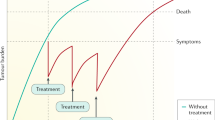
Abstract
Mouse models are the basis of preclinical and translational research in hepatocellular carcinoma (HCC). Multiple methods exist to induce tumour formation in mice, including genetically engineered mouse models, chemotoxic agents, intrahepatic or intrasplenic injection of tumour cells and xenograft approaches. Additionally, as HCC generally develops in the context of diseased liver, methods exist to induce liver disease in mice to mimic viral hepatitis, fatty liver disease, fibrosis, alcohol-induced liver disease and cholestasis. Similar to HCC in humans, response to therapy in mouse models is monitored with imaging modalities such as CT or MRI, as well as additional techniques involving bioluminescence. As immunotherapy is increasingly applied to HCC, mouse models for these approaches are required for preclinical data. In studying cancer immunotherapy, it is important to consider aspects of antitumour immune responses and to produce a model that mimics the complexity of the immune system. This Review provides an overview of the different mouse models of HCC, presenting techniques to prepare an HCC mouse model and discussing different approaches to help researchers choose an appropriate model for a specific hypothesis. Specific aspects of immunotherapy research in HCC and the applied mouse models in this field are also highlighted.
Key points
-
Hepatocellular carcinoma (HCC) is a heterogeneous tumour and requires genetically engineered models to study different genetic mutations in detail; however, these models are limited to one specific driver mutation.
-
Liver-specific induction of HCC can be achieved by transgenic modification using liver-specific promoters, plasmid injection via liver-specific viruses, hydrodynamic injection or orthotopic implantation of tumour cells.
-
Comorbid liver disease must be considered and can be modelled by diets, injection of chemotoxic agents or expression of inflammation promoting genes.
-
Only syngeneic orthotopic and subcutaneous mouse models can be utilized to investigate immunotherapy for HCC, whereas orthotopic models in HCC mimic the tumour microenvironment more accurately.
-
Xenograft models in immunocompromised mice are useful to study specific treatment effects but lack a full immune response that includes all immune cell subsets, lymphangiogenesis and chemokine signalling.
-
Immunologically humanized mouse models might overcome the shortcomings of traditional xenograft models, but the techniques to create these models are challenging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fitzmaurice, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 3, 524–548 (2017).
Duffy, A. G. & Greten, T. F. Liver cancer: Regorafenib as second-line therapy in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 14, 141–142 (2017).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2, 16018 (2016).
Schambach, S. J., Bag, S., Schilling, L., Groden, C. & Brockmann, M. A. Application of micro-CT in small animal imaging. Methods 50, 2–13 (2010).
Rothe, J. H. et al. Time course of contrast enhancement by micro-CT with dedicated contrast agents in normal mice and mice with hepatocellular carcinoma: comparison of one iodinated and two nanoparticle-based agents. Academ. Radiol. 22, 169–178 (2015).
Fiebig, T. et al. Three-dimensional in vivo imaging of the murine liver: a micro-computed tomography-based anatomical study. PLoS ONE 7, e31179 (2012).
Heindryckx, F., Colle, I. & Van Vlierberghe, H. Experimental mouse models for hepatocellular carcinoma research. Int. J. Exp. Pathol. 90, 367–386 (2009).
Newell, P., Villanueva, A., Friedman, S. L., Koike, K. & Llovet, J. M. Experimental models of hepatocellular carcinoma. J. Hepatol. 48, 858–879 (2008).
Ju, H. L., Han, K. H., Lee, J. D. & Ro, S. W. Transgenic mouse models generated by hydrodynamic transfection for genetic studies of liver cancer and preclinical testing of anti-cancer therapy. International journal of cancer. J. Int. Cancer 138, 1601–1608 (2016).
Li, Y., Tang, Z. Y. & Hou, J. X. Hepatocellular carcinoma: insight from animal models. Nat. Rev. Gastroenterol. Hepatol. 9, 32–43 (2011).
The Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e23 (2017). This paper presents multiplex genomic profiling of human HCCs from the TCGA Research Network.
