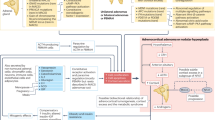
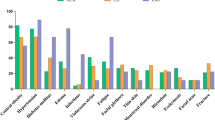
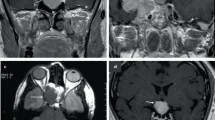
Abstract
Cushing disease caused by an adrenocorticotropic hormone (ACTH)-secreting pituitary corticotroph adenoma leads to hypercortisolaemia with high mortality due to metabolic, cardiovascular, immunological, neurocognitive, haematological and infectious conditions. The disorder is challenging to diagnose because of its common and heterogenous presenting features and the biochemical pitfalls of testing levels of hormones in the hypothalamic–pituitary–adrenal axis. Several late-night salivary cortisol and 24-h urinary free cortisol tests are usually required as well as serum levels of cortisol after a dexamethasone suppression test. MRI might only identify an adenoma in 60–75% of patients and many adenomas are small. Therefore, inferior petrosal sinus sampling remains the gold standard for confirmation of ACTH secretion from a pituitary source. Initial treatment is usually transsphenoidal adenoma resection, but preoperative medical therapy is increasingly being used in some countries and regions. Other management approaches are required if Cushing disease persists or recurs following surgery, including medications to modulate ACTH or block cortisol secretion or actions, pituitary radiation, and/or bilateral adrenalectomy. All patients require lifelong surveillance for persistent comorbidities, clinical and biochemical recurrence, and treatment-related adverse effects (including development of treatment-associated hypopituitarism). In this Review, we discuss challenges in the management of Cushing disease in adults and provide information to guide clinicians when planning an integrated and individualized approach for each patient.
Key points
-
Cushing disease, an adrenocorticotropic hormone-secreting pituitary adenoma, is the most frequent cause of endogenous Cushing syndrome; sustained hypercortisolism produced by increased levels of adrenocorticotropic hormone leads to substantial morbidity and mortality.
-
Surgery is the first-line treatment for most patients, with preoperative medical therapy increasingly being used in some countries; biochemical postoperative remission largely depends on adenoma size and surgeon experience.
-
Up to 35% of cases recur after surgery, and recurrence might be delayed by decades; patient management should be individualized and lifelong follow-up is required.
-
Practice standards for the use of pituitary-directed agents, adrenal steroidogenesis inhibitors and glucocorticoid receptor antagonists as well as for repeat surgery and for radiotherapy differ and are often specific to the centre and region.
-
Bilateral adrenalectomy is often recommended for patients desiring pregnancy and those with severe persistent hypercortisolaemia; resultant permanent adrenal insufficiency and potential for corticotroph adenoma progression are precautionary clinical considerations.
-
Clinical and biochemical responses to therapy and quality-of-life changes should be assessed within weeks of treatment initiation; all patients require lifelong monitoring and management of comorbidities and treatment-related adverse effects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fleseriu, M. et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 9, 847–875 (2021).
Jones, P. S. & Swearingen, B. Pituitary surgery in Cushing’s disease: first line treatment and role of reoperation. Pituitary 25, 713–717 (2022).
Melmed, S. Pituitary-tumor endocrinopathies. N. Engl. J. Med. 382, 937–950 (2020).
Ragnarsson, O. et al. The incidence of Cushing’s disease: a nationwide Swedish study. Pituitary 22, 179–186 (2019).
Broder, M. S., Neary, M. P., Chang, E., Cherepanov, D. & Ludlam, W. H. Incidence of Cushing’s syndrome and Cushing’s disease in commercially-insured patients <65 years old in the United States. Pituitary 18, 283–289 (2015).
Melmed, S. et al. Clinical biology of the pituitary adenoma. Endocr. Rev. 43, 1003–1037 (2022).
Limumpornpetch, P. et al. The effect of endogenous Cushing syndrome on all-cause and cause-specific mortality. J. Clin. Endocrinol. Metab. 107, 2377–2388 (2022).
