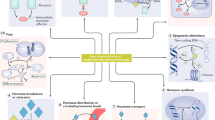
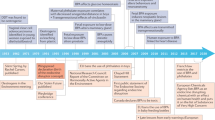
Abstract
Endocrine-disrupting chemicals (EDCs) are substances generated by human industrial activities that are detrimental to human health through their effects on the endocrine system. The global societal and economic burden posed by EDCs is substantial. Poorly defined or unenforced policies can increase human exposure to EDCs, thereby contributing to human disease, disability and economic damage. Researchers have shown that policies and interventions implemented at both individual and government levels have the potential to reduce exposure to EDCs. This Review describes a set of evidence-based policy actions to manage, minimize or even eliminate the widespread use of these chemicals and better protect human health and society. A number of specific challenges exist: defining, identifying and prioritizing EDCs; considering the non-linear or non-monotonic properties of EDCs; accounting for EDC exposure effects that are latent and do not appear until later in life; and updating testing paradigms to reflect ‘real-world’ mixtures of chemicals and cumulative exposure. A sound strategy also requires partnering with health-care providers to integrate strategies to prevent EDC exposure in clinical care. Critical next steps include addressing EDCs within global policy frameworks by integrating EDC exposure prevention into emerging climate policy.
Key points
-
Poorly defined or unenforced policies can increase global human exposure to endocrine-disrupting chemicals (EDCs), contributing to substantial human disease, disability and economic damage.
-
Regulatory bodies have drawn from leading scientific and health organizations to define EDC properties but have not operationalized a consistent definition.
-
Current risk-based paradigms do not consider the non-linear and/or non-monotonic properties of EDCs: default toxicology methods to measure minimum levels do not adequately protect from EDC exposure.
-
Policies also need to account for latent EDC exposure effects that do not appear until later in life.
-
EDC testing paradigms should reflect real-world mixtures and cumulative exposures.
-
Many EDCs are manufactured from fossil fuels, linking their fate with our ability to develop sound policy to address the grand societal challenge of climate change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Carson R., Lear L., Wilson E. O. Silent Spring Anniversary edition (Houghton Mifflin, 2002).
Herbst, A. L., Ulfelder, H. & Poskanzer, D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 284, 878–881 (1971).
Attina, T. M. et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 4, 996–1003 (2016).
Hotchkiss, A. K. et al. Fifteen years after “Wingspread” — environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol. Sci. 105, 235–259 (2008).
World Health Organization. State of the Science of Endocrine Disrupting Chemicals. Inter-Organization Programme for the Sound Management of Chemicals https://apps.who.int/iris/handle/10665/78101 (2013).
Diamanti-Kandarakis, E. et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342 (2009).
Gore, A. C. et al. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, E1–E150 (2015). This manuscript remains the most authoritative review and analysis of the effects of EDCs on health outcomes.
Trasande, L., Shaffer, R. M. & Sathyanarayana, S. Food additives and child health. Pediatrics 142, e20181410 (2018).
Di Renzo, G. C. et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int. J. Gynaecol. Obstet. 131, 219–225 (2015).
Malits, J., Naidu, M. & Trasande, L. Exposure to endocrine disrupting chemicals in Canada: population-based estimates of disease burden and economic costs. Toxics 10, 146 (2022).
Bellanger, M., Demeneix, B., Grandjean, P., Zoeller, R. T. & Trasande, L. Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 100, 1256–1266 (2015).
Hauser, R. et al. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 100, 1267–1277 (2015).
Hunt, P. A., Sathyanarayana, S., Fowler, P. A. & Trasande, L. Female reproductive disorders, diseases, and costs of exposure to endocrine disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 101, 1562–1570 (2016).
Legler, J. et al. Trasande L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 100, 1278–1288 (2015).
Trasande, L. et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 100, 1245–1255 (2015). The first assessment demonstrating that EDC exposures were contributing to significant cost burdens.
Trasande, L. et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology 4, 565–572 (2016).
Obsekov, V., Kahn, L. G. & Trasande, L. Leveraging systematic reviews to explore disease burden and costs of per- and polyfluoroalkyl substance exposures in the United States. Expo. Health 15, 373–394 (2023).
