Abstract
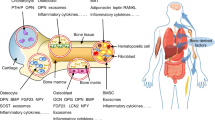
Adipose tissue is a dynamic component of the bone marrow, regulating skeletal remodelling and secreting paracrine and endocrine factors that can affect haematopoiesis, as well as potentially nourishing the bone marrow during periods of stress. Bone marrow adipose tissue is regulated by multiple factors, but particularly nutrient status. In this Review, we examine how bone marrow adipocytes originate, their function in normal and pathological states and how bone marrow adipose tissue modulates whole-body homoeostasis through actions on bone cells, haematopoietic stem cells and extra-medullary adipocytes during nutritional challenges. We focus on both rodent models and human studies to help understand the unique marrow adipocyte, its response to the external nutrient environment and its effects on the skeleton. We finish by addressing some critical questions that to date remain unanswered.
Key points
-
Adipocytes are critical cellular components of the bone marrow that are regulated by local and systemic factors.
-
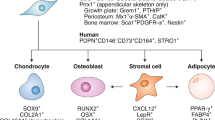
Bone marrow adipocytes have unique origins and distinct functions that are distinguishable from extra-medullary adipocytes.
-
In mice and humans, both axial and appendicular bone marrow adipose tissue increase with age and in response to environmental, nutritional and endocrine factors.
-
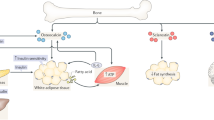
Both a high-fat diet and caloric restriction enhance the recruitment and differentiation of marrow adipocytes, although their function might differ by nutrient stores.
-
A unique marrow adipocyte-like precursor probably serves as a source of mature bone marrow adipocytes.
-
Increased bone marrow adipose tissue can drive bone loss during high dietary intake or can protect the skeleton during caloric restriction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van de Vyver, M. Immunology of chronic low-grade inflammation: relationship with metabolic function. J. Endocrinol. 257, e220271 (2023).
Nicolay, N. H., Lopez Perez, R., Debus, J. & Huber, P. E. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 366, 133–140 (2015).
Nehlin, J. O., Jafari, A., Tencerova, M. & Kassem, M. Aging and lineage allocation changes of bone marrow skeletal (stromal) stem cells. Bone 123, 265–273 (2019).
Wang, L. et al. Bone marrow adipocytes: a critical player in the bone marrow microenvironment. Front. Cell Dev. Biol. 9, 770705 (2021).
Zhou, W. et al. Ablation of fat cells in adult mice induces massive bone gain. Cell Metab. 32, 801–813.e6 (2020). To our knowledge, the first evidence that marrow adipocytes secrete factors that block osteoblast differentiation.
Zhong et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. eLife 9, e54695 (2020).
Matsushita, Y., Ono, W. & Ono, N. Toward marrow adipocytes: adipogenic trajectory of the bone marrow stromal cell lineage. Front. Endocrinol. 13, 882297 (2022).
Bianco, P. & Robey, P. G. Skeletal stem cells. Development 142, 1023–1027 (2015).
Friedenstein, A. J., Chailakhjan, R. K. & Lalykina, K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 3, 393–403 (1970).
Bennet, J. H. et al. Adipocytic cells cultured from marrow have osteogenic potential. J. Cell Sci. 9, 131–139 (1991).
Bianco, P., Robey, P. G. & Simmons, P. J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2, 313–319 (2008). A classic review paper that defines SSCs.
Morikawa et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 206, 2483–2496 (2009).
Park, D. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 (2012).
Jeong, J. H. Adipose stem cells as a clinically available and effective source of adult stem cell therapy. Int. J. Stem Cell 1, 43–48 (2008).
Guasti, L., New, S. E., Hadjidemetriou, I., Palmiero, M. & Ferretti, P. Plasticity of human adipose-derived stem cells — relevance to tissue repair. Int. J. Dev. Biol. 62, 431–439 (2018).
Gimble, J. M. et al. Adipose-derived stromal/stem cells: a primer. Organogenesis 9, 3–10 (2013).
Rauch, A. et al. Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nat. Genet. 51, 716–727 (2019).
Ambrosi, T. H. et al. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. eLife 10, e66063 (2021).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014). A major discovery of the importance of LEPR as a marker of SSCs.
Logan, M. et al. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77–80 (2002).