Nieman, L. K. Molecular derangements and the diagnosis of ACTH-dependent Cushing’s syndrome. Endocr. Rev. 43, 852–877 (2022).
Asa, S. L., Mete, O., Perry, A. & Osamura, R. Y. Overview of the 2022 WHO classification of pituitary tumors. Endocr. Pathol. 33, 6–26 (2022).
Reincke, M. et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 47, 31–38 (2015).
Fukuoka, H. et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Invest. 121, 4712–4721 (2011).
Neou, M. et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell 37, 123–134 e125 (2020).
Stroud, A. et al. Outcomes of pituitary surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary 23, 595–609 (2020).
Honegger, J. & Grimm, F. The experience with transsphenoidal surgery and its importance to outcomes. Pituitary 21, 545–555 (2018).
Zamanipoor Najafabadi, A. H. et al. Starting point for benchmarking outcomes and reporting of pituitary adenoma surgery within the European Reference Network on Rare Endocrine Conditions (Endo-ERN): results from a meta-analysis and survey study. Endocr. Connect. 12, e220349 (2023).
Casanueva, F. F. et al. Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a pituitary society statement. Pituitary 20, 489–498 (2017).
Couselo, M., Frara, S., Giustina, A. & Casanueva, F. F. Pituitary Tumor Centers of Excellence for Cushing’s disease. Pituitary 25, 772–775 (2022).
Yang, A. B., Henderson, F. Jr. & Schwartz, T. H. Surgical strategies in the treatment of MR-negative Cushing’s disease: a systematic review and treatment algorithm. Pituitary 25, 551–562 (2022).
Sharifi, G. et al. MRI-negative Cushing’s disease: management strategy and outcomes in 15 cases utilizing a pure endoscopic endonasal approach. BMC Endocr. Disord. 22, 154 (2022).
Wind, J. J. et al. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 98, 2285–2293 (2013).
Oldfield, E. H. Surgical management of Cushing’s disease: a personal perspective. Clin. Neurosurg. 58, 13–26 (2011).
Akirov, A. et al. Clinical study and systematic review of pituitary microadenomas vs. macroadenomas in Cushing’s disease: does size matter? J. Clin. Med. 11, 1558 (2022).
Balomenaki, M., Vassiliadi, D. A. & Tsagarakis, S. Cushing’s disease: risk of recurrence following trans-sphenoidal surgery, timing and methods for evaluation. Pituitary 25, 718–721 (2022).
Paluzzi, A. et al. Endoscopic endonasal infrasellar approach to the sellar and suprasellar regions: technical note. Skull Base 21, 335–342 (2011).
Broersen, L. H. A. et al. Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary 21, 524–534 (2018).
Zhang, T., Zhang, B., Yuan, L., Song, Y. & Wang, F. Superiority of endoscopic transsphenoidal pituitary surgery to microscopic transseptal pituitary surgery for treatment of Cushing’s disease. Rev. Assoc. Med. Bras. 67, 1687–1691 (2021).
Sabahi, M. et al. MRI-negative Cushing’s disease: a review on therapeutic management. World Neurosurg. 162, 126–137 (2022).
Monteith, S. J., Starke, R. M., Jane, J. A. Jr. & Oldfield, E. H. Use of the histological pseudocapsule in surgery for Cushing disease: rapid postoperative cortisol decline predicting complete tumor resection. J. Neurosurg. 116, 721–727 (2012).
Lonser, R. R., Nieman, L. & Oldfield, E. H. Cushing’s disease: pathobiology, diagnosis, and management. J. Neurosurg. 126, 404–417 (2017).
Lim, J. S., Lee, S. K., Kim, S. H., Lee, E. J. & Kim, S. H. Intraoperative multiple-staged resection and tumor tissue identification using frozen sections provide the best result for the accurate localization and complete resection of tumors in Cushing’s disease. Endocrine 40, 452–461 (2011).
