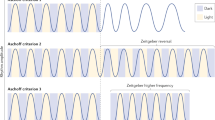
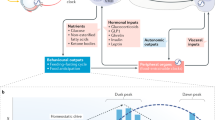
Abstract
Despite the growing popular interest in sleep and diet, many gaps exist in our scientific understanding of the interaction between circadian rhythms and metabolism. In this Review, we explore a promising, bidirectional role for ghrelin in mediating this interaction. Ghrelin both influences and is influenced by central and peripheral circadian systems. Specifically, we focus on how ghrelin impacts outputs of circadian rhythm, including neuronal activity, circulating growth hormone levels, locomotor activity and eating behaviour. We also consider the effects of circadian rhythms on ghrelin expression and the consequences of disrupted circadian patterns, such as shift work and jet lag, on ghrelin secretion. Our Review is aimed at both the casual reader interested in gaining more insight into the scientific context surrounding the trending topics of sleep and metabolism, as well as experienced scientists in the fields of ghrelin and circadian biology seeking inspiration and a comprehensive overview of how these fields are related.
Key points
-
Disrupted circadian and metabolic systems are a growing cause of societal health burden.
-
The ghrelin system regulates many key metabolic functions including food intake, body weight, blood glucose, food anticipatory activity, growth hormone secretion and body temperature.
-
Ghrelin and GHSR (growth hormone secretagogue receptor, which serves as the receptor for ghrelin) mRNA and protein expression are regulated by the circadian system.
-
Ghrelin affects neuronal firing, food anticipatory activity and thermoregulation in a circadian-dependent manner.
-
Given the expansion in scientific technology in the past 25 years, many more tools now exist to better understand the role of the ghrelin system in metabolism and circadian biology.
-
Future studies are needed to elucidate further the connections between the ghrelin system, metabolism and circadian systems, and to provide novel avenues for therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Müller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Sánchez, J., Oliver, P., Picó, C. & Palou, A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflügers Arch. 448, 500–506 (2004).
Cummings, D. E. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001).
Frecka, J. M. & Mattes, R. D. Possible entrainment of ghrelin to habitual meal patterns in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G699–G707 (2008). Plasma ghrelin measurements in humans indicate that ghrelin fluctuates across 24-h both as a function of mealtime as well as in a diurnal rhythm.
Inui, A. Ghrelin: An orexigenic and somatotrophic signal from the stomach. Nat. Rev. Neurosci. 2, 551–560 (2001).
Mani, B. K., Shankar, K. & Zigman, J. M. Ghrelin’s relationship to blood glucose. Endocrinology 160, 1247–1261 (2019).
Sakata, I. et al. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am. J. Physiol. Endocrinol. Metab. 302, E1300–E1310 (2012).
Shankar, K. et al. Acyl-ghrelin is permissive for the normal counterregulatory response to insulin-induced hypoglycemia. Diabetes 69, 228–237 (2020).
Decarie-Spain, L. & Kanoski, S. E. Ghrelin and glucagon-like peptide-1: a gut-brain axis battle for food reward. Nutrients 13, 977 (2021).
Farokhnia, M., Faulkner, M. L., Piacentino, D., Lee, M. R. & Leggio, L. Ghrelin: from a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol. Behav. 204, 49–57 (2019).
Tian, J., Wang, T. & Du, H. Ghrelin system in Alzheimer’s disease. Curr. Opin. Neurobiol. 78, 102655 (2023).
Diano, S. et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 9, 381–388 (2006).
Bahinipati, J., Sarangi, R., Pathak, M. & Mohapatra, S. Effect of night shift on development of metabolic syndrome among health care workers. J. Fam. Med. Prim. Care 11, 1710–1715 (2022).
Wang, Y., Guo, H. & He, F. Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets. Cancer Metastasis Rev. 42, 297–322 (2023).
Hibi, M. et al. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R94–R101 (2013).