Lin, H. H. et al. Inhibition of the Wnt/beta-catenin signaling pathway improves the anti-tumor effects of sorafenib against hepatocellular carcinoma. Cancer Lett. 381, 58–66 (2016).
Katz, S. F. et al. Disruption of Trp53 in livers of mice induces formation of carcinomas with bilineal differentiation. Gastroenterology 142, 1229–1239.e3 (2012).
Liu, Y. et al. CRISPR/Cas9-mediated p53 and Pten dual mutation accelerates hepatocarcinogenesis in adult hepatitis B virus transgenic mice. Sci. Rep. 7, 2796 (2017).
Xue, W. et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–384 (2014). This is the first paper that uses hydrodynamic injection of the CRISPR–Cas system to create a liver-specific genetic knockout to induce HCC.
Suda, T. & Liu, D. Hydrodynamic gene delivery: its principles and applications. Mol. Ther. 15, 2063–2069 (2007).
Shibata, T. & Aburatani, H. Exploration of liver cancer genomes. Nat. Rev. Gastroenterol. Hepatol. 11, 340–349 (2014).
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Jain, M. et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104 (2002).
Shachaf, C. M. et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 431, 1112–1117 (2004).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA 89, 5547–5551 (1992).
Conner, E. A. et al. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene 19, 5054–5062 (2000).
Conner, E. A., Lemmer, E. R., Sanchez, A., Factor, V. M. & Thorgeirsson, S. S. E2F1 blocks and c-Myc accelerates hepatic ploidy in transgenic mouse models. Biochem. Biophys. Res. Commun. 302, 114–120 (2003).
Calvisi, D. F. et al. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J. Hepatol. 42, 842–849 (2005).
Zender, L. & Hemann, M. Reconstitution of mice with modified liver stem cells. Cold Spring Harb. Protoc. 2015, 685–688 (2015).
Nitou, M., Sugiyama, Y., Ishikawa, K. & Shiojiri, N. Purification of fetal mouse hepatoblasts by magnetic beads coated with monoclonal anti-e-cadherin antibodies and their in vitro culture. Exp. Cell Res. 279, 330–343 (2002).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Pitot, H. C. & Dragan, Y. P. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 5, 2280–2286 (1991).
Vesselinovitch, S. D. & Mihailovich, N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 43, 4253–4259 (1983).
Kawanishi, S., Hiraku, Y., Murata, M. & Oikawa, S. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic. Biol. Med. 32, 822–832 (2002).
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M. & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40 (2006).
Verna, L., Whysner, J. & Williams, G. M. N-Nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 71, 57–81 (1996).
Rao, K. V. & Vesselinovitch, S. D. Age- and sex-associated diethylnitrosamine dealkylation activity of the mouse liver and hepatocarcinogenesis. Cancer Res. 33, 1625–1627 (1973). This is an important paper about the influence of age and sex on HCC induction by DEN.
Bakiri, L. & Wagner, E. F. Mouse models for liver cancer. Mol. Oncol. 7, 206–223 (2013).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010). This paper presents the influence of IL-6 and TNF in obesity-promoted HCC in a mouse model using DEN and an HFD.
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996).
He, L., Tian, D. A., Li, P. Y. & He, X. X. Mouse models of liver cancer: progress and recommendations. Oncotarget 6, 23306–23322 (2015).
Hernandez-Gea, V., Toffanin, S., Friedman, S. L. & Llovet, J. M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 144, 512–527 (2013).
Kerbel, R. S. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2, S134–S139 (2003).
Jung, J. Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicol. Res. 30, 1–5 (2014).
Richmond, A. & Su, Y. Mouse xenograft models versus GEM models for human cancer therapeutics. Dis. Model. Mech. 1, 78–82 (2008).
Morton, J. J., Bird, G., Refaeli, Y. & Jimeno, A. Humanized mouse xenograft models: narrowing the tumor-microenvironment gap. Cancer Res. 76, 6153–6158 (2016).
Zhou, Q., Facciponte, J., Jin, M., Shen, Q. & Lin, Q. Humanized NOD-SCID IL2rg−/− mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 344, 13–19 (2014).
Friedman, D. et al. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol. Res. 47, 702–714 (2016).