Patel, V. et al. Ultra-high field magnetic resonance imaging for localization of corticotropin-secreting pituitary adenomas. Neuroradiology 62, 1051–1054 (2020).
Grober, Y., Grober, H., Wintermark, M., Jane, J. A. & Oldfield, E. H. Comparison of MRI techniques for detecting microadenomas in Cushing’s disease. J. Neurosurg. 128, 1051–1057 (2018).
Guo, Q., Young, W. F., Erickson, D. & Erickson, B. Usefulness of dynamic MRI enhancement measures for the diagnosis of ACTH-producing pituitary adenomas. Clin. Endocrinol. 82, 267–273 (2015).
Chatain, G. P. et al. Potential utility of FLAIR in MRI-negative Cushing’s disease. J. Neurosurg. 129, 620–628 (2018).
Bashari, W. A. et al. Modern imaging in Cushing’s disease. Pituitary 25, 709–712 (2022).
Koh, C. H. et al. The clinical outcomes of imaging modalities for surgical management Cushing’s disease — a systematic review and meta-analysis. Front. Endocrinol. 13, 1090144 (2022).
Boyle, J. et al. CRH stimulation improves 18F-FDG-PET detection of pituitary adenomas in Cushing’s disease. Endocrine 65, 155–165 (2019).
Senanayake, R. et al. New types of localization methods for adrenocorticotropic hormone-dependent Cushing’s syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101513 (2021).
Koulouri, O. et al. A role for 11C-methionine PET imaging in ACTH-dependent Cushing’s syndrome. Eur. J. Endocrinol. 173, M107–M120 (2015).
Berkmann, S. et al. Selective resection of cushing microadenoma guided by preoperative hybrid 18-fluoroethyl-L-tyrosine and 11-C-methionine PET/MRI. Pituitary 24, 878–886 (2021).
Walia, R. et al. Molecular imaging targeting corticotropin-releasing hormone receptor for corticotropinoma: a changing paradigm. J. Clin. Endocrinol. Metab. 106, e1816–e1826 (2021).
Valassi, E. et al. Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 95, 601–610 (2010).
Fan, Y. et al. Development of machine learning models for predicting postoperative delayed remission in patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 106, e217–e231 (2021).
Esposito, F. et al. Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J. Clin. Endocrinol. Metab. 91, 7–13 (2006).
Hameed, N. et al. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16, 452–458 (2013).
Wang, F. et al. Postoperative day 1 morning cortisol value as a biomarker to predict long-term remission of cushing disease. J. Clin. Endocrinol. Metab. 106, e94–e102 (2021).
Lindsay, J. R., Oldfield, E. H., Stratakis, C. A. & Nieman, L. K. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J. Clin. Endocrinol. Metab. 96, 2057–2064 (2011).
Bou Khalil, R. et al. Sequential hormonal changes in 21 patients with recurrent Cushing’s disease after successful pituitary surgery. Eur. J. Endocrinol. 165, 729–737 (2011).
Amlashi, F. G. et al. Accuracy of late-night salivary cortisol in evaluating postoperative remission and recurrence in Cushing’s disease. J. Clin. Endocrinol. Metab. 100, 3770–3777 (2015).
Carroll, T. B., Javorsky, B. R. & Findling, J. W. Postsurgical recurrent Cushing disease: clinical benefit of early intervention in patients with normal urinary free cortisol. Endocr. Pract. 22, 1216–1223 (2016).
Braun, L. T. et al. Recurrence after pituitary surgery in adult Cushing’s disease: a systematic review on diagnosis and treatment. Endocrine 70, 218–231 (2020).
Sandouk, Z. et al. Variability of late-night salivary cortisol in Cushing disease: a prospective study. J. Clin. Endocrinol. Metab. 103, 983–990 (2018).
Petersenn, S. et al. High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin. Endocrinol. 80, 261–269 (2014).