Vujović, N. et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 34, 1486–1498.e7 (2022).
Birketvedt, G. S. et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA 282, 657–663 (1999).
Goel, N. et al. Circadian rhythm profiles in women with night eating syndrome. J. Biol. Rhythm. 24, 85–94 (2009).
Chaput, J. P. et al. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 19, 82–97 (2023).
Reinke, H. & Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 20, 227–241 (2019).
Marcheva, B. et al. in Circadian Clocks (eds Kramer, A. & Merrow, M.) 127–155 [Series ed. Michel, M. C. Handbook of Experimental Pharmacology Vol. 217] (Springer, 2013).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005).
Oishi, K. et al. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 580, 127–130 (2006).
Marcheva, B., Ramsey, K. M., Affinati, A. & Bass, J. Clock genes and disease. J. Appl. Physiol. 107, 1638–1646 (2009).
Coomans, C. P. et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 62, 1102–1108 (2013).
Kolbe, I., Leinweber, B., Brandenburger, M. & Oster, H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol. Metab. 30, 140–151 (2019).
Cedernaes, J. et al. Transcriptional basis for rhythmic control of hunger and metabolism within the AgRP neuron. Cell Metab. 29, 1078–1091.e5 (2019).
Yi, C.-X. et al. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–294 (2006).
Gupta, D. et al. “A LEAP 2 conclusions? Targeting the ghrelin system to treat obesity and diabetes”. Mol. Metab. 46, 101128 (2021).
Ariyasu, H. et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 86, 4753–4758 (2001).
Mani, B. K., Osborne-Lawrence, S., Vijayaraghavan, P., Hepler, C. & Zigman, J. M. β1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J. Clin. Invest. 126, 3467–3478 (2016).
Tschöp, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Liu, J. et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J. Clin. Endocrinol. Metab. 93, 1980–1987 (2008).
Gupta, D. et al. β1-adrenergic receptors mediate plasma acyl-ghrelin elevation and depressive-like behavior induced by chronic psychosocial stress. Neuropsychopharmacology 44, 1319–1327 (2019).
Gong, Z. et al. G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am. J. Physiol. Endocrinol. Metab. 306, E28–E35 (2014).
Koyama, H. et al. Comprehensive profiling of GPCR expression in ghrelin-producing cells. Endocrinology 157, 692–704 (2016).
Uchida, A. et al. Altered ghrelin secretion in mice in response to diet-induced obesity and Roux-en-Y gastric bypass. Mol. Metab. 3, 717–730 (2014).
Shankar, K. et al. Ghrelin cell-expressed insulin receptors mediate meal- and obesity-induced declines in plasma ghrelin. JCI Insight 6, e146983 (2021).
Engelstoft, M. S. et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2, 376–392 (2013).
Mani, B. K. et al. The role of ghrelin-responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Mol. Metab. 6, 882–896 (2017).
Davies, J. S. et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol. Endocrinol. 23, 914–924 (2009).
Dallak, M. A. Acylated ghrelin induces but deacylated ghrelin prevents hepatic steatosis and insulin resistance in lean rats: effects on DAG/PKC/JNK pathway. Biomed. Pharmacother. 105, 299–311 (2018).
Li, Z. et al. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARγ signaling pathway. Proc. Natl Acad. Sci. USA 111, 13163–13168 (2014).
Asakawa, A. et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74, 143–147 (2001).
Theander-Carrillo, C. et al. Ghrelin action in the brain controls adipocyte metabolism. J. Clin. Invest. 116, 1983–1993 (2006).
Perello, M. et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry 67, 880–886 (2010).
Perello, M. & Zigman, J. M. The role of ghrelin in reward-based eating. Biol. Psychiatry 72, 347–353 (2012).
Skibicka, K. P., Hansson, C., Egecioglu, E. & Dickson, S. L. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict. Biol. 17, 95–107 (2012).