Wu, T. et al. Multimodal imaging of a humanized orthotopic model of hepatocellular carcinoma in immunodeficient mice. Sci. Rep. 6, 35230 (2016). This is an overview of imaging techniques to monitor HCC in mice.
Sun, F. X. et al. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. International journal of cancer. J. Int. Cancer 66, 239–243 (1996).
Yan, M. et al. Establishment of NOD/SCID mouse models of human hepatocellular carcinoma via subcutaneous transplantation of histologically intact tumor tissue. Chin. J. Cancer Res. 25, 289–298 (2013).
Golebiewska, A., Brons, N. H., Bjerkvig, R. & Niclou, S. P. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell 8, 136–147 (2011).
Behbod, F. & Vivanco, M. D. Side population. Methods Mol. Biol. 1293, 73–81 (2015).
Xia, H. et al. Hepatocellular carcinoma-propagating cells are detectable by side population analysis and possess an expression profile reflective of a primitive origin. Sci. Rep. 6, 34856 (2016).
Chow, A. K. et al. The Enhanced metastatic potential of hepatocellular carcinoma (HCC) cells with sorafenib resistance. PLoS ONE 8, e78675 (2013).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Shultz, L. D. et al. Human cancer growth and therapy In NOD/SCID/IL2Rγ(null) (NSG) mice. Cold Spring Harb. Protoc. 2014, 694–708 (2014).
Martin-Padura, I., Agliano, A., Marighetti, P., Porretti, L. & Bertolini, F. Sex-related efficiency in NSG mouse engraftment. Blood 116, 2616–2617 (2010).
Notta, F., Doulatov, S. & Dick, J. E. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 115, 3704–3707 (2010).
Wilson, E. M. et al. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 13, 404–412 (2014).
Greten, T. F., Duffy, A. G. & Korangy, F. Hepatocellular carcinoma from an immunologic perspective. Clin. Cancer Res. 19, 6678–6685 (2013).
Akinyemiju, T. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 3, 1683–1691 (2017).
Constandinou, C., Henderson, N. & Iredale, J. P. Modeling liver fibrosis in rodents. Methods Mol. Med. 117, 237–250 (2005).
Liu, Y. et al. Animal models of chronic liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G449–G468 (2013).
Starkel, P. & Leclercq, I. A. Animal models for the study of hepatic fibrosis. Best practice and research. Clin. Gastroenterol. 25, 319–333 (2011).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Salguero Palacios, R. et al. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab. Invest. 88, 1192–1203 (2008).
Kuriyama, S. et al. Hepatocellular carcinoma in an orthotopic mouse model metastasizes intrahepatically in cirrhotic but not in normal liver. Int. J. Cancer 80, 471–476 (1999).
Li, X., Benjamin, I. S. & Alexander, B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J. Hepatol. 36, 488–493 (2002).
Farazi, P. A. & DePinho, R. A. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat. Rev. Cancer 6, 674–687 (2006).
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Sandgren, E. P. et al. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 66, 245–256 (1991).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). This is an important paper showing that the IgA + cell-mediated suppression of cytotoxic CD8 + T cells promotes tumorigenesis in several mouse models of NASH-mediated HCC.
Smit, J. J. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993).
Katzenellenbogen, M. et al. Molecular mechanisms of liver carcinogenesis in the mdr2-knockout mice. Mol. Cancer Res. 5, 1159–1170 (2007).
Mauad, T. H. et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 145, 1237–1245 (1994).
Katzenellenbogen, M. et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 66, 4001–4010 (2006).
Popov, Y., Patsenker, E., Fickert, P., Trauner, M. & Schuppan, D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J. Hepatol. 43, 1045–1054 (2005).
Endig, J. et al. Dual role of the adaptive immune system in liver injury and hepatocellular carcinoma development. Cancer Cell 30, 308–323 (2016).
Grompe, M. et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 7, 2298–2307 (1993).
Marhenke, S. et al. Activation of nuclear factor E2-related factor 2 in hereditary tyrosinemia type 1 and its role in survival and tumor development. Hepatology 48, 487–496 (2008).