Cambos, S. et al. Persistent cortisol response to desmopressin predicts recurrence of Cushing’s disease in patients with post-operative corticotropic insufficiency. Eur. J. Endocrinol. 182, 489–498 (2020).
Abellan-Galiana, P. et al. Prognostic usefulness of ACTH in the postoperative period of Cushing’s disease. Endocr. Connect. 8, 1262–1272 (2019).
Albani, A. et al. Improved pasireotide response in USP8 mutant corticotroph tumours in vitro. Endocr. Relat. Cancer 29, 503–511 (2022).
Zoli, M. et al. Machine learning-based prediction of outcomes of the endoscopic endonasal approach in Cushing disease: is the future coming? Neurosurg. Focus 48, E5 (2020).
Nadezhdina, E. Y. et al. Prediction of recurrence and remission within 3 years in patients with Cushing disease after successful transnasal adenomectomy. Pituitary 22, 574–580 (2019).
Shahrestani, S. et al. Neural network modeling for prediction of recurrence, progression, and hormonal non-remission in patients following resection of functional pituitary adenomas. Pituitary 24, 523–529 (2021).
Nieman, L. K. et al. Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 2807–2831 (2015).
Melmed, S. Medical progress: acromegaly. N. Engl. J. Med. 355, 2558–2573 (2006).
Fleseriu, M. et al. Long-term outcomes of osilodrostat in Cushing’s disease: LINC 3 study extension. Eur. J. Endocrinol. 187, 531–541 (2022).
Fleseriu, M. et al. Long-term efficacy and safety of once-monthly pasireotide in Cushing’s disease: a Phase III extension study. Clin. Endocrinol. 91, 776–785 (2019).
Fleseriu, M. et al. Levoketoconazole treatment in endogenous Cushing’s syndrome: extended evaluation of clinical, biochemical, and radiologic outcomes. Eur. J. Endocrinol. 187, 859–871 (2022).
Ferriere, A. et al. Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur. J. Endocrinol. 176, 305–314 (2017).
Simoes Correa Galendi, J., Correa Neto, A. N. S., Demetres, M., Boguszewski, C. L. & Nogueira, V. Effectiveness of medical treatment of Cushing’s disease: a systematic review and meta-analysis. Front. Endocrinol. 12, 732240 (2021).
Pivonello, R., Pivonello, C., Simeoli, C., De Martino, M. C. & Colao, A. The dopaminergic control of Cushing’s syndrome. J. Endocrinol. Invest. 45, 1297–1315 (2022).
Varlamov, E. V., Han, A. J. & Fleseriu, M. Updates in adrenal steroidogenesis inhibitors for Cushing’s syndrome — a practical guide. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101490 (2021).
Biller, B. M. et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93, 2454–2462 (2008).
Raverot, G. et al. European Society of Endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 178, G1–G24 (2018).
Lasolle, H., Vasiljevic, A., Jouanneau, E., Ilie, M. D. & Raverot, G. Aggressive corticotroph tumors and carcinomas. J. Neuroendocrinol. 34, e13169 (2022).
Colao, A. et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366, 914–924 (2012).
Lacroix, A. et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 6, 17–26 (2018).
Manetti, L. et al. Long-term safety and efficacy of subcutaneous pasireotide in patients with Cushing’s disease: Interim results from a long-term real-world evidence study. Pituitary 22, 542–551 (2019).
Lacroix, A. et al. Long-acting pasireotide improves clinical signs and quality of life in Cushing’s disease: Results from a phase III study. J. Endocrinol. Invest. 43, 1613–1622 (2020).
Pivonello, R. et al. Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a phase III study. Clin. Endocrinol. 81, 408–417 (2014).
Newell-Price, J. et al. Use of late-night salivary cortisol to monitor response to medical treatment in Cushing’s disease. Eur. J. Endocrinol. 182, 207–217 (2020).
Mondin, A. et al. Pasireotide-induced shrinkage in GH and ACTH secreting pituitary adenoma: a systematic review and meta-analysis. Front. Endocrinol. 13, 935759 (2022).