Trasande, L., Liu, B. & Bao, W. Phthalates and attributable mortality: a population-based longitudinal cohort study and cost analysis. Environ. Pollut. 292, 118021 (2022).
Ruiz, D., Becerra, M., Jagai, J. S., Ard, K. & Sargis, R. M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 41, 193–205 (2018).
Abellan, A. et al. In utero exposure to bisphenols and asthma, wheeze, and lung function in school-age children: a prospective meta-analysis of 8 European birth cohorts. Environ. Int. 162, 107178 (2022).
Lam, J. et al. The navigation guide — evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ. Health Perspect. 122, 1040–1051 (2014).
Fan, X. et al. Global exposure to per- and polyfluoroalkyl substances and associated burden of low birthweight. Environ. Sci. Technol. 56, 4282–4294 (2022).
Barbosa, K. L. et al. Tributyltin and the female hypothalamic-pituitary-gonadal disruption. Toxicol. Sci. 186, 179–189 (2022).
Uc-Peraza, R. G., Castro, Í. B. & Fillmann, G. An absurd scenario in 2021: banned TBT-based antifouling products still available on the market. Sci. Total Environ. 805, 150377 (2022).
Trasande, L. When enough data are not enough to enact policy: the failure to ban chlorpyrifos. PLoS Biol. 15, e2003671 (2017).
Malo, S. 3M, others reach $65 mln deal with NY town over PFOA in drinking water. Reuters https://www.reuters.com/legal/litigation/3m-others-reach-65-mln-deal-with-ny-town-over-pfoa-drinking-water-2021-07-22/ (2021).
Bradman, A. et al. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ. Health Perspect. 123, 1086–1093 (2015). An outstanding intervention study showing that lifestyle changes can impact exposures to EDCs.
Harley, K. G. et al. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ. Health Perspect. 124, 1600–1607 (2016).
Rudel, R. A. et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Perspect. 119, 914–920 (2011).
Young, A. S. et al. Impact of “healthier” materials interventions on dust concentrations of per- and polyfluoroalkyl substances, polybrominated diphenyl ethers, and organophosphate esters. Environ. Int. 150, 106151 (2021).
Li, R. et al. Temporal trends in risk of bisphenol A, benzophenone-3 and triclosan exposure among U.S. children and adolescents aged 6-19 years: findings from the National Health and Nutrition Examination Survey 2005-2016. Environ. Res. 216, 114474 (2023).
Zoeller, R. T. et al. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ. Health 13, 118 (2014).
International Programme on Chemical Safety. Global Assessment of the State of the Science of Endocrine Disruptors. World Health Organization https://apps.who.int/iris/handle/10665/67357 (2002).
Woodruff, T. J. et al. Meeting report: moving upstream-evaluating adverse upstream end points for improved risk assessment and decision-making. Environ. Health Perspect. 116, 1568–1575 (2008).
Maffini, M. V. & Vandenberg, L. N. Failure to launch: the endocrine disruptor screening program at the U.S. Environmental Protection Agency. Front. Toxicol. 4, 908439 (2022). A new analysis demonstrating how and why the EPA screening programme for EDCs has failed to protect the public from chemicals with endocrine-disrupting properties.
Knudsen, T. B. et al. FutureTox II: in vitro data and in silico models for predictive toxicology. Toxicol. Sci. 143, 256–267 (2015).
Malo, N., Hanley, J. A., Cerquozzi, S., Pelletier, J. & Nadon, R. Statistical practice in high-throughput screening data analysis. Nat. Biotechnol. 24, 167–175 (2006).
Richard, A. M. et al. ToxCast chemical landscape: paving the road to 21st century toxicology. Chem. Res. Toxicol. 29, 1225–1251 (2016).
Environmental Protection Agency. Availability of New Approach Methodologies (NAMs) in the Endocrine Disruptor Screening Program (EDSP) https://www.regulations.gov/document/EPA-HQ-OPP-2021-0756-0002 (2023).
European Agency for Safety and Health at Work. Regulation (EC) No 1907/2006 — Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Safety and Health at Work EU-OSHA https://osha.europa.eu/en/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (2021).
Endocrine Disruptor Lists. Substances Identified as Endocrine Disruptors at EU Level https://edlists.org/the-ed-lists/list-i-substances-identified-as-endocrine-disruptors-by-the-eu (2022).