Chisari, F. V. et al. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl Acad. Sci. USA 84, 6909–6913 (1987).
Chisari, F. V. et al. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J. Virol. 60, 880–887 (1986).
Chisari, F. V. et al. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science 230, 1157–1160 (1985). This is the first design and description of a mouse model to study hepatitis B infection.
Dunsford, H. A., Sell, S. & Chisari, F. V. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 50, 3400–3407 (1990).
Wang, Y. et al. HBsAg and HBx knocked into the p21 locus causes hepatocellular carcinoma in mice. Hepatology 39, 318–324 (2004).
Ye, H. et al. Synergistic function of Kras mutation and HBx in initiation and progression of hepatocellular carcinoma in mice. Oncogene 33, 5133–5138 (2014).
Koike, K. et al. Expression of hepatitis C virus envelope proteins in transgenic mice. J. Gen. Virol. 76, 3031–3038 (1995).
Kamegaya, Y. et al. Hepatitis C virus acts as a tumor accelerator by blocking apoptosis in a mouse model of hepatocarcinogenesis. Hepatology 41, 660–667 (2005).
Chen, J. et al. Persistent hepatitis C virus infections and hepatopathological manifestations in immune-competent humanized mice. Cell Res. 24, 1050–1066 (2014).
Wang, Z., Wu, N., Tesfaye, A., Feinstone, S. & Kumar, A. HCV infection-associated hepatocellular carcinoma in humanized mice. Infect. Agents Cancer 10, 24 (2015).
Tesfaye, A. et al. Chimeric mouse model for the infection of hepatitis B and C viruses. PLoS ONE 8, e77298 (2013).
Ploss, A. et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886 (2009).
Dorner, M. et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature 501, 237–241 (2013).
McKillop, I. H. & Schrum, L. W. Role of alcohol in liver carcinogenesis. Semin. Liver Dis. 29, 222–232 (2009).
Jinjuvadia, R. & Liangpunsakul, S. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J. Clin. Gastroenterol. 49, 506–511 (2015).
Singal, A. K., Kamath, P. S., Gores, G. J. & Shah, V. H. Alcoholic hepatitis: current challenges and future directions. Clin. Gastroenterol. Hepatol. 12, 555–564 (2014).
Seitz, H. K. & Becker, P. Alcohol metabolism and cancer risk. Alcohol Res. Health 30, 38–41 (2007).
Lu, Y. & Cederbaum, A. I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 44, 723–738 (2008).
Brooks, P. J. & Theruvathu, J. A. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol 35, 187–193 (2005).
Eriksson, C. J. The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol. Clin. Exp. Res. 25 (Suppl.), 15S–32S (2001).
Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947 (2014).
Bode, C. & Bode, J. C. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol. Clin. Exp. Res. 29 (Suppl.), 166S–171S (2005).
Thurman, R. G. I. I. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Physiol. 275, G605–G611 (1998).
Nagata, K., Suzuki, H. & Sakaguchi, S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J. Toxicol. Sci. 32, 453–468 (2007).
Lieber, C. S. & DeCarli, L. M. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol. Clin. Exp. Res. 6, 523–531 (1982).
Bertola, A., Mathews, S., Ki, S. H., Wang, H. & Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637 (2013).
Mandrekar, P., Ambade, A., Lim, A., Szabo, G. & Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197 (2011).
Cohen, J. I., Roychowdhury, S., McMullen, M. R., Stavitsky, A. B. & Nagy, L. E. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology 139, 664–674 (2010).
Tsukamoto, H. et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology 5, 224–232 (1985).
Ueno, A. et al. Mouse intragastric infusion (iG) model. Nat. Protoc. 7, 771–781 (2012).
Beltran-Sanchez, H., Harhay, M. O., Harhay, M. M. & McElligott, S. Prevalence and trends of metabolic syndrome in the adult U. S. population, 1999–2010. J. Am. Coll. Cardiol. 62, 697–703 (2013).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease — meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Ibrahim, S. H., Hirsova, P., Malhi, H. & Gores, G. J. Animal models of nonalcoholic steatohepatitis: eat, delete, and inflame. Dig. Dis. Sci. 61, 1325–1336 (2016).