Samson, S. L. et al. Managing pasireotide-associated hyperglycemia: a randomized, open-label, phase IV study. Pituitary 24, 887–903 (2021).
Pivonello, R., Simeoli, C., Di Paola, N. & Colao, A. Cushing’s disease: adrenal steroidogenesis inhibitors. Pituitary 25, 726–732 (2022).
Castinetti, F. et al. Ketoconazole in Cushing’s disease: is it worth a try? J. Clin. Endocrinol. Metab. 99, 1623–1630 (2014).
Viecceli, C. et al. Evaluation of ketoconazole as a treatment for Cushing’s disease in a retrospective cohort. Front. Endocrinol. 13, 1017331 (2022).
Ollivier, M., Haissaguerre, M., Ferriere, A. & Tabarin, A. Should we avoid using ketoconazole in patients with severe Cushing’s syndrome and increased levels of liver enzymes? Eur. J. Endocrinol. 179, L1–L2 (2018).
Young, J. et al. Hepatic safety of ketoconazole in Cushing’s syndrome: results of a compassionate use programme in france. Eur. J. Endocrinol. 178, 447–458 (2018).
Creemers, S. G. et al. Levoketoconazole, the 2S,4R enantiomer of ketoconazole, a new steroidogenesis inhibitor for Cushing’s syndrome treatment. J. Clin. Endocrinol. Metab. 106, e1618–e1630 (2021).
Pivonello, R. et al. Levoketoconazole in the treatment of patients with endogenous Cushing’s syndrome: a double-blind, placebo-controlled, randomized withdrawal study (LOGICS). Pituitary 25, 911–926 (2022).
Fleseriu, M. et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol. 7, 855–865 (2019).
Pivonello, R. et al. Levoketoconazole in the treatment of patients with Cushing’s syndrome and diabetes mellitus: results from the SONICS phase 3 study. Front. Endocrinol. 12, 595894 (2021).
Pivonello, R. et al. Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 8, 748–761 (2020).
Gadelha, M. et al. Randomized trial of osilodrostat for the treatment of cushing disease. J. Clin. Endocrinol. Metab. 107, e2882–e2895 (2022).
Fleseriu, M. et al. Long-term efficacy and safety of osilodrostat in Cushing’s disease: final results from a Phase II study with an optional extension phase (LINC 2). Pituitary 25, 959–970 (2022).
He, X., Findling, J. W. & Auchus, R. J. Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary 25, 393–403 (2022).
Fontaine-Sylvestre, C., Letourneau-Guillon, L., Moumdjian, R. A., Berthelet, F. & Lacroix, A. Corticotroph tumor progression during long-term therapy with osilodrostat in a patient with persistent Cushing’s disease. Pituitary 24, 207–215 (2021).
Nowotny, H. F. et al. 11-Oxygenated C19 steroids are the predominant androgens responsible for hyperandrogenemia in Cushing’s disease. Eur. J. Endocrinol. 187, 663–673 (2022).
Bonnet-Serrano, F. et al. Differences in the spectrum of steroidogenic enzyme inhibition between osilodrostat and metyrapone in ACTH-dependent Cushing syndrome patients. Eur. J. Endocrinol. 187, 315–322 (2022).
Daniel, E. et al. Effectiveness of metyrapone in treating Cushing’s syndrome: a retrospective multicenter study in 195 patients. J. Clin. Endocrinol. Metab. 100, 4146–4154 (2015).
Nieman, L. K. et al. Metyrapone treatment in endogenous Cushing’s syndrome: results at week 12 from PROMPT, a prospective international multicenter, open-label, phase III/IV study. J. Endocr. Soc. 5, A515 (2021).
Constantinescu, S. M. et al. Etomidate infusion at low doses is an effective and safe treatment for severe Cushing’s syndrome outside intensive care. Eur. J. Endocrinol. 183, 161–167 (2020).