Wang, Z., Walker, G. W., Muir, D. C. G. & Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 54, 2575–2584 (2020).
Neltner, T. G. et al. Navigating the U.S. Food Additive Regulatory Program. Compr. Rev. Food Sci. Food Saf. 10, 342–368 (2011).
Encarnação, T., Pais, A. A., Campos, M. G. & Burrows, H. D. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci. Prog. 102, 3–42 (2019).
Neltner, T. G., Alger, H. M., Leonard, J. E. & Maffini, M. V. Data gaps in toxicity testing of chemicals allowed in food in the United States. Reprod. Toxicol. 42, 85–94 (2013).
Janesick, A. S. et al. On the utility of ToxCastTM and ToxPi as methods for identifying new obesogens. Environ. Health Perspect. 124, 1214–1226 (2016).
Filer, D., Patisaul, H. B., Schug, T., Reif, D. & Thayer, K. Test driving ToxCast: endocrine profiling for 1858 chemicals included in phase II. Curr. Opin. Pharmacol. 19, 145–152 (2014).
NRDC. Court Rules EPA Must Regulate Perchlorate https://www.nrdc.org/press-releases/court-rules-epa-must-regulate-perchlorate (2023).
Filer, D. L., Hoffman, K., Sargis, R. M., Trasande, L. & Kassotis, C. D. On the utility of ToxCast-based predictive models to evaluate potential metabolic disruption by environmental chemicals. Env. Health Perspect. 130, 57005 (2022).
Martin, M. T. et al. Predictive model of rat reproductive toxicity from ToxCast high throughput screening. Biol. Reprod. 85, 327–339 (2011).
Sipes, N. S. et al. Predictive models of prenatal developmental toxicity from ToxCast high-throughput screening data. Toxicol. Sci. 124, 109–127 (2011).
Liu, J. et al. Predicting hepatotoxicity using ToxCast in vitro bioactivity and chemical structure. Chem. Res. Toxicol. 28, 738–751 (2015).
Schwarzman, M. R. et al. Screening for chemical contributions to breast cancer risk: a case study for chemical safety evaluation. Environ. Health Perspect. 123, 1255–1264 (2015).
Maffini, M. V., Trasande, L. & Neltner, T. G. Perchlorate and diet: human exposures, risks, and mitigation strategies. Curr. Environ. Health Rep. 3, 107–117 (2016).
EFSA. Public Consultation: Re-evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs https://connect.efsa.europa.eu/RM/s/publicconsultation2/a0l1v00000E8BRD/pc0109 (2022).
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP) et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs.EFSA J. 21, e06857 (2023).
FDA. GRAS Notices: Soy Isoflavone Extract https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=1 (1998).
FDA. GRAS Notices: trans-Resveratrol https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=224 (2007).
Maffini, M. V. & Neltner, T. G. How the FDA Ignores the Law When Approving New Chemical Additives to Food. Environmental Health News https://www.ehn.org/health-issues-associated-with-food-additives-2649620272.html (2020).
Maffini, M. V. et al. Advancing the science on chemical classes. Environ. Health 21, 120 (2023). A new policy analysis that reviews the approaches that are available to regulate environmental chemicals in classes rather than as individual chemicals.
Zimmermann, L. et al. Implementing the EU chemicals strategy for sustainability: the case of food contact chemicals of concern. J. Hazard. Mater. 437, 129167 (2022).
Solecki, R. et al. Scientific principles for the identification of endocrine-disrupting chemicals: a consensus statement. Arch. Toxicol. 91, 1001–1006 (2017).
ANSES’s Working Group on Endocrine Disruptors et al. Concerns related to ED-mediated effects of Bisphenol A and their regulatory consideration. Mol. Cell Endocrinol. 475, 92–106 (2018).
Vandenberg, L. N. et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455 (2012).
Hill, C. E., Myers, J. P. & Vandenberg, L. N. Nonmonotonic dose–response curves occur in dose ranges that are relevant to regulatory decision-making. Dose Response 16, 1559325818798282 (2018).
Do, R. P., Stahlhut, R. W., Ponzi, D., Vom Saal, F. S. & Taylor, J. A. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod. Toxicol. 34, 614–621 (2012).