Santhekadur, P. K., Kumar, D. P. & Sanyal, A. J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 68, 230–237 (2018). This is an overview of preclinical models of NASH.
Dela Pena, A. et al. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 129, 1663–1674 (2005).
Ip, E., Farrell, G., Hall, P., Robertson, G. & Leclercq, I. Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 39, 1286–1296 (2004).
Rinella, M. E. & Green, R. M. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 40, 47–51 (2004).
Nakae, D. et al. Comparative changes in the liver of female Fischer-344 rats after short-term feeding of a semipurified or a semisynthetic L-amino acid-defined choline-deficient diet. Toxicol. Pathol. 23, 583–590 (1995).
Hebbard, L. & George, J. Animal models of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 8, 35–44 (2011).
Kodama, Y. et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology 137, 1467–1477.e5 (2009).
Deng, Q. G. et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology 42, 905–914 (2005).
Ito, M. et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol. Res. 37, 50–57 (2007).
Charlton, M. et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G825–G834 (2011).
Chheda, T. K. et al. Fast food diet with CCl4 micro-dose induced hepatic-fibrosis — a novel animal model. BMC Gastroenterol. 14, 89 (2014).
Ouyang, X. et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 48, 993–999 (2008).
Sanches, S. C., Ramalho, L. N., Augusto, M. J., da Silva, D. M. & Ramalho, F. S. Nonalcoholic steatohepatitis: a search for factual animal models. BioMed Res. Int. 2015, 574832 (2015).
Spruss, A. et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50, 1094–1104 (2009).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103 (2013).
Yamazaki, Y. et al. Interstrain differences in susceptibility to non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 23, 276–282 (2008).
Brennan, A. M. & Mantzoros, C. S. Drug Insight: the role of leptin in human physiology and pathophysiology — emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2, 318–327 (2006).
Diehl, A. M. Lessons from animal models of NASH. Hepatol. Res. 33, 138–144 (2005).
Mayer, J., Bates, M. W. & Dickie, M. M. Hereditary diabetes in genetically obese mice. Science 113, 746–747 (1951).
Takahashi, Y., Soejima, Y. & Fukusato, T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 18, 2300–2308 (2012).
Leclercq, I. A., Farrell, G. C., Schriemer, R. & Robertson, G. R. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J. Hepatol. 37, 206–213 (2002).
Wortham, M., He, L., Gyamfi, M., Copple, B. L. & Wan, Y. J. The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig. Dis. Sci. 53, 2761–2774 (2008).
Horie, Y. et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Invest. 113, 1774–1783 (2004).
Stiles, B. et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc. Natl Acad. Sci. USA 101, 2082–2087 (2004).
Xu, H. E. et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell 3, 397–403 (1999).
Costet, P. et al. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J. Biol. Chem. 273, 29577–29585 (1998).
Okumura, K. et al. Exacerbation of dietary steatohepatitis and fibrosis in obese, diabetic KK-A(y) mice. Hepatol. Res. 36, 217–228 (2006).
Tsuchida, T. et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. https://doi.org/10.1016/j.jhep.2018.03.011 (2018).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012).
Uehara, T. et al. Molecular mechanisms of fibrosis-associated promotion of liver carcinogenesis. Toxicol. Sci. 132, 53–63 (2013).
Uehara, T., Pogribny, I. P. & Rusyn, I. The DEN and CCl4-induced mouse model of fibrosis and inflammation-associated hepatocellular carcinoma. Curr. Protoc. Pharmacol. 66, 14.30.1–14.30.10 (2014).
Reiberger, T. et al. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat. Protoc. 10, 1264–1274 (2015).
Li, G. et al. Successful chemoimmunotherapy against hepatocellular cancer in a novel murine model. J. Hepatol. 66, 75–85 (2017).
Ambade, A., Satishchandran, A., Gyongyosi, B., Lowe, P. & Szabo, G. Adult mouse model of early hepatocellular carcinoma promoted by alcoholic liver disease. World J. Gastroenterol. 22, 4091–4108 (2016).