Sanchez-Gurmaches, J., Hsiao, W. Y. & Guertin, D. Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Rep. 4, 541–550 (2015). A critical paper for lineage studies defining Prrx1 as an early marker of mesenchymal progenitors.
Horowitz, M. C. et al. Bone marrow adipocytes. Adipocytes 6, 193–204 (2017).
Li, Z. et al. Constitutive bone marrow adipocytes suppress local bone formation. JCI Insight 7, e160915 (2022).
He, W. et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl Acad. Sci. USA 26, 15712–15717 (2003).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 (2013).
Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009). An important paper adding to the delineation of the marrow adipocyte as a negative regulator of haematopoiesis.
Greenbaum, A. et al. CXCL12 production by early mesenchymal progenitors is required for hematopoietic stem cell maintenance. Nature 495, 227–230 (2013).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stroma; progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Liu, Y. et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS ONE 8, e71318 (2013).
Ambrosi, T. H. et al. Adipocyte accumulation in bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784 (2017). An important paper to show the effect of marrow adipocytes on haematopoietic and skeletal progenitors with ageing.
Chan, C. K. F. et al. Identification and specification of the mouse skeletal stem. Cell 160, 285–298 (2015).
Shao, M. et al. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab. 23, 1167–1184 (2016).
Chandra, A. et al. Suppression of sclerostin alleviates radiation-induced bone loss by protecting bone-forming cells and their progenitors through distinct mechanisms. J. Bone Miner. Res. 32, 360–372 (2017).
Vieira, R. et al. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome — review of classical and new compounds: part-I. Pharmaceuticals 12, 152 (2019).
Worthley, D. L. et al. Gremlin 1 identifies a skeletal stem cell with bone cartilage, and reticular stromal potential. Cell 160, 269–284 (2015).
Scheller, E. L. et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat. Commun. 6, 7808 (2015).
Boyd, A. L. et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat. Cell Biol. 19, 1336–1347 (2017).
Boroumand, P. et al. Bone marrow adipocytes drive the development of tissue invasive Ly6C high monocytes during obesity. eLife 11, e65553 (2022).
Zhong, L. et al. Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone. eLife 12, e82112 (2023).
Galic, S., Oakhill, J. S. & Steinberg, G. R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 316, 129–139 (2010).
Sun, K., Kusminski, C. M. & Scherer, P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 (2011).
Tavassoli, M., Eastlund, D. T., Yam, L. T., Neiman, R. S. & Finkel, H. Gelatinous transformation of bone marrow in prolonged self-induced starvation. Scand. J. Haematol. 16, 311–319 (1976).
Devlin, M. J. Why does starvation make bones fat? Am. J. Hum. Biol. 23, 577–85 (2011).
Animal Disease Diagnostic Laboratory: Purdue University. Bone marrow fat analysis as a measure of starvation in animals. Animal Disease Diagnostic Laboratory https://www.addl.purdue.edu/newsletters/2006/Winter/bmfa.htm (2006).
Bermudez, B., Ishii, T., Wu, Y. H., Carpenter, R. D. & Sherk, V. D. Energy balance and bone health: a nutrient availability perspective. Curr. Osteoporos. Rep. 21, 77–84 (2023).
Ali, D. et al. High-fat diet-induced obesity augments the deleterious effects of estrogen deficiency on bone: evidence from ovariectomized mice. Aging Cell 12, e13726 (2022).
Tencerova, M. et al. High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J. Bone Min. Res. 6, 1154–1165 (2018). One of the important studies tying obesity to marrow adiposity.
Dimitri, P., Bishop, N., Walsh, J. S. & Eastell, R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone 50, 457–466 (2012).
Rosen, C. J. & Bouxsein, M. L. Mechanisms of disease: is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2, 35–43 (2006).
de Araujo, I. M. et al. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur. J. Endocrinol. 176, 21–30 (2017).
Villareal, D. T. et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Min. Res. 31, 40–51 (2016). A classic paper showing that caloric restriction improves surrogates of cardiovascular health but causes bone loss.
Zibellini, J. et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J. Bone Min. Res. 30, 2168–2178 (2015).
Hadi, Y. B. et al. Metabolic bone disease and fracture risk after gastric bypass and sleeve gastrectomy: comparative analysis of a multi-institutional research network. Surg. Obes. Relat. Dis. 18, 604–609 (2022).