Lee, D. H. et al. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case–control study. Environ. Health Perspect. 118, 1235–1242 (2010).
Lanphear, B. P. et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ. Health Perspect. 113, 894–899 (2005).
Grandjean, P. et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am. J. Epidemiol. 150, 301–305 (1999).
Bouchard, M. F. et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 119, 1189–1195 (2011).
Rauh, V. A. et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118, e1845–e1859 (2006).
Mancini, F. R. et al. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: findings from the E3N cohort study. Int. J. Hyg. Environ. Health 221, 1054–1060 (2018).
Chen, A. et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ. Health Perspect. 122, 856–862 (2014).
Herbstman, J. B. et al. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 118, 712–719 (2010).
Varshavsky, J. R. et al. Current practice and recommendations for advancing how human variability and susceptibility are considered in chemical risk assessment. Environ. Health 21, 133 (2023). A new policy analysis that illustrates best practices for using measures of variability and susceptibility in models for risk assessment.
Demeneix, B., Vandenberg, L. N., Ivell, R. & Zoeller, R. T. Thresholds and endocrine disruptors: an endocrine society policy perspective. J. Endocr. Soc. 4, bvaa085 (2020).
Hall, A. M. & Braun, J. M. Per- and polyfluoroalkyl substances and outcomes related to metabolic syndrome: a review of the literature and current recommendations for clinicians. Am. J. Lifestyle Med. https://doi.org/10.1177/15598276231162802 (2023).
Jackson, A. A., Langley-Evans, S. C. & McCarthy, H. D. Nutritional influences in early life upon obesity and body proportions. CIBA Found. Symp. 201, 118–129 (1996).
Kahn, H. S., Graff, M., Stein, A. D. & Lumey, L. H. A fingerprint marker from early gestation associated with diabetes in middle age: the Dutch Hunger Winter Families Study. Int. J. Epidemiol. 38, 101–109 (2009).
Schulz, L. C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl Acad. Sci. USA 107, 16757–16758 (2010).
Ghassabian, A., Vandenberg, L., Kannan, K. & Trasande, L. Endocrine-disrupting chemicals and child health. Annu. Rev. Pharmacol. Toxicol. 62, 573–594 (2022).
Newbold, R. R., Padilla-Banks, E., Snyder, R. J. & Jefferson, W. N. Perinatal exposure to environmental estrogens and the development of obesity. Mol. Nutr. Food Res. 51, 912–917 (2007).
Somm, E. et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 117, 1549–1555 (2009).
Hao, C. J., Cheng, X. J., Xia, H. F. & Ma, X. The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell Physiol. Biochem. 30, 382–394 (2012).
Patisaul, H. B. et al. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol. 27, 124–136 (2013).
Kassotis, C. D. et al. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 156, 4458–4473 (2015).
Kassotis, C. D. et al. Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology 157, 3469–3481 (2016).
Balise, V. D. et al. Preconceptional, gestational, and lactational exposure to an unconventional oil and gas chemical mixture alters energy expenditure in adult female mice. Front. Endocrinol. 10, 323 (2019).
Balise, V. D. et al. Developmental exposure to a mixture of unconventional oil and gas chemicals increased risk-taking behavior, activity and Energy expenditure in aged female mice after a metabolic challenge. Front. Endocrinol. 10, 460 (2019).
Vandenberg, L. N. Endocrine disrupting chemicals and the mammary gland. Adv. Pharmacol. 92, 237–277 (2021).
Neal-Kluever, A. et al. Infant toxicology: State of the science and considerations in evaluation of safety. Food Chem. Toxicol. 70, 68–83 (2014).
Cameron, J., & Abouchar, J. The precautionary principle: a fundamental principle of law and policy for the protection of the global environment. 14 BC Int’l & Comp. L. Rev. 1 (1991).
Tsai, P. L. & Hatfield, T. H. Global benefits from the phaseout of leaded fuel. J. Environ. Health 74, 8–15 (2011).
Silva, E., Rajapakse, N. & Kortenkamp, A. Something from “nothing”-eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 36, 1751–1756 (2002).
Rajapakse, N., Silva, E. & Kortenkamp, A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ. Health Perspect. 110, 917–921 (2002).