Kanoski, S. E., Fortin, S. M., Ricks, K. M. & Grill, H. J. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 73, 915–923 (2013).
Barnett, B. P. et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 330, 1689–1692 (2010).
Esler, W. P. et al. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology 148, 5175–5185 (2007).
Shearman, L. P. et al. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology 147, 1517–1526 (2006).
Zorrilla, E. P. et al. Vaccination against weight gain. Proc. Natl Acad. Sci. USA 103, 13226–13231 (2006).
Abegg, K. et al. Ghrelin receptor inverse agonists as a novel therapeutic approach against obesity-related metabolic disease. Diabetes Obes. Metab. 19, 1740–1750 (2017).
Sun, Y., Wang, P., Zheng, H. & Smith, R. G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl Acad. Sci. USA 101, 4679–4684 (2004).
Zigman, J. M. et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 115, 3564–3572 (2005).
Ge, X. et al. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 27, 461–469.e6 (2018).
Yi, C.-X. et al. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS ONE 7, e32100 (2012).
Mani, B. K. et al. Ghrelin mediates exercise endurance and the feeding response post-exercise. Mol. Metab. 9, 114–130 (2018).
Chuang, J. C. et al. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 121, 2684–2692 (2011).
Perello, M. & Dickson, S. L. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J. Neuroendocrinol. 27, 424–434 (2015).
Cornejo, M. P. et al. Growth hormone secretagogue receptor signalling affects high-fat intake independently of plasma levels of ghrelin and LEAP2, in a 4-day binge eating model. J. Neuroendocrinol. 31, e12785 (2019).
Mani, B. K. et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Invest. 129, 3909 (2019).
Shankar, K. et al. LEAP2 deletion in mice enhances ghrelin’s actions as an orexigen and growth hormone secretagogue. Mol. Metab. 53, 101327 (2021).
Islam, M. N. et al. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 244, 13–23 (2020).
Wald, H. S., Ghidewon, M. Y., Hayes, M. R. & Grill, H. J. Hindbrain ghrelin and liver-expressed antimicrobial peptide 2, ligands for growth hormone secretagogue receptor, bidirectionally control food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 324, R547–R555 (2023).
Hagemann, C. A. et al. LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell Rep. Med. 3, 100582 (2022).
Bhargava, R. et al. Postprandial increases in liver-gut hormone LEAP2 correlate with attenuated eating behavior in adults without obesity. J. Endocr. Soc. 7, bvad061 (2023).
Guan, X.-M. et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol. Brain Res. 48, 23–29 (1997).
Zigman, J. M., Jones, J. E., Lee, C. E., Saper, C. B. & Elmquist, J. K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 494, 528–548 (2006).
Ferrini, F., Salio, C., Lossi, L. & Merighi, A. Ghrelin in central neurons. Curr. Neuropharmacol. 7, 37–49 (2009).
Kanoski, S. E. & Grill, H. J. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81, 748–756 (2017).
Faulconbridge, L. F., Cummings, D. E., Kaplan, J. M. & Grill, H. J. Hyperphagic effects of brainstem ghrelin administration. Diabetes 52, 2260–2265 (2003).
Andrews, Z. B. The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci. 34, 31–40 (2011).
Zhou, L. et al. Activation of growth hormone secretagogue receptor induces time-dependent clock phase delay in mice. Am. J. Physiol. Endocrinol. Metab. 307, E515–E526 (2014). Bolus injection of ghrelin mimetic at CT12 produces phase delay under DD; this effect is abolished by pretreatment with a GHSR agonist.
Tokizawa, K., Onoue, Y., Uchida, Y. & Nagashima, K. Ghrelin induces time-dependent modulation of thermoregulation in the cold. Chronobiol. Int. 29, 736–746 (2012). Wild-type animals exposed to ghrelin during the early light phase display diminished ability to protect their body temperature following cold exposure; this effect is abolished when ghrelin is instead administered during the early dark phase.
