Abstract
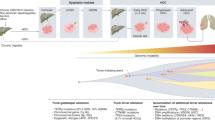
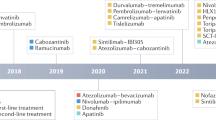
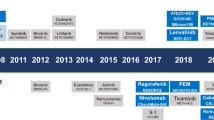
Systemic treatment for hepatocellular carcinoma (HCC) has been boosted by the incorporation of new agents after many negative phase III trials in the decade since the approval of sorafenib. Sorafenib introduced the concept that targeting specific hallmarks of hepatocarcinogenesis could modify the dismal prognosis of this disease, with the drug remaining a cornerstone in the upfront therapy for advanced HCC. The design of clinical trials in this malignancy is complicated by important obstacles related to patient selection, prognostic assessment and the need for endpoints that correlate with improvement in survival outcomes. In addition, the currently used criteria to determine treatment response or progression might prevent physicians from making appropriate clinical judgements and interpreting evidence arising from trials. In this Review, we discuss the advances in systemic therapy for HCC and critically review trial designs in HCC. Although novel therapies, such as new targeted agents and immunotherapies, are being rapidly incorporated, it is paramount to design future clinical trials based on the lessons learned from past failures and successes.
Key points
-
The changing landscape of hepatocellular carcinoma treatment demands a critical interpretation of how therapies have evolved and what future challenges lie ahead.
-
Improving overall survival is the main objective in advanced hepatocellular carcinoma, and the use of surrogate endpoints, such as response rate, time to progression or progression-free survival, lacks scientific evidence.
-
Criteria for response to treatment should evolve so that validated signals of activity prime transition into phase III trials.
-
Trial design and analysis should include novel clinical characteristics such as pattern of progression and pattern of adverse events with prior therapy.
-
Phase III trials in ill-defined target populations with limited clinical characterization might provide unreliable (positive or negative) results.
-
Molecular stratification is hampered by tumour heterogeneity and lacks prognostic power and linkage to treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
International Agency for Research on Cancer. Population fact sheets. IARC http://gco.iarc.fr/today/fact-sheets-populations (2018).
Schulze, K., Nault, J.-C. & Villanueva, A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J. Hepatol. 65, 1031–1042 (2016).
Galle, P. R. et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018).
Johnson, P. J., Williams, R., Thomas, H., Sherlock, S. & Murray-Lyon, I. M. Induction of remission in hepatocellular carcinoma with doxorubicin. Lancet 1, 1006–1009 (1978).
Melia, W. M., Johnson, P. J. & Williams, R. Controlled clinical trial of doxorubicin and tamoxifen versus doxorubicin alone in hepatocellular carcinoma. Cancer Treat. Rep. 71, 1213–1216 (1987).
Gish, R. G. et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J. Clin. Oncol. 25, 3069–3075 (2007).
Qin, S. et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 31, 3501–3508 (2013).
Lai, C. L., Wu, P. C., Chan, G. C., Lok, A. S. & Lin, H. J. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 62, 479–483 (1988).
Llovet, J. M. & Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37, 429–442 (2003).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Rimassa, L. et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 19, 682–693 (2018).
Llovet, J. M. et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J. Clin. Oncol. 31, 3509–3516 (2013).
Vilgrain, V. et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 18, 1624–1636 (2017).
Chow, P. K. H. et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J. Clin. Oncol. 36, 1913–1921 (2018).
Johnson, P. J. et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 31, 3517–3524 (2013).
Zhu, A. X. et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib. JAMA 312, 57 (2014).
Zhu, A. X. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16, 859–870 (2015).
Abou-Alfa, G. K. et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 29, 1402–1408 (2018).
Cainap, C. et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 33, 172–179 (2015).
Abou-Alfa, G. K. et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J. Clin. Oncol. 34, 192–192 (2016).
Zhu, A. X. et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 33, 559–566 (2015).
Cheng, A.-L. et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol. 31, 4067–4075 (2013).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 20, 282–296 (2019).
