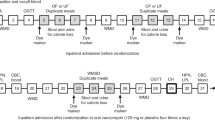
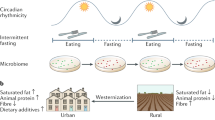
Abstract
Antibiotic use disrupts microbial composition and activity in humans, but whether this disruption in turn affects host metabolic health is unclear. Cohort studies show associations between antibiotic use and an increased risk of developing obesity and type 2 diabetes mellitus. Here, we review available clinical trials and show the disruptive effect of antibiotic use on the gut microbiome in humans, as well as its impact on bile acid metabolism and microbial metabolites such as short-chain fatty acids. Placebo-controlled human studies do not show a consistent effect of antibiotic use on body weight and insulin sensitivity at a population level, but rather an individual-specific or subgroup-specific response. This response to antibiotic use is affected by the resistance and resilience of the gut microbiome, factors that determine the extent of disruption and the speed of recovery afterwards. Nutritional strategies to improve the composition and functionality of the gut microbiome, as well as its recovery after antibiotic use (for instance, with prebiotics), require a personalized approach to increase their efficacy. Improved insights into key factors that influence the individual-specific response to antibiotics and dietary intervention may lead to better efficacy in reversing or preventing antibiotic-induced microbial dysbiosis as well as strategies for preventing cardiometabolic diseases.
Key points
-
Antibiotic use disrupts gut microbial composition and diversity in humans.
-
Observational studies show associations between antibiotic use and an increased risk of weight gain and development of type 2 diabetes mellitus.
-
Short-term placebo-controlled studies do not show a consistent effect of antibiotic-induced disruptions of the gut microbiome on body weight and insulin sensitivity in humans but suggest an individual-specific or subgroup-specific response.
-
The individual-specific or subgroup-specific response to antibiotic use is determined by the resistance and resilience of the gut microbiome, among other factors.
-
Dietary substrates such as prebiotics modulate gut microbial composition and function and might be used to limit the detrimental effects of antibiotic use, but will require a personalized approach to increase their efficacy.
-
More insight into the personalized interaction between the gut microbiome and host metabolism in response to prebiotics and antibiotics might lead to effective prevention of cardiometabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
World Obesity Federation. World Obesity Atlas https://data.worldobesity.org/publications/?cat (2023).
Haslam, D. W. & James, W. P. T. Obesity. Lancet 366, 1197–1209 (2005).
Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 121, 21–33 (2009).
World Health Organization (WHO) Diabetes — Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/diabetes (2022).
Snel, M. et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012, 983814 (2012).
Blüher, M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 27, 163–177 (2013).
Canfora, E. E., Meex, R. C. R., Venema, K. & Blaak, E. E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273 (2019).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Loftfield, E. et al. Association of body mass index with fecal microbial diversity and metabolites in the northern Finland birth cohort. Cancer Epidemiol. Biomark. Prev. 29, 2289–2299 (2020).
Thursby, E. & Juge, N. Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 (2017).
Browne, A. J. et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet. Health 5, e893–e904 (2021).
Rinninella, E. et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7, 14 (2019).
Lloyd-Price, J., Abu-Ali, G. & Huttenhower, C. The healthy human microbiome. Genome Med. 8, 51–51 (2016).
Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Kalbermatter, C., Fernandez Trigo, N., Christensen, S. & Ganal-Vonarburg, S. C. Maternal microbiota, early life colonization and breast milk drive immune development in the newborn. Front. Immunol. 12, 683022 (2021).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Heintz-Buschart, A. & Wilmes, P. Human gut microbiome: function matters. Trends Microbiol. 26, 563–574 (2018).
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Gibson, G. R. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Hamer, H. M., De Preter, V., Windey, K. & Verbeke, K. Functional analysis of colonic bacterial metabolism: relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1–G9 (2012).
Russell, W. R. et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 93, 1062–1072 (2011).
Diether, N. E. & Willing, B. P. Microbial fermentation of dietary protein: an important factor in diet–microbe–host interaction. Microorganisms 7, 19 (2019).
Gawałko, M. et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc. Res. 118, 2415–2427 (2022).
Agus, A., Clément, K. & Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70, 1174–1182 (2021).