Baudry, C. et al. Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur. J. Endocrinol. 167, 473–481 (2012).
Fleseriu, M. et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 97, 2039–2049 (2012).
Fleseriu, M. et al. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing’s disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J. Clin. Endocrinol. Metab. 99, 3718–3727 (2014).
Kamenicky, P. et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 96, 2796–2804 (2011).
Valassi, E., Crespo, I., Gich, I., Rodriguez, J. & Webb, S. M. A reappraisal of the medical therapy with steroidogenesis inhibitors in Cushing’s syndrome. Clin. Endocrinol. 77, 735–742 (2012).
Feelders, R. A. et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N. Engl. J. Med. 362, 1846–1848 (2010).
Vilar, L. et al. Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary 13, 123–129 (2010).
Barbot, M. et al. Combination therapy for Cushing’s disease: effectiveness of two schedules of treatment: should we start with cabergoline or ketoconazole? Pituitary 17, 109–117 (2014).
Feelders, R. et al. Prospective phase ii study (CAPACITY) of pasireotide monotherapy or in combination with cabergoline in patients with Cushing’s disease (Poster). Endocr. Rev. 38 (Suppl. 1), i1–i1431 (2017).
Broersen, L. H. A., Jha, M., Biermasz, N. R., Pereira, A. M. & Dekkers, O. M. Effectiveness of medical treatment for Cushing’s syndrome: a systematic review and meta-analysis. Pituitary 21, 631–641 (2018).
Gadelha, M. R., Wildemberg, L. E. & Shimon, I. Pituitary acting drugs: cabergoline and pasireotide. Pituitary 25, 722–725 (2022).
Gatto, F., Arvigo, M. & Ferone, D. Somatostatin receptor expression and patients’ response to targeted medical treatment in pituitary tumors: evidences and controversies. J. Endocrinol. Invest. 43, 1543–1553 (2020).
Greenblatt, H. K. & Greenblatt, D. J. Liver injury associated with ketoconazole: review of the published evidence. J. Clin. Pharmacol. 54, 1321–1329 (2014).
Findling, J. W. et al. Late-night salivary cortisol may be valuable for assessing treatment response in patients with Cushing’s disease: 12-month, phase III pasireotide study. Endocrine 54, 516–523 (2016).
Dormoy, A. et al. Efficacy and safety of osilodrostat in paraneoplastic Cushing’s syndrome: a real-world multicenter study in France. J. Clin. Endocrinol. Metab. 108, 1475–1487 (2023).
Bessiene, L. et al. Rapid control of severe ectopic Cushing’s syndrome by oral osilodrostat monotherapy. Eur. J. Endocrinol. 184, L13–L15 (2021).
Valassi, E. et al. Preoperative medical treatment in Cushing’s syndrome: frequency of use and its impact on postoperative assessment: data from ERCUSYN. Eur. J. Endocrinol. 178, 399–409 (2018).
Liu, N. A. et al. Treatment of Cushing’s disease with pituitary-targeting seliciclib. J. Clin. Endocrinol. Metab. 108, 726–735 (2023).
Krasner, A. et al. Inhibition of Basal and ACTH-Stimulated Cortisol Secretion in Humans Using an Oral, Nonpeptide ACTH Antagonist (CRN04894) ENDO 2022. Poster presentation (2022).
Feldhaus, A. L. et al. ALD1613, a novel long-acting monoclonal antibody to control ACTH-driven pharmacology. Endocrinology 158, 1–8 (2017).
Ben-Shlomo, A. & Cooper, O. Role of tyrosine kinase inhibitors in the treatment of pituitary tumours: From bench to bedside. Curr. Opin. Endocrinol. Diabetes Obes. 24, 301–305 (2017).
Sugiyama, A. et al. Inhibition of heat shock protein 90 decreases ACTH production and cell proliferation in AtT-20 cells. Pituitary 18, 542–553 (2015).