Christiansen, S. et al. Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int. J. Androl. 31, 241–248 (2008).
Orton, F., Rosivatz, E., Scholze, M. & Kortenkamp, A. Competitive androgen receptor antagonism as a factor determining the predictability of cumulative antiandrogenic effects of widely used pesticides. Environ. Health Perspect. 120, 1578–1584 (2012).
Thrupp, T. J. et al. The consequences of exposure to mixtures of chemicals: something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Sci. Total Environ. 619–620, 1482–1492 (2018).
Yang, M., Park, M. S. & Lee, H. S. Endocrine disrupting chemicals: human exposure and health risks. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 24, 183–224 (2006).
Conley, J. M. et al. A mixture of 15 phthalates and pesticides below individual chemical no observed adverse effect levels (NOAELs) produces reproductive tract malformations in the male rat. Environ. Int. 156, 106615 (2021). An important study of chemical mixtures, highlighting that chemicals at doses that individually have no adverse effects can produce serious adverse effects when combined.
Martin, O. et al. Ten years of research on synergisms and antagonisms in chemical mixtures: a systematic review and quantitative reappraisal of mixture studies. Environ. Int. 146, 106206 (2021).
Centers for Disease Control and Prevention. National Report on Human Exposure to Environmental Chemicals https://www.cdc.gov/exposurereport/index.html (2015).
Houlihan, J. Body Burden: The Pollution in Newborns. Environmental Working Group https://www.ewg.org/research/body-burden-pollution-newborns (2005).
EFSA Scientific Committee et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 17, e05634 (2019).
Anzenbacher, P. & Anzenbacherová, E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol. Life Sci. 58, 737–747 (2001).
Markowitz, J. S. et al. Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. J. Am. Med. Assoc. 290, 1500–1504 (2003).
Kassotis, C. D., Tillitt, D. E., Lin, C. H., McElroy, J. A. & Nagel, S. C. Endocrine-disrupting chemicals and oil and natural gas operations: potential environmental contamination and recommendations to assess complex environmental mixtures. Environ. Health Perspect. 124, 256–264 (2016).
Gauger, K. J. et al. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ. Health Perspect. 115, 1623–1630 (2007).
Hoover, G., Kar, S., Guffey, S., Leszczynski, J. & Sepúlveda, M. S. In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 233, 25–33 (2019).
Ojo, A. F., Peng, C. & Ng, J. C. Combined effects and toxicological interactions of perfluoroalkyl and polyfluoroalkyl substances mixtures in human liver cells (HepG2). Environ. Pollut. 263, 114182 (2020).
Ding, G. et al. Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch. Environ. Contam. Toxicol. 64, 668–675 (2013).
Marques, E. S. et al. The role of maternal high fat diet on mouse pup metabolic endpoints following perinatal PFAS and PFAS mixture exposure. Toxicology 462, 152921 (2021).
Liew, Z. et al. Maternal plasma perfluoroalkyl substances and miscarriage: a nested case-control study in the Danish National Birth Cohort. Environ. Health Perspect. 128, 47007 (2020).
Shih, Y. H., Blomberg, A. J., Jørgensen, L. H., Weihe, P. & Grandjean, P. Early-life exposure to perfluoroalkyl substances in relation to serum adipokines in a longitudinal birth cohort. Environ. Res 204, 111905 (2022).
Guo, J. et al. Umbilical cord serum perfluoroalkyl substance mixtures in relation to thyroid function of newborns: findings from Sheyang Mini Birth Cohort Study. Chemosphere 273, 129664 (2021).
Zhuang L. H. et al. Effects of gestational exposures to chemical mixtures on birth weight using Bayesian factor analysis in the Health Outcome and Measures of Environment (HOME) Study. Environ Epidemiol. 2021;5:e159.
Preston, E. V. et al. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: a mixtures approach. Environ. Int. 139, 105728 (2020).
Luo, D. et al. Associations of prenatal exposure to per- and polyfluoroalkyl substances with the neonatal birth size and hormones in the growth hormone/insulin-like growth factor axis. Environ. Sci. Technol. 55, 11859–11873 (2021).