Fazeli, P. K. et al. The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight 6, e138636 (2021).
Holanda, N. et al. Musculoskeletal effects of obesity and bariatric surgery — a narrative review. Arch. Endocrinol. Metab. 66, 621–632 (2022).
Doucette, C. R. et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J. Cell. Physiol. 230, 2032–2037 (2015).
Devlin, M. J. et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. 25, 2078–2088 (2010).
Li, Z. et al. G-CSF partially mediates effects of sleeve gastrectomy on the bone marrow niche. J. Clin. Invest. 129, 2404–2416 (2019). One of the mechanisms of gastric-bypass-induced bone loss is mediated through G-CSF.
Bornstein, S. et al. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J. Endocrinology 155, 3516–3526 (2014).
Bachmann, K. N. et al. Vertebral volumetric bone density and strength are impaired in women with low-weight and atypical anorexia nervosa. J. Clin. Endocrinol. Metab. 102, 57–68 (2017).
Cawthorn, W. P. et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology 157, 508–521 (2016).
Fazeli, P. K. et al. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J. Clin. Endocrinol. Metab. 95, 407–413 (2010).
Milos, G. et al. Positive effect of teriparatide on areal bone mineral density in young women with anorexia nervosa: a pilot study. Calcif. Tissue Int. 108, 595–604 (2021).
Fazeli, P. K. et al. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J. Clin. Endocrinol. Metab. 99, 1322–1329 (2014).
Colling, C. et al. Changes in serum cortisol 10 days after overfeeding or fasting. Am. J. Physiol. Endocrinol. Metab. 324, E506–E513 (2023).
Fan, Y. et al. PTH directs bone marrow mesenchymal cell fate. Cell Metab. 25, 661–672 (2017).
Zhong, L. et al. Transient expansion and myofibroblast conversion of adipogenic lineage precursors mediate bone marrow repair after radiation. JCI Insight 7, e150323 (2022).
Piotrowska, K. & Tarnowski, M. Bone marrow adipocytes — role in physiology and various nutritional conditions in human and animal models. Nutrients 13, 1412 (2021).
Reitman, M. L. & Gavrilova, O. A-ZIP/F-1 mice lacking white fat: a model for understanding lipoatrophic diabetes. Int. J. Obes. Relat. Metab. Disord. 24, S11–S14 (2000).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and hematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 (2017). A critical paper that delineates how bone marrow adipocytes might enhance haematopoiesis.
Qiu, W. et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J. Bone Min. Res. 22, 1720–1731 (2007).
Li, Z. et al. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. eLife 11, e78496 (2022). A seminal paper that defines how, during caloric restriction, regulated marrow adipose tissue might protect the skeleton.
Ables, G. P. & Johnson, J. E. Pleiotropic responses to methionine restriction. Exp. Gerontol. 94, 83–88 (2017).
Plummer, J., Park, M., Perodin, F., Horowitz, M. C. & Hens, J. R. Methionine-restricted diet increases miRNAs that can target RUNX2 expression and alters bone structure in young mice. J. Cell. Biochem. 118, 31–42 (2017).
Ouattara, A., Cooke, D., Gopalakrishnan, R., Huang, T.-H. & Ables, G. P. Methionine restriction alters bone morphology and affects osteoblast differentiation. Bone Rep. 5, 33–42 (2016).
Richie, J. P. et al. Dietary methionine and total sulfur amino acid restriction in healthy adults. J. Nutr. Health Aging 27, 111–123 (2023).
Meunier, P., Aaron, J., Edouard, C. & Vignon, G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 80, 147–154 (1971). To our knowledge, the first description of the relationship between marrow adipose tissue and bone.
Yeung, D. K. et al. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J. Magn. Reson. Imaging 22, 279–285 (2005).
Shen, W. et al. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos. Int. 18, 641–647 (2007).
Syed, F. A. et al. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos. Int. 19, 1323–1330 (2008).
Aron, N. et al. Adipsin promotes bone marrow adiposity by priming mesenchymal stem cells. eLife 10, e69209 (2021).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Rosen, C. J. Extensive expertise in endocrinology. My quarter century quest to understand the paradox of marrow adiposity. Eur. J. Endocrinol. 187, R17–R26 (2022).