Sena, E. S., van der Worp, H. B., Bath, P. M. W., Howells, D. W. & Macleod, M. R. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLOS Biol. 8, e1000344 (2010).
Common ground on the critical path [Editorial]. Nat. Rev. Drug Discov. 5, 267 (2006).
Deininger, M., Buchdunger, E. & Druker, B. J. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 105, 2640–2653 (2005).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–716 (2004).
Ruggeri, B. A., Camp, F. & Miknyoczki, S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol. 87, 150–161 (2014).
Kaitin, K. I. & DiMasi, J. A. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin. Pharmacol. Ther. 89, 183–188 (2011).
Delcò, F., Tchambaz, L., Schlienger, R., Drewe, J. & Krähenbühl, S. Dose adjustment in patients with liver disease. Drug Saf. 28, 529–545 (2005).
Tannock, I. F. et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J. Clin. Oncol. 14, 1756–1764 (1996).
Power, D. G. & Kemeny, N. E. Long-term outcome of unresectable metastatic colorectal cancer: does “adjuvant” chemotherapy play a role after resection? Ann. Surg. 250, 654–655 (2009).
Lencioni, R. et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J. Hepatol. 66, 1166–1172 (2017).
Abou-Alfa, G. K. et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 24, 4293–4300 (2006).
Zhu, A. et al. A study of ramucirumab (LY3009806) versus placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J. Clin. Oncol. 36, 4003 (2018).
Finn, R. S. et al. IMbrave150: a randomized phase III study of 1L atezolizumab plus bevacizumab versus sorafenib in locally advanced or metastatic hepatocellular carcinoma. J. Clin. Oncol. 36, TPS4141 (2018).
Pishvaian, M. J. et al. Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Presented at the 2018 ESMO Congress (2018).
US Food & Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. FDA.gov https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm577166.htm (2017).
US Food & Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. FDA.gov https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm625705.htm (2018).
Finn, R. S. et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 37, 4004–4004 (2019).
Bristol-Myers Squibb. Bristol-Myers Squibb Announces Results from CheckMate -459 Study Evaluating Opdivo (nivolumab) as a First-Line Treatment for Patients with Unresectable Hepatocellular Carcinoma. bms.com https://news.bms.com/press-release/bmy/bristol-myers-squibb-announces-results-checkmate-459-study-evaluating-opdivo-nivol (2019).
US Food & Drug Administration. Table of surrogate endpoints that were the basis of drug approval or licensure. FDA.gov https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm613636.htm (2019).
Huang, L. et al. Weak correlation of overall survival and time to progression in advanced hepatocellular carcinoma. J. Clin. Oncol. 35, 233 (2017).
Terashima, T. et al. Surrogacy of time to progression for overall survival in advanced hepatocellular carcinoma treated with systemic therapy: a systematic review and meta-analysis of randomized controlled trials. Liver Cancer 8, 130–139 (2018).
Tan, A., Porcher, R., Crequit, P., Ravaud, P. & Dechartres, A. Differences in treatment effect size between overall survival and progression-free survival in immunotherapy trials: a meta-epidemiologic study of trials with results posted at ClinicalTrials.gov. J. Clin. Oncol. 35, 1686–1694 (2017).
Llovet, J. M., Montal, R. & Villanueva, A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J. Hepatol. 70, 1262–1277 (2019).
Moertel, C. G., Hanley, J. A. & Johnson, L. A. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 303, 1189–1194 (1980).
Moertel, C. G. & Hanley, J. A. The effect of measuring error on the results of therapeutic trials in advanced cancer. Cancer 38, 388–394 (1976).
Miller, A. B., Hoogstraten, B., Staquet, M. & Winkler, A. Reporting results of cancer treatment. Cancer 47, 207–214 (1981).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Raoul, J.-L. et al. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat. Rev. 72, 28–36 (2019).