Shah, S. et al. Physical activity-induced alterations of the gut microbiota are BMI dependent. FASEB J. 37, e22882 (2023).
Estaki, M. et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 4, 42 (2016).
Claesson, M. J. et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108, 4586–4591 (2011).
Xu, C., Zhu, H. & Qiu, P. Aging progression of human gut microbiota. BMC Microbiol. 19, 236 (2019).
Melander, R. J., Zurawski, D. V. & Melander, C. Narrow-spectrum antibacterial agents. MedChemComm 9, 12–21 (2018).
Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 13, 423–434 (2020).
Fassarella, M. et al. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut 70, 595–605 (2021).
Lange, K., Buerger, M., Stallmach, A. & Bruns, T. Effects of antibiotics on gut microbiota. Dig. Dis. 34, 260–268 (2016).
Gentile, C. L. & Weir, T. L. The gut microbiota at the intersection of diet and human health. Science 362, 776 (2018).
European Centre for Disease Prevention and Control (ECDC) Antimicrobial Consumption in the EU/EEA (ESAC-Net) — Annual Epidemiological Report 2022 (ECDC, 2023).
Kapoor, G., Saigal, S. & Elongavan, A. Action and resistance mechanisms of antibiotics: a guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 33, 300–305 (2017).
Durack, J. & Lynch, S. V. The gut microbiome: relationships with disease and opportunities for therapy. J. Exp. Med. 216, 20–40 (2019).
Sommer, F., Anderson, J. M., Bharti, R., Raes, J. & Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15, 630–638 (2017).
Nel Van Zyl, K., Matukane, S. R., Hamman, B. L., Whitelaw, A. C. & Newton-Foot, M. Effect of antibiotics on the human microbiome: a systematic review. Int. J. Antimicrob. Agents 59, 106502 (2022).
Ferrer, M., Méndez-García, C., Rojo, D., Barbas, C. & Moya, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 134, 114–126 (2017).
McDonnell, L. et al. Association between antibiotics and gut microbiome dysbiosis in children: systematic review and meta-analysis. Gut Microbes 13, 1–18 (2021).
Ainonen, S. et al. Antibiotics at birth and later antibiotic courses: effects on gut microbiota. Pediatr. Res. 91, 154–162 (2022).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Utzschneider, K. M., Kratz, M., Damman, C. J. & Hullar, M. Mechanisms linking the gut microbiome and glucose metabolism. J. Clin. Endocrinol. Metab. 101, 1445–1454 (2016).
Mills, S., Stanton, C., Lane, J. A., Smith, G. J. & Ross, R. P. Precision nutrition and the microbiome, part I: current state of the science. Nutrients 11, 923 (2019).
Russell, D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 (2003).
Pols, T. W. H., Noriega, L. G., Nomura, M., Auwerx, J. & Schoonjans, K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig. Dis. 29, 37–44 (2011).
Staels, B. & Fonseca, V. A. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32, S237–S245 (2009).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009).
Winston, J. A. & Theriot, C. M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 11, 158–171 (2020).
de Aguiar Vallim, T. Q., Tarling, E. J. & Edwards, P. A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 17, 657–669 (2013).
Stamler, J. et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 284, 311–318 (2000).
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Chambers, E. S. et al. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes. Metab. 20, 1034–1039 (2018).
van der Beek, C. M. et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 130, 2073–2082 (2016).
Canfora, E. E. et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci. Rep. 7, 2360 (2017).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591 (2015).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455 (2020).
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Karra, E., Chandarana, K. & Batterham, R. L. The role of peptide YY in appetite regulation and obesity. J. Physiol. 587, 19–25 (2009).
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Rowlands, J., Heng, J., Newsholme, P. & Carlessi, R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 9, 672 (2018).
Feng, Y. et al. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 14, e0218384 (2019).
Lee, S. H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest. Res. 13, 11–18 (2015).
Moreira, A. P. B., Texeira, T. F. S., Ferreira, A. B., do Carmo Gouveia Peluzio, M. & de Cássia Gonçalves Alfenas, R. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 108, 801–809 (2012).
Mehta, N. N. et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59, 172–181 (2010).