Ma, C. et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257 (2016). This paper presents the influence of fatty acids on immune-mediated HCC progression in livers with NASH.
Hill-Baskin, A. E. et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum. Mol. Genet. 18, 2975–2988 (2009).
Wolf, M. J. et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014). This paper describes the CD8 + T cell-mediated and NKT cell-mediated liver damage and progression to HCC in a mouse model of CD-HFD.
Asgharpour, A. et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 65, 579–588 (2016).
Klevorn, L. E. & Teague, R. M. Adapting cancer immunotherapy models for the real world. Trends Immunol. 37, 354–363 (2016).
Crispe, I. N. Liver antigen-presenting cells. J. Hepatol. 54, 357–365 (2011).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Petrizzo, A. et al. Identification and validation of HCC-specific gene transcriptional signature for tumor antigen discovery. Sci. Rep. 6, 29258 (2016).
Bernstein, M. B., Krishnan, S., Hodge, J. W. & Chang, J. Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat. Rev. Clin. Oncol. 13, 516–524 (2016).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 15, 660–660 (2016).
Lee, J. H. et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148, 1383–1391.e6 (2015).
Zhang, Q. et al. CAR-T cell therapy in gastrointestinal tumors and hepatic carcinoma: from bench to bedside. Oncoimmunology 5, e1251539 (2016).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263–274 (2005).
Waldmann, T. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
Gabeen, A. A., Abdel-Hamid, F. F., El-Houseini, M. E. & Fathy, S. A. Potential immunotherapeutic role of interleukin-2 and interleukin-12 combination in patients with hepatocellular carcinoma. J. Hepatocell. Carcinoma 1, 55–63 (2014).
Subleski, J. J. et al. Serum-based tracking of de novo initiated liver cancer progression reveals early immunoregulation and response to therapy. J. Hepatol. 63, 1181–1189 (2015).
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Kapanadze, T. et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J. Hepatol. 59, 1007–1013 (2013).
Kapanadze, T. et al. Tumor-induced CD11b(+) Gr-1(+) myeloid-derived suppressor cells exacerbate immune-mediated hepatitis in mice in a CD40-dependent manner. Eur. J. Immunol. 45, 1148–1158 (2015).
Tu, J. F. et al. Regulatory T cells, especially ICOS(+) FOXP3(+) regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci. Rep. 6, 35056 (2016).
Zschaler, J., Schlorke, D. & Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 34, 433–454 (2014).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Bogdanos, D. P., Gao, B. & Gershwin, M. E. Liver Immunology. Compr. Physiol. 3, 567–598 (2013).
Tian, Z., Chen, Y. & Gao, B. Natural killer cells in liver disease. Hepatology 57, 1654–1662 (2013).
Colucci, F., Di Santo, J. P. & Leibson, P. J. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat. Immunol. 3, 807–813 (2002).
Haley, P. J. Species differences in the structure and function of the immune system. Toxicology 188, 49–71 (2003).
Duffy, A. G. et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66, 545–551 (2017).
Seung, S. K. et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2 — tumor and immunological responses. Sci. Transl Med. 4, 137ra74 (2012).
Baird, J. R. et al. Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 76, 50–61 (2016).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Woller, N. et al. Viral Infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T cell responses. Mol. Ther. 23, 1630–1640 (2015).
Gurlevik, E. et al. Adjuvant gemcitabine therapy improves survival in a locally induced, R0-resectable model of metastatic intrahepatic cholangiocarcinoma. Hepatology 58, 1031–1041 (2013).
Boozari, B. et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut 59, 1416–1426 (2010).
Zhang, H. et al. Targeting VCP enhances anticancer activity of oncolytic virus M1 in hepatocellular carcinoma. Sci. Transl Med. 9, eaam7996 (2017).
Wepsic, H. T. Overview of oncofetal antigens in cancer. Ann. Clin. Lab. Sci. 13, 261–266 (1983).
Stern, P. L. in Encyclopedia of Cancer (ed. Schwab, M.) 2610–2613 (Springer, Berlin Heidelberg, 2011).
Liu, C. et al. Value of alpha-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J. Gastroenterol. 19, 1811–1819 (2013).