Maridas, D. E., DeMambro, V. E., Le, P., Mohan, S. & Rosen, C. J. IGFBP-4 is required for adipogenesis and adipose distribution. Endocrinology 158, 3488–3500 (2017).
Zou, Q. et al. Risk of fracture following gastric surgery for benign and malignant conditions: a study level pooled analysis of population-based cohort studies. Front. Oncol. 12, 1001662 (2022).
Aaseth, J. O. & Alexander, J. Postoperative osteoporosis in subjects with morbid obesity undergoing bariatric surgery with gastric bypass or sleeve gastrectomy. Nutrients 15, 1302 (2023).
Sanchez-Gurmaches, J. & Guertin, D. A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5, 4099 (2014).
Chapman, J. & Vega, F. Incidental brown adipose tissue in bone marrow biopsy. Blood 130, 952 (2017).
Dannheim, K. & Bhargava, P. A rare finding of brown fat in bone marrow as a mimic for metastatic disease. Am. J. Hematol. 91, 545–546 (2016).
Rahman, S. et al. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154, 2687–2701 (2013).
Krings, A. et al. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 50, 546–552 (2012).
Scheller, E. L. et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Meth. Enzymol. 537, 123–139 (2014).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Craft, C. S. et al. Bone marrow adipose tissue does not express UCP1 during development or adrenergic-induced remodeling. Sci. Rep. 9, 17427 (2019).
Thompson J. C., Carvalho S., Marean C. W. & Alemseged, Z., Origins of the human predatory pattern: the transition to large-animals exploitation by early hominins. Curr. Anthropol. https://doi.org/10.1086/701477 (2019).
Plummer, T. W. et al. Expanded geographic distribution and dietary strategies of the earliest Oldoman hominins and Paranthropus. Science 379, 561–566 (2023).
Steele R. Hygenic Physiology: Skin and Bone (1883).
Feussner, J. R., Shelburne, J. D., Bredehoeft, S. & Cohen, H. J. Arsenic-induced bone marrow toxicity: ultrastructural and electron-probe analysis. Blood 53, 820–827 (1979).
Tavassoli, M. & Crosby, W. H. Bone marrow histogenesis: a comparison of fatty and red marrow. Science 169, 291–293 (1970). To our knowledge, the first characterization of marrow adipocytes and their relationship to bone marrow haematopoiesis.
Feng, C. S. Gelatinous transformation of marrow in a case of acute myelogenous leukemia post-chemotherapy. Am. J. Hematol. 38, 220–222 (1991).
Bredella, M. A. et al. Increased bone marrow fat in anorexia nervosa. J. Clin. Endocrinol. Metab. 94, 2129–2136 (2009). The paradoxical increase in marrow adipose tissue with peripheral adipose tissue loss drove the field of nutrition, endocrinology and bone biology.
Gao, Y. et al. Magnetic resonance imaging-measured bone marrow adipose tissue area is inversely related to cortical bone area in children and adolescents aged 5–18 years. J. Clin. Densitom. 18, 203–208 (2015).
Griffith, J. F., Yeung, D. K., Chow, S. K., Leung, J. C. & Leung, P. C. Reproducibility of MR perfusion and 1H spectroscopy of bone marrow. J. Magn. Reson. Imaging 29, 1438–1442 (2009).
Tencerova, M. et al. Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell Rep. 27, 2050–2062 (2019).
Akune, T. et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113, 846–855 (2004).
Velickovic, K. et al. Low temperature exposure induces browning of bone marrow stem cell derived adipocytes in vitro. Sci. Rep. 8, 4974 (2018).
Balani, D. H. & Kronenbergy, H. M. Withdrawal of parathyroid hormone after prolonged administration leads to adipogenic differentiation of mesenchymal precursors in vivo. Bone 118, 16–19 (2019). A classic paper demonstrating the effect of PTH on skeletal progenitor lineage.
Acknowledgements
The authors acknowledge the support of National Institute of Diabetes and Digestive and Kidney Diseases R24 092759-09.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Frederic Deschaseaux, Moustapha Kassem and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rosen, C.J., Horowitz, M.C. Nutrient regulation of bone marrow adipose tissue: skeletal implications of weight loss. Nat Rev Endocrinol 19, 626–638 (2023). https://doi.org/10.1038/s41574-023-00879-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00879-4