Steimle, A., Autenrieth, I. B. & Frick, J.-S. Structure and function: lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 306, 290–301 (2016).
Anhê, F. F., Barra, N. G., Cavallari, J. F., Henriksbo, B. D. & Schertzer, J. D. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 36, 109691 (2021).
Ghosh, S. S., Wang, J., Yannie, P. J. & Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 4, bvz039 (2020).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761 (2007).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470 (2008).
Wolf, A. J. & Underhill, D. M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 18, 243–254 (2018).
Clasen, S. J. et al. Silent recognition of flagellins from human gut commensal bacteria by toll-like receptor 5. Sci. Immunol. 8, eabq7001 (2023).
Creely, S. J. et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 292, E740–E747 (2007).
Kallio, K. A. E. et al. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 52, 395–404 (2015).
Geng, J., Ni, Q., Sun, W., Li, L. & Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 147, 112678 (2022).
Natividad, J. M. et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 28, 737–749.e4 (2018).
Mahana, D. et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 8, 48 (2016).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Nobel, Y. R. et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 6, 7486–7486 (2015).
Membrez, M. et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 22, 2416–2426 (2008).
Carvalho, B. M. et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 55, 2823–2834 (2012).
Murphy, E. F. et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 62, 220–226 (2013).
Rodrigues, R. R. et al. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front. Microbiol. 8, 2306–2306 (2017).
Rausch, P. et al. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int. J. Med. Microbiol. 306, 343–355 (2016).
Hild, B. et al. Neonatal exposure to a wild-derived microbiome protects mice against diet-induced obesity. Nat. Metab. 3, 1042–1057 (2021).
Sprockett, D., Fukami, T. & Relman, D. A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 15, 197–205 (2018).
Jian, C. et al. Early-life gut microbiota and its connection to metabolic health in children: perspective on ecological drivers and need for quantitative approach. eBioMedicine 69, 103475 (2021).
Principi, N. & Esposito, S. Antibiotic administration and the development of obesity in children. Int. J. Antimicrob. Agents 47, 171–177 (2016).
Shao, X. et al. Antibiotic exposure in early life increases risk of childhood obesity: a systematic review and meta-analysis. Front. Endocrinol. 8, 170 (2017).
Rasmussen, S. H. et al. Antibiotic exposure in early life and childhood overweight and obesity: a systematic review and meta-analysis. Diabetes Obes. Metab. 20, 1508–1514 (2018).
Miller, S. A., Wu, R. K. S. & Oremus, M. The association between antibiotic use in infancy and childhood overweight or obesity: a systematic review and meta-analysis. Obes. Rev. 19, 1463–1475 (2018).
Mor, A. et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. Int. J. Obes. 39, 1450–1455 (2015).
Aversa, Z. et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 96, 66–77 (2021).
Furlong, M., Deming-Halverson, S. & Sandler, D. P. Chronic antibiotic use during adulthood and weight change in the Sister Study. PLoS ONE 14, e0216959 (2019).
Mikkelsen, K. H., Knop, F. K., Frost, M., Hallas, J. & Pottegård, A. Use of antibiotics and risk of type 2 diabetes: a population-based case–control study. J. Clin. Endocrinol. Metab. 100, 3633–3640 (2015).
Ye, M. et al. Systemic use of antibiotics and risk of diabetes in adults: a nested case–control study of Alberta’s Tomorrow Project. Diabetes Obes. Metab. 20, 849–857 (2018).
Boursi, B., Mamtani, R., Haynes, K. & Yang, Y.-X. The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol. 172, 639–648 (2015).
Kummeling, I. & Thijs, C. Reverse causation and confounding-by-indication: do they or do they not explain the association between childhood antibiotic treatment and subsequent development of respiratory illness? Clin. Exp. Allergy 38, 1249–1251 (2008).
Vrieze, A. et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831 (2014).
Reijnders, D. et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab. 24, 63–74 (2016).
Reijnders, D. et al. Short-term microbiota manipulation and forearm substrate metabolism in obese men: a randomized, double-blind, placebo-controlled trial. Obes. Facts 11, 318–326 (2018).