Woods, M. M., Lanphear, B. P., Braun, J. M. & McCandless, L. C. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ. Health 16, 115 (2017).
Liang, H. et al. Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environ. Health 19, 127 (2020).
Slama, R. et al. Scientific issues relevant to setting regulatory criteria to identify endocrine-disrupting substances in the European Union. Environ. Health Perspect. 124, 1497–1503 (2016).
Bourguignon, J. P. et al. Science-based regulation of endocrine disrupting chemicals in Europe: which approach? Lancet Diabetes Endocrinol. 4, 643–646 (2016).
European Chemical Agency (ECHA) and European Food Safety Authority (EFSA) with the technical support of the Joint Research Centre (JRC) et al. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 16, e05311 (2018).
European Commission. Chemicals Strategy for Sustainability Towards a Toxic-Free Environment https://environment.ec.europa.eu/strategy/chemicals-strategy_en (2020).
O’Reilly, J. T. What REACH can teach us about TSCA: retrospectives of America’s failed Toxics Statute. Eur. J. Risk Regul. 1, 40–50 (2010).
Environmental Protection Agency. Cumulative Assessment of Risk from Pesticides https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/cumulative-assessment-risk-pesticides (2015).
Maffini, M. V. & Neltner, T. G. Brain drain: the cost of neglected responsibilities in evaluating cumulative effects of environmental chemicals.J. Epidemiol. Community Health 69, 496–499 (2015).
US Food and Drug Administration. CFR — Code of Federal Regulations Title 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=170.18 (2022).
Federal Register. Indirect Food Additives: Paper and Paperboard Components https://www.federalregister.gov/documents/2016/01/04/2015-33026/indirect-food-additives-paper-and-paperboard-components (2016).
Keefe D. M. RE: Pre-Notification Consultation (PNC) 2422. US Food and Drug Administration https://blogs.edf.org/health/files/2021/04/Daikin-PNC-2422-PFAS_Daikin-Final-10-1-2019-and-response-combined.pdf (2019).
Brennan, N. M., Evans, A. T., Fritz, M. K., Peak, S. A. & von Holst, H. E. Trends in the regulation of Per- and polyfluoroalkyl substances (PFAS): a scoping review. Int. J. Environ. Res. Public Health 18, 10900 (2021).
ECHA. ECHA Publishes PFAS Restriction Proposal. ECHA All News. https://echa.europa.eu/-/echa-publishes-pfas-restriction-proposal (2023).
US Consumer Product Safety Commission. The Consumer Product Safety Improvement Act of 2008 https://www.cpsc.gov/s3fs-public/pdfs/blk_pdf_cpsia.pdf (2008).
National Academies of Sciences, Engineering, and Medicine. A Class Approach to Hazard Assessment of Organohalogen Flame Retardants. National Academies Press http://www.ncbi.nlm.nih.gov/books/NBK545458/ (2019).
Babich M. A. Commission Briefing Package: Project Plan: Organohalogen Flame Retardant Chemicals Assessment. US Consumer Product Safety Commission https://www.cpsc.gov/content/Commission-Briefing-Package-Project-Plan-Organohalogen-Flame-Retardant-Chemicals-Assessment (2020).
Bevington, C. et al. Development of a flame retardant and an organohalogen flame retardant chemical inventory. Sci. Data 9, 295 (2022).
Payne, J., Rajapakse, N., Wilkins, M. & Kortenkamp, A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Env. Health Perspect. 108, 983–987 (2000).
Miller, M. F., Chernyak, S. M., Batterman, S. & Loch-Caruso, R. Polybrominated diphenyl ethers in human gestational membranes from women in Southeast Michigan (USA). Environ. Sci. Technol. 43, 3042–3046 (2009).
Jacobs, D. E. Lead screening update from the US Preventive Services Task Force. J. Pediatr. 212, 243 (2019).
National Academies of Sciences, Engineering, and Medicine. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up https://nap.nationalacademies.org/catalog/26156/guidance-on-pfas-exposure-testing-and-clinical-follow-up (2022).
Gosetti, F. et al. Study of endocrine disrupting compound release from different medical devices through an on-line SPE UHPLC-MS/MS method. Analytica Chim. Acta 1042, 141–154 (2018).