Litière, S., Collette, S., de Vries, E. G. E., Seymour, L. & Bogaerts, J. RECIST — learning from the past to build the future. Nat. Rev. Clin. Oncol. 14, 187–192 (2017).
Reig, M. et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology 58, 2023–2031 (2013).
Sorbye, H., Kohne, C.-H., Sargent, D. J. & Glimelius, B. Patient characteristics and stratification in medical treatment studies for metastatic colorectal cancer: a proposal for standardization of patient characteristic reporting and stratification. Ann. Oncol. 18, 1666–1672 (2007).
Takagi, T. et al. Prognostic markers for refined stratification of IMDC intermediate-risk metastatic clear cell renal cell carcinoma treated with first-line tyrosine kinase inhibitor therapy. Target. Oncol. 14, 179–186 (2019).
Bruix, J. et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J. Hepatol. 35, 421–430 (2001).
Lencioni, R. & Llovet, J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 30, 052–060 (2010).
Reig, M. et al. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin. Liver Dis. 34, 444–455 (2014).
Zhao, Y. et al. Which criteria applied in multi-phasic CT can predict early tumor response in patients with hepatocellular carcinoma treated using conventional TACE: RECIST, mRECIST, EASL or qEASL? Cardiovasc. Intervent. Radiol. 41, 433–442 (2018).
Mejias, M. et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49, 1245–1256 (2009).
Pinter, M. et al. The effects of sorafenib on the portal hypertensive syndrome in patients with liver cirrhosis and hepatocellular carcinoma—a pilot study. Aliment. Pharmacol. Ther. 35, 83–91 (2012).
Fernandez, M. et al. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology 46, 1208–1217 (2007).
Reig, M. & Bruix, J. Lenvatinib: can a non-inferiority trial change clinical practice? Lancet 391, 1123–1124 (2018).
Tugues, S. et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology 46, 1919–1926 (2007).
Bosch, J., Abraldes, J. G., Fernández, M. & García-Pagán, J. C. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J. Hepatol. 53, 558–567 (2010).
Bruix, J., Reig, M. & Sangro, B. Assessment of treatment efficacy in hepatocellular carcinoma: response rate, delay in progression or none of them. J. Hepatol. 66, 1114–1117 (2017).
Amit, O. et al. Blinded independent central review of progression in cancer clinical trials: results from a meta-analysis. Eur. J. Cancer 47, 1772–1778 (2011).
US Food & Drug Administration. Memorandum to the file BLA 125085 Avastin (bevacizumab). FDA.gov https://www.fda.gov/media/79525/download (2010).
Brufsky, A. Is there room for bevacizumab in metastatic breast cancer? Lancet Oncol. 17, 1175–1176 (2016).
Aghajanian, C. et al. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 139, 10–16 (2015).
Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379, 1926–1936 (2018).
Maindrault-Gœbel, F. et al. Oxaliplatin reintroduction in patients previously treated with leucovorin, fluorouracil and oxaliplatin for metastatic colorectal cancer. Ann. Oncol. 15, 1210–1214 (2004).
Extra, J.-M. et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist 15, 799–809 (2010).
Mathur, A. K. et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch. Surg. 145, 1158 (2010).
Díaz-González, Á. et al. Systematic review with meta-analysis: the critical role of dermatological events in patients with hepatocellular carcinoma treated with sorafenib. Aliment. Pharmacol. Ther. 49, 482–491 (2019).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Kaye, F. J., Jantz, M. A. & Dallas, J. Erlotinib or gefitinib for non–small-cell lung cancer. N. Engl. J. Med. 364, 2367–2368 (2011).
Fornasier, G., Francescon, S. & Baldo, P. An update of efficacy and safety of cetuximab in metastatic colorectal cancer: a narrative review. Adv. Ther. 35, 1497–1509 (2018).
Larkin, J. et al. Vemurafenib in patients with BRAFV600 mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 15, 436–444 (2014).
Horwitz, E. et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 4, 730–743 (2014).
Llovet, J. M. et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 18, 2290–2300 (2012).