Pivonello, R. et al. Relacorilant, a selective glucocorticoid receptor modulator, induces clinical improvements in patients with Cushing syndrome: results from a prospective, open-label phase 2 study. Front. Endocrinol. 12, 662865 (2021).
Oda, S. et al. An open-label phase I/IIA clinical trial of 11BETA-HSD1 inhibitor for Cushing’s syndrome and autonomous cortisol secretion. J. Clin. Endocrinol. Metab. 106, e3865–e3880 (2021).
Katznelson, L. Role of radiation in the treatment of Cushing disease. Pituitary 25, 740–742 (2022).
Gheorghiu, M. L. Updates in the outcomes of radiation therapy for Cushing’s disease. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101514 (2021).
Sherry, A. D. et al. Outcomes of stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for refractory Cushing’s disease. Pituitary 22, 607–613 (2019).
Mehta, G. U. et al. Stereotactic radiosurgery for Cushing disease: results of an international, multicenter study. J. Clin. Endocrinol. Metab. 102, 4284–4291 (2017).
Mathieu, D. et al. Stereotactic radiosurgery for secretory pituitary adenomas: systematic review and international stereotactic radiosurgery society practice recommendations. J. Neurosurg. 136, 801–812 (2022).
Bunevicius, A. et al. Early versus late Gamma Knife radiosurgery for Cushing’s disease after prior resection: results of an international, multicenter study. J. Neurosurg. 134, 807–815 (2020).
Lee, C. C. et al. Whole-sellar stereotactic radiosurgery for functioning pituitary adenomas. Neurosurgery 75, 227–237 (2014).
Shepard, M. J. et al. Technique of whole-sellar stereotactic radiosurgery for cushing disease: results from a multicenter. Int. Cohort Study World Neurosurg. 116, e670–e679 (2018).
Ironside, N. et al. Effects of neuroanatomic structural distances on pituitary function after stereotactic radiosurgery: a multicenter study. Neurosurgery 92, 1035–1042 (2023).
Burman, P., van Beek, A. P., Biller, B. M., Camacho-Hubner, C. & Mattsson, A. F. Radiotherapy, especially at young age, increases the risk for de novo brain tumors in patients treated for pituitary/sellar lesions. J. Clin. Endocrinol. Metab. 102, 1051–1058 (2017).
van Varsseveld, N. C. et al. Cerebrovascular events, secondary intracranial tumors, and mortality after radiotherapy for nonfunctioning pituitary adenomas: a subanalysis from the Dutch National Registry of Growth Hormone Treatment in Adults. J. Clin. Endocrinol. Metab. 100, 1104–1112 (2015).
Hamblin, R. et al. Risk of second brain tumour after radiotherapy for pituitary adenoma or craniopharyngioma: a retrospective, multicentre, cohort study of 3679 patients with long-term imaging surveillance. Lancet Diabetes Endocrinol. 10, 581–588 (2022).
Wolf, A. et al. Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol. 20, 159–164 (2019).
Reincke, M. et al. A critical reappraisal of bilateral adrenalectomy for ACTH-dependent Cushing’s syndrome. Eur. J. Endocrinol. 173, M23–M32 (2015).
Ritzel, K. et al. Clinical review: outcome of bilateral adrenalectomy in Cushing’s syndrome: a systematic review. J. Clin. Endocrinol. Metab. 98, 3939–3948 (2013).
Bertherat, J. Cushing’s disease: role of bilateral adrenalectomy. Pituitary 25, 743–745 (2022).
Reibetanz, J. et al. Differences in morbidity and mortality between unilateral adrenalectomy for adrenal Cushing’s syndrome and bilateral adrenalectomy for therapy refractory extra-adrenal Cushing’s syndrome. Langenbecks Arch. Surg. 407, 2481–2488 (2022).
Papakokkinou, E. et al. Prevalence of Nelson’s syndrome after bilateral adrenalectomy in patients with cushing’s disease: a systematic review and meta-analysis. Pituitary 24, 797–809 (2021).