Genco, M., Anderson-Shaw, L. & Sargis, R. M. Unwitting accomplices: endocrine disruptors confounding clinical care. J. Clin. Endocrinol. Metab. 105, e3822–e3827 (2020). Essential reading for the clinician who is concerned about exposures to EDCs in the medical setting, and how these exposures might be affecting the health of patients.
Demeneix, B. A. How fossil fuel-derived pesticides and plastics harm health, biodiversity, and the climate. Lancet Diabetes Endocrinol. 8, 462–464 (2020).
DeCourten, B. M. & Brander, S. M. Combined effects of increased temperature and endocrine disrupting pollutants on sex determination, survival, and development across generations. Sci. Rep. 7, 9310 (2017).
Brown, A. R. et al. Climate change and pollution speed declines in zebrafish populations. Proc. Natl Acad. Sci. 112, E1237–E1246 (2015).
Kulkarni, M. A., Duguay, C. & Ost, K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: a scoping review of reviews. Globalization Health 18, 1 (2022).
IPCC. Masson-Delmotte, V. et al. Special Report: Global Warming of 1.5°C. Summary for Policymakers 3-24 (Cambridge University Press, 2018). https://www.ipcc.ch/sr15/chapter/spm/.
d’Ambrières, W. Plastics recycling worldwide: current overview and desirable changes. Field Actions Sci. Rep. 19, 12–21 (2019).
Turner, A. & Filella, M. Hazardous metal additives in plastics and their environmental impacts. Environ. Int. 156, 106622 (2021).
Kassotis, C. D. et al. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 8, 719–730 (2020). An important contribution showing how EDC policies and regulations are handled in different jurisdictions.
Kahn, L. G., Philippat, C., Nakayama, S. F., Slama, R. & Trasande, L. Endocrine-disrupting chemicals: implications for human health.Lancet Diabetes Endocrinol. 8, 703–718 (2020).
Kuruto-Niwa, R., Nozawa, R., Miyakoshi, T., Shiozawa, T. & Terao, Y. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ. Toxicol. Pharmacol. 19, 121–130 (2005).
Chen, M. Y., Ike, M. & Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 17, 80–86 (2002).
Yoshihara, S. et al. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: their structures and estrogenic potency. Toxicol. Sci. 78, 50–59 (2004).
Okuda, K., Fukuuchi, T., Takiguchi, M. & Yoshihara, S. Novel pathway of metabolic activation of bisphenol A-related compounds for estrogenic activity. Drug Metab. Dispos. 39, 1696–1703 (2011).
Audebert, M., Dolo, L., Perdu, E., Cravedi, J. P. & Zalko, D. Use of the γH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch. Toxicol. 85, 1463–1473 (2011).
Viñas, R. & Watson, C. S. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ. Health Perspect. 121, 352–358 (2013).
Kunikane, H. et al. Double-blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy-induced anemia in patients with non-small cell lung cancer. Int. J. Clin. Oncol. 6, 296–301 (2001).
Brendel, S., Fetter, É., Staude, C., Vierke, L. & Biegel-Engler, A. Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 30, 9 (2018).
Acknowledgements
The authors would like to share their appreciation for their support networks during the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
C.D.-L. and M.V.M. researched data for the article and wrote the article. L.T. wrote the article and reviewed and/or edited the manuscript before submission. C.D.K. contributed substantially to the discussion of content and wrote the article. L.N.V. researched data for the article, contributed substantially to the discussion of content, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Mariana Fátima Fernández and Jones Bernardes Graceli for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Food Packaging Forum: https://www.foodpackagingforum.org/fcch-project
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duh-Leong, C., Maffini, M.V., Kassotis, C.D. et al. The regulation of endocrine-disrupting chemicals to minimize their impact on health. Nat Rev Endocrinol 19, 600–614 (2023). https://doi.org/10.1038/s41574-023-00872-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00872-x
This article is cited by
-
Development of an environmentally friendly method for the determination of prednisolone in natural waters using unmodified boron-doped diamond electrode
Journal of Solid State Electrochemistry (2024)
-
8-OHdG mediates the association of co-exposure to fifty-five typical endocrine-disrupting chemicals with renal function: a cross-section investigation in Southern Chinese adults
Environmental Science and Pollution Research (2024)