Villanueva, A. et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology 140, 1501–1512 (2011).
Pinyol, R. et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut 68, 1065–1075 (2018).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Nakashima, T. & Kojiro, M. Hepatocellular Carcinoma: An Atlas of Its Pathology (Springer Japan, 1987).
Chen, Y. J. et al. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology 119, 431–440 (2000).
Morimoto, O. et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J. Hepatol. 39, 215–221 (2003).
Saeki, R. et al. Intratumoral genomic heterogeneity in human hepatocellular carcinoma detected by restriction landmark genomic scanning. J. Hepatol. 33, 99–105 (2000).
Friemel, J. et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin. Cancer Res. 21, 1951–1961 (2015).
Zucman-Rossi, J., Villanueva, A., Nault, J.-C. & Llovet, J. M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149, 1226–1239 (2015).
Rimassa, L. et al. Tumor biopsy and patient enrollment in clinical trials for advanced hepatocellular carcinoma. World J. Gastroenterol. 23, 2448 (2017).
Champiat, S. et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat. Rev. Clin. Oncol. 15, 748–762 (2018).
Goumard, C. et al. Low levels of microsatellite instability at simple repeated sequences commonly occur in human hepatocellular carcinoma. Cancer Genomics Proteomics 14, 329–339 (2017).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018).
Trotti, A. et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 13, 176–181 (2003).
Bruix, J. et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J. Hepatol. 57, 821–829 (2012).
Cheng, A.-L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Bruix, J. et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J. Hepatol. 67, 999–1008 (2017).
Finn, R. S. et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J. Hepatol. 69, 353–358 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02576509 (2019).
Acknowledgements
M.R. received grant support from Instituto de Salud Carlos III (PI15/00145). J.B. received grant support from Instituto de Salud Carlos III (PI18/00768), AECC (PI044031), Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2014 SGR 605) and WCR (AICR) 16–0026. CIBERehd is funded by the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Contributions
J.B. mentored the ideas and concepts of the manuscript. All authors contributed to all aspects of the preparation of the manuscript, including writing, editing and discussion the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.B. has consulted for Arqule, Bayer-Shering Pharma, Novartis, BMS, BTG-Biocompatibles, Eisai, Kowa, Terumo, Gilead, Bio-Alliance, Roche, AbbVie, Merck, Roche, Sirtex, Ipsen, Astra-Medimmune, Incyte, Quirem, Adaptimmune, Lilly, Basilea and Nerviano; and has received research and educational grants from Bayer and BTG. L.d.F. has received travel grants from Bayer and speaker fees from Bayer-Shering Pharma, BTG-Biocompatibles, Eisai, Terumo, Sirtex and Ipsen. M.R. has received speaker fees from Gilead, BMS, BTG, Lilly and Bayer, and consultancy fees for Bayer, BMS and AstraZeneca.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks P. Galle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bruix, J., da Fonseca, L.G. & Reig, M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 16, 617–630 (2019). https://doi.org/10.1038/s41575-019-0179-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0179-x
This article is cited by
-
Integrating single-cell and bulk RNA sequencing reveals CK19 + cancer stem cells and their specific SPP1 + tumor-associated macrophage niche in HBV-related hepatocellular carcinoma
Hepatology International (2024)
-
Machine learning integrations develop an antigen-presenting-cells and T-Cells-Infiltration derived LncRNA signature for improving clinical outcomes in hepatocellular carcinoma
BMC Cancer (2023)
-
Combining bulk and single-cell RNA-sequencing data to develop an NK cell-related prognostic signature for hepatocellular carcinoma based on an integrated machine learning framework
European Journal of Medical Research (2023)
-
Prognostic stratification based on HIF-1α signaling for evaluating hypoxia status and immune landscape in hepatocellular carcinoma
Journal of Big Data (2023)
-
A pyroptosis-related gene signature provides an alternative for predicting the prognosis of patients with hepatocellular carcinoma
BMC Medical Genomics (2023)