes in preterm pigs. J. Proteome Res. 16, 3547–3557 (2017).
Call, L. et al. Metabolomic signatures distinguish the impact of formula carbohydrates on disease outcome in a preterm piglet model of NEC. Microbiome 6, 111 (2018).
Underwood, M. A. Probiotics and the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 54, 405–412 (2019).
Patel, R. M. & Underwood, M. A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 27, 39–46 (2018).
Underwood, M. A. Arguments for routine administration of probiotics for NEC prevention. Curr. Opin. Pediatr. 31, 188–194 (2019).
Underwood, M. A. et al. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 76, 326–333 (2014).
Olson, J. K. et al. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G408–G419 (2018).
Olson, J. K. et al. Harvesting the benefits of biofilms: a novel probiotic delivery system for the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 51, 936–941 (2016).
Kersey, J. H. Fifty years of studies of the biology and therapy of childhood leukemia. Blood 90, 4243–4251 (1997).
Caplan, M. S. et al. Necrotizing enterocolitis: using regulatory science and drug development to improve outcomes. J. Pediatr. 212, 208–215.e1. https://doi.org/10.1016/j.jpeds.2019.05.032 (2019).
Gordon, P. V. & Swanson, J. R. Necrotizing enterocolitis is one disease with many origins and potential means of prevention. Pathophysiology 21, 13–19 (2014).
Gephart, S. M. et al. Changing the paradigm of defining, detecting, and diagnosing NEC: Perspectives on Bell’s stages and biomarkers for NEC. Semin. Pediatr. Surg. 27, 3–10 (2018).
Ares, G. J., McElroy, S. J. & Hunter, C. J. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin. Pediatr. Surg. 27, 29–33 (2018).
Acknowledgements
This publication of this article was sponsored by the Necrotizing Enterocolitis (NEC) Society, Patient-Centered Outcomes Research Institute, and National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Author information
Authors and Affiliations
Contributions
R.D.S., R.R., G.E.B., and S.J.M. all made substantial contributions to conception, drafting, critical revisions, and approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.E.B. receives support from Scioto Biosciences, holds stock options in the company, and receives royalties for US Patent No. 5811393. S.J.M. has received consulting and lecture fees from Abbott, and grant support from Evolve Biosystems and the Carver College of Medicine at the University of Iowa. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. R.D.S. and R.R. declared no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shelby, R.D., Raab, R., Besner, G.E. et al. Hope on the horizon: promising novel therapies for necrotizing enterocolitis. Pediatr Res 88 (Suppl 1), 30–34 (2020). https://doi.org/10.1038/s41390-020-1077-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1077-1