Butterfield, L. H. et al. T cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin. Cancer Res. 9, 5902–5908 (2003).
Wang, X. P. et al. Recombinant heat shock protein 70 functional peptide and alpha-fetoprotein epitope peptide vaccine elicits specific anti-tumor immunity. Oncotarget 7, 71274–71284 (2016).
Walker, K. B., Keeble, J. & Colaco, C. Mycobacterial heat shock proteins as vaccines - a model of facilitated antigen presentation. Curr. Mol. Med. 7, 339–350 (2007).
Singh-Jasuja, H. et al. The heat shock protein gp96: a receptor-targeted cross-priming carrier and activator of dendritic cells. Cell Stress Chaperones 5, 462–470 (2000).
Binder, R. J., Han, D. K. & Srivastava, P. K. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 1, 151–155 (2000).
Su, H., Li, B., Zheng, L., Wang, H. & Zhang, L. Immunotherapy based on dendritic cells pulsed with CTPFoxM1 fusion protein protects against the development of hepatocellular carcinoma. Oncotarget 7, 48401–48411 (2016).
Capurro, M. et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 125, 89–97 (2003).
Baumhoer, D. et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am. J. Clin. Pathol. 129, 899–906 (2008).
Zhu, Z. W. et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 48, 558–564 (2001).
Shirakawa, H. et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 100, 1403–1407 (2009).
Dargel, C. et al. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology 149, 1042–1052 (2015).
Chmielewski, M., Hombach, A. A. & Abken, H. Antigen-specific T-cell activation independently of the MHC: chimeric antigen receptor-redirected T cells. Front. Immunol. 4, 371 (2013).
Kershaw, M. H., Westwood, J. A., Slaney, C. Y. & Darcy, P. K. Clinical application of genetically modified T cells in cancer therapy. Clin. Transl Immunol. 3, e16 (2014).
Kershaw, M. H., Westwood, J. A. & Darcy, P. K. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 13, 525–541 (2013).
Gao, H. et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. 20, 6418–6428 (2014).
Feng, M. et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 110, E1083–E1091 (2013).
Llovet, J. M. et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 131, 1758–1767 (2006).
Gauttier, V. et al. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. International journal of cancer. J. Int. Cancer 135, 2857–2867 (2014).
Makkouk, A., Chester, C. & Kohrt, H. E. Rationale for anti-CD137 cancer immunotherapy. Eur. J. Cancer 54, 112–119 (2016).
Subleski, J. J., Hall, V. L., Back, T. C., Ortaldo, J. R. & Wiltrout, R. H. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 66, 11005–11012 (2006).
Dinarello, C. A. & Fantuzzi, G. Interleukin-18 and host defense against infection. J. Infecti. Diseases 187 (Suppl. 2), S370–S384 (2003).
Eggert, T. et al. Immune studies in a mouse model of MET and CAT induced liver tumors [abstract]. J. Immunother. Cancer 2 (Suppl. 3), P202 (2014).
Sangro, B. et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59, 81–88 (2013).
Wolchok, J. D. PD-1 Blockers. Cell 162, 937 (2015).
Chen, Y. et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61, 1591–1602 (2015).
Zhou, D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009).
Teufel, A. et al. Comparison of gene expression patterns between mouse models of nonalcoholic fatty liver disease and liver tissues from patients. Gastroenterology 151, 513–525.e10 (2016).
Allweiss, L. & Dandri, M. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J. Hepatol. 64, S17–31 (2016).
Kimura, K. & Kohara, M. An experimental mouse model for hepatitis C virus. Exp. Animals 60, 93–100 (2011).
Dorner, M. et al. A genetically humanized mouse model for hepatitis C virus infection. Nature 474, 208–211 (2011).
Vucur, M. et al. Mouse models of hepatocarcinogenesis: what can we learn for the prevention of human hepatocellular carcinoma? Oncotarget 1, 373–378 (2010).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Xiao, Y. & Freeman, G. J. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 5, 16–18 (2015).
Chen, K., Ahmed, S., Adeyi, O., Dick, J. E. & Ghanekar, A. Human solid tumor xenografts in immunodeficient mice are vulnerable to lymphomagenesis associated with Epstein-Barr virus. PLoS ONE 7, e39294 (2012).