Basolo, A. et al. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat. Med. 26, 589–598 (2020).
Palleja, A. et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3, 1255–1265 (2018).
Mikkelsen, K. H. et al. Effect of antibiotics on gut microbiota, gut hormones and glucose metabolism. PLoS ONE 10, e0142352 (2015).
Raymond, F. et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 10, 707–720 (2016).
Vliex, L. M. M. et al. Fecal carriage of vanB antibiotic resistance gene affects adipose tissue function under vancomycin use. Gut Microbes 14, 2083905 (2022).
Stogios, P. J. & Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 29, 654–669 (2020).
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Wan, Y. et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68, 1417–1429 (2019).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Fragiadakis, G. K. et al. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 111, 1127–1136 (2020).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Müller, M. et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: a randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes 12, 1704141 (2020).
Hjorth, M. F. et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 42, 580–583 (2018).
Hjorth, M. F. et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int. J. Obes. 43, 149–157 (2019).
Cronin, P., Joyce, S. A., O’Toole, P. W. & O’Connor, E. M. Dietary fibre modulates the gut microbiota. Nutrients 13, 1655 (2021).
Healey, G. et al. Habitual dietary fibre intake influences gut microbiota response to an insulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 119, 176–189 (2018).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619.e6 (2017).
Jardon, K. M., Canfora, E. E., Goossens, G. H. & Blaak, E. E. Dietary macronutrients and the gut microbiome: a precision nutrition approach to improve cardiometabolic health. Gut 71, 1214–1226 (2022).
Roager, H. M. & Christensen, L. H. Personal diet–microbiota interactions and weight loss. Proc. Nutr. Soc. 81, 243–254 (2022).
Liu, H. et al. Butyrate: a double-edged sword for health? Adv. Nutr. 9, 21–29 (2018).
Pushpass, R.-A. G., Alzoufairi, S., Jackson, K. G. & Lovegrove, J. A. Circulating bile acids as a link between the gut microbiota and cardiovascular health: impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 35, 161–180 (2022).
Delzenne, N. M., Neyrinck, A. M. & Cani, P. D. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Fact. 10, S10 (2011).
Canfora, E. E. et al. Fiber mixture-specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes 14, 2009297 (2022).
Holmes, Z. C. et al. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome 10, 114 (2022).
Ladirat, S. E. et al. Exploring the effects of galacto-oligosaccharides on the gut microbiota of healthy adults receiving amoxicillin treatment. Br. J. Nutr. 112, 536–546 (2014).
Zierer, J. et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 50, 790–795 (2018).
ClinicalTrials.gov. US National Library of Medicine. https://www.clinicaltrials.gov/study/NCT04561284 (2022).
Author information
Authors and Affiliations
Contributions
E.E.B. and J.P. made a substantial contribution to discussion of content and reviewed/edited the manuscript before submission. L.M.M.V. researched data for the article, made a substantial contribution to discussion of the content and wrote the article. A.N. and E.G.Z. reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.N. is an employee of FrieslandCampina. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Martin Blaser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Antibiotic
-
A compound with antibacterial activity. Can be bactericidal (kills bacteria) or bacteriostatic (stops growth).
- Human gut microbiome
-
The ecosystem of bacteria, fungi, archaea and viruses residing in the gastrointestinal tract, especially the intestines and its ‘theatre of activity’.
- Microbial dysbiosis
-
An imbalance in the regular bacterial composition, distribution or activity.
- Perturbations
-
A trigger that can lead to changes in the ecosystem. Can be caused by antibiotic use or a dietary change, among other things.
- Prebiotic
-
A substrate that is selectively utilized by host microorganisms conferring a healthy benefit (defined by the International Association for Probiotics and Prebiotics).
- Recovery
-
The ability of an ecosystem to return to its initial state after a perturbation.
- Resilience
-
The speed at and extent to which the ecosystem returns to its initial state after a perturbation.
- Resistance
-
The ability of an ecosystem to be unaffected by a perturbation and therefore remain in its initial state.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vliex, L.M.M., Penders, J., Nauta, A. et al. The individual response to antibiotics and diet — insights into gut microbial resilience and host metabolism. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-00966-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-024-00966-0