Yi, C. X. et al. A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats. Eur. J. Neurosci. 27, 1965–1972 (2008).
Yannielli, P. C., Molyneux, P. C., Harrington, M. E. & Golombek, D. A. Ghrelin effects on the circadian system of mice. J. Neurosci. 27, 2890–2895 (2007). Slice physiology of the SCN shows a 3-h advance in the rhythm of spontaneous neuronal firing when sections are treated with ghrelin at ZT6.
Lamont, E. W., Bruton, J., Blum, I. D. & Abizaid, A. Ghrelin receptor-knockout mice display alterations in circadian rhythms of activity and feeding under constant lighting conditions. Eur. J. Neurosci. 39, 207–217 (2014).
Merkestein, M. et al. GHS-R1a signaling in the DMH and VMH contributes to food anticipatory activity. Int. J. Obes. 38, 610–618 (2014).
Mistlberger, R. E. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 104, 535–545 (2011).
LeSauter, J., Hoque, N., Weintraub, M., Pfaff, D. W. & Silver, R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc. Natl Acad. Sci. USA 106, 13582–13587 (2009). Food anticipatory activity is diminished in GHSR-knockout mice, and in ghrelin-containing oxyntic cells, ghrelin express is rhythmic, anti-phase to clock proteins, Per1 and Per2, and does not require light input.
Blum, I. D. et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 164, 351–359 (2009).
Nisembaum, L. G., de Pedro, N., Delgado, M. J. & Isorna, E. Crosstalking between the “gut-brain” hormone ghrelin and the circadian system in the goldfish. Effects on clock gene expression and food anticipatory activity. Gen. Comp. Endocrinol. 205, 287–295 (2014).
Gunapala, K. M., Gallardo, C. M., Hsu, C. T. & Steele, A. D. Single gene deletions of orexin, leptin, neuropeptide Y, and ghrelin do not appreciably alter food anticipatory activity in mice. PLoS ONE 6, e18377 (2011).
Gooley, J. J., Schomer, A. & Saper, C. B. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 9, 398–407 (2006).
Kanamoto, N. et al. Genomic structure and characterization of the 5′-flanking region of the human ghrelin gene. Endocrinology 145, 4144–4153 (2004).
Herzog, E. D. Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8, 790–802 (2007).
Foster-Schubert, K. E. et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 93, 1971–1979 (2008).
Bodosi, B. et al. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1071–R1079 (2004). Time-restricted feeding of rats to just the light phase shifts ghrelin peak from the light phase to the end of the dark phase.
Kumar, J. et al. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite 64, 81–88 (2013).
Cabral, A., López Soto, E. J., Epelbaum, J. & Perelló, M. Is ghrelin synthesized in the central nervous system? Int. J. Mol. Sci. 18, 638 (2017).
Manoogian, E. N. C. & Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 39, 59–67 (2017).
Verbaeys, I. et al. Scheduled feeding results in adipogenesis and increased acylated ghrelin. Am. J. Physiol. Endocrinol. Metab. 300, E1103–E1111 (2011).
Oishi, K. & Hashimoto, C. Short-term time-restricted feeding during the resting phase is sufficient to induce leptin resistance that contributes to development of obesity and metabolic disorders in mice. Chronobiol. Int. 35, 1576–1594 (2018).
Sorrell, J. et al. The central melanocortin system mediates the benefits of time-restricted feeding on energy balance. Physiol. Behav. 227, 113132 (2020).
Drazen, D. L., Vahl, T. P., D’Alessio, D. A., Seeley, R. J. & Woods, S. C. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147, 23–30 (2006).
Merkestein, M. et al. Ghrelin mediates anticipation to a palatable meal in rats. Obesity 20, 963–971 (2012).
Saper, C. B. in Hypothalamic Integration of Energy Metabolism (eds Kalsbeek et al.) 243–252 (Elsevier, 2006). [Series Progress in Brain Research Vol. 153].