Sangro, B. et al. A randomized, multicenter, phase 3 study of nivolumab versus sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J. Clin. Oncol. https://doi.org/10.1200/JCO.2016.34.15_suppl.TPS4147 (2016).
Boll, H. et al. Micro-CT based experimental liver imaging using a nanoparticulate contrast agent: a longitudinal study in mice. PLoS ONE 6, e25692 (2011).
Freimuth, J. et al. Application of magnetic resonance imaging in transgenic and chemical mouse models of hepatocellular carcinoma. Mol. Cancer 9, 94 (2010).
Thaker, A. A. et al. Combination therapy of radiofrequency ablation and bevacizumab monitored with power Doppler ultrasound in a murine model of hepatocellular carcinoma. Int. J. Hyperthermia 28, 766–775 (2012).
Lee, T. K., Na, K. S., Kim, J. & Jeong, H. J. Establishment of animal models with orthotopic hepatocellular carcinoma. Nuclear Med. Mol. Imag. 48, 173–179 (2014).
Fleten, K. G. et al. Use of non-invasive imaging to monitor response to aflibercept treatment in murine models of colorectal cancer liver metastases. Clin. Exp. Metastasis 34, 51–62 (2017).
Lee, W. C. et al. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J. Immunother. 28, 496–504 (2005).
Lee, J.-H. et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br. J. Cancer 113, 1666–1676 (2015).
Palmer, D. H. et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49, 124–132 (2009).
Acknowledgements
The authors thank the Mouse Imaging Facility (MIF) at the NIH for preparing and providing pictures for imaging in hepatocellular carcinoma mouse models.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Glossary
- Immunocompetent
-
Having a fully functional immune system.
- Humanized
-
A mouse model with modifications to incorporate or mimic human cells or tissue.
- Cre–Lox recombination
-
A method of site-specific recombination enabling investigators to manipulate genes at targeted locations in the DNA.
- CRISPR–Cas9
-
Enables RNA-guided cutting of DNA for targeting genes to be deleted or inserted.
- Hydrodynamic injection
-
Injection of 10% volume per mouse weight and with high pressure to cause swelling of the liver to allow for better plasmid delivery.
- Plasmids
-
Small DNA molecules distinct from the host DNA that can be induced as a vector for genetic engineering.
- Latency period
-
The time between the exposure of a chemotoxic agent or the manipulation of a gene or genes to the development of tumour.
- Orthotopic
-
A procedure that occurs in the original place, such as inducing a tumour within its tissue of origin.
- Heterotopic
-
A procedure occurring outside the original place, such as inducing a tumour in a foreign tissue.
- Syngeneic
-
Genetically similar cells arising from the same species.
- Xenograft
-
Tissue or cells from one species transplanted into a different species.
- Immunodeficient
-
An organism in which certain immune subsets are absent or nonfunctional, rendering the organism unable to mount a full immune response.
- Sleeping Beauty Transposon
-
A synthetic DNA transposon that enables incorporation of introduced genetic material into the host DNA.
Rights and permissions
About this article
Cite this article
Brown, Z.J., Heinrich, B. & Greten, T.F. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol 15, 536–554 (2018). https://doi.org/10.1038/s41575-018-0033-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0033-6
This article is cited by
-
Translational research on drug development and biomarker discovery for hepatocellular carcinoma
Journal of Biomedical Science (2024)
-
An overview of mouse models of hepatocellular carcinoma
Infectious Agents and Cancer (2023)
-
Small extrachromosomal circular DNA harboring targeted tumor suppressor gene mutations supports intratumor heterogeneity in mouse liver cancer induced by multiplexed CRISPR/Cas9
Genome Medicine (2023)
-
Tumor-mediated microbiota alteration impairs synaptic tagging/capture in the hippocampal CA1 area via IL-1β production
Communications Biology (2023)
-
Eicosapentaenoic acid loaded silica nanoemulsion attenuates hepatic inflammation through the enhancement of cell membrane components
Biological Procedures Online (2022)