Palyha, O. C. et al. Ligand activation domain of human orphan growth hormone (GH) secretagogue receptor (GHS-R) conserved from pufferfish to humans. Mol. Endocrinol. 14, 160–169 (2000).
Kojima, M. & Kangawa, K. Ghrelin: structure and function. Physiol. Rev. 85, 495–522 (2005).
Denney, W. S., Sonnenberg, G. E., Carvajal-Gonzalez, S., Tuthill, T. & Jackson, V. M. Pharmacokinetics and pharmacodynamics of PF-05190457: the first oral ghrelin receptor inverse agonist to be profiled in healthy subjects. Br. J. Clin. Pharmacol. 83, 326–338 (2017).
Wierup, N., Sundler, F. & Heller, R. S. The islet ghrelin cell. J. Mol. Endocrinol. 52, R35–R49 (2014).
Garaulet, M. et al. Ghrelin, sleep reduction and evening preference: relationships to CLOCK 3111 T/C SNP and weight loss. PLoS ONE 6, e17435 (2011). Individuals with overweight and obesity and a specific CLOCK single nucleotide polymorphism had higher plasma ghrelin concentrations.
Spiegel, K., Tasali, E., Penev, P. & Van Cauter, E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004).
Desmet, L., Thijs, T., Segers, A., Verbeke, K. & Depoortere, I. Chronodisruption by chronic jetlag impacts metabolic and gastrointestinal homeostasis in male mice. Acta Physiol. 233, e13703 (2021). Mouse model of chronic jetlag displays disturbed circadian rhythmicity of ghrelin mRNA expression in stomach mucosa.
Luo, S. et al. Experimental dopaminergic neuron lesion at the area of the biological clock pacemaker, suprachiasmatic nuclei (SCN) induces metabolic syndrome in rats. Diabetol. Metab. Syndr. 13, 11 (2021).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152 (2013).
Gupta, D. et al. Disrupting the ghrelin-growth hormone axis limits ghrelin’s orexigenic but not glucoregulatory actions. Mol. Metab. 53, 101258 (2021).
Nass, R. et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J. Clin. Endocrinol. Metab. 93, 1988–1994 (2008).
Zizzari, P., Hassouna, R., Grouselle, D., Epelbaum, J. & Tolle, V. Physiological roles of preproghrelin-derived peptides in GH secretion and feeding. Peptides 32, 2274–2282 (2011).
Serin, Y. & Acar Tek, N. Effect of circadian rhythm on metabolic processes and the regulation of energy balance. Ann. Nutr. Metab. 74, 322–330 (2019).
Wang, W. et al. Rotating day and night disturb growth hormone secretion profiles, body energy metabolism, and insulin levels in mice. Neuroendocrinology 112, 481–492 (2022).
Richards, J. & Gumz, M. L. Advances in understanding the peripheral circadian clocks. FASEB J. 26, 3602–3613 (2012).
Vitaterna, M. H., Takahashi, J. S. & Turek, F. W. Overview of circadian rhythms. Alcohol. Res. Health 25, 85–93 (2001).
Bass, J. & Takahashi, J. S. Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010).
Heyde, I. & Oster, H. Induction of internal circadian desynchrony by misaligning zeitgebers. Sci. Rep. 12, 1601 (2022).
Acknowledgements
The authors’ works is supported through research grants from the NIH (R01 DK103884, P01DK119130 and 5T32GM008014).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. S.S.K. and J.M.Z. contributed to the discussion of content, wrote the article and reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
J.M.Z. owns stock in Medtronic, Eli Lilly and Novo Nordisk, and received research funding from Novo Nordisk for another project during the writing of this Review. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Yu Zhou and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulkarni, S.S., Singh, O. & Zigman, J.M. The intersection between ghrelin, metabolism and circadian rhythms. Nat Rev Endocrinol 20, 228–238 (2024). https://doi.org/10.1038/s41574-023-00927-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00927-z