Das, L. et al. ACTH increment post total bilateral adrenalectomy for Cushing’s disease: a consistent biosignature for predicting Nelson’s syndrome. Pituitary 23, 488–497 (2020).
Reincke, M. et al. Corticotroph tumor progression after bilateral adrenalectomy (Nelson’s syndrome): systematic review and expert consensus recommendations. Eur. J. Endocrinol. 184, P1–P16 (2021).
Suarez, M. G. et al. Hypercoagulability in Cushing syndrome, prevalence of thrombotic events: a large, single-center, retrospective study. J. Endocr. Soc. 4, bvz033 (2020).
Varlamov, E. V., Langlois, F., Vila, G. & Fleseriu, M. Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing’s syndrome: a practical approach. Eur. J. Endocrinol. 184, R207–R224 (2021).
Feelders, R. A. & Nieman, L. K. Hypercoagulability in Cushing’s syndrome: incidence, pathogenesis and need for thromboprophylaxis protocols. Pituitary 25, 746–749 (2022).
Bunevicius, A., Lavezzo, K., Smith, P. W., Vance, M. L. & Sheehan, J. Stereotactic radiosurgery before bilateral adrenalectomy is associated with lowered risk of Nelson’s syndrome in refractory Cushing’s disease patients. Acta Neurochir. 163, 1949–1956 (2021).
Losa, M. et al. Gamma knife radiosurgery in patients with Nelson’s syndrome. J. Endocrinol. Invest. 44, 2243–2251 (2021).
Amodru, V. et al. Cushing’s syndrome in the elderly: data from the european registry on Cushing’s syndrome. Eur. J. Endocrinol. 188, 395–406 (2023).
Qiao, N., Swearingen, B. & Tritos, N. A. Cushing’s disease in older patients: presentation and outcome. Clin. Endocrinol. 89, 444–453 (2018).
van Haalen, F. M. et al. Current clinical practice for thromboprophylaxis management in patients with Cushing’s syndrome across reference centers of the European Reference Network on rare endocrine conditions (Endo-ERN). Orphanet J. Rare Dis. 17, 178 (2022).
Boscaro, M. et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s syndrome. J. Clin. Endocrinol. Metab. 87, 3662–3666 (2002).
Dekkers, A. J. et al. Long-term effects of glucocorticoid excess on the brain. J. Neuroendocrinol. 34, e13142 (2022).
Braun, L. T. et al. Whom should we screen for cushing syndrome? the endocrine society practice guideline recommendations 2008 revisited. J. Clin. Endocrinol. Metab. 107, e3723–e3730 (2022).
Vogel, F. et al. Persisting muscle dysfunction in Cushing’s syndrome despite biochemical remission. J. Clin. Endocrinol. Metab. 105, e4490–e4498 (2020).
Webb, S. M. & Valassi, E. Quality of life impairment after a diagnosis of Cushing’s syndrome. Pituitary 25, 768–771 (2022).
Gumaste, N., Shah, L., Cheesman, K. C. & Geer, E. B. Evaluating patient-reported outcomes in Cushing’s syndrome. Endocrinol. Metab. Clin. North Am. 51, 691–707 (2022).
Pupier, E. et al. Impaired quality of life, but not cognition, is linked to a history of chronic hypercortisolism in patients with Cushing’s disease in remission. Front. Endocrinol. 13, 934347 (2022).
Schernthaner-Reiter, M. H. et al. Acute and life-threatening complications in cushing syndrome: prevalence, predictors, and mortality. J. Clin. Endocrinol. Metab. 106, e2035–e2046 (2021).
Ebbehoj, A. et al. The socioeconomic consequences of Cushing’s syndrome: a nationwide cohort study. J. Clin. Endocrinol. Metab. 107, e2921–e2929 (2022).
Santos, A. et al. Quality of life in patients with Cushing’s disease. Front. Endocrinol. 10, 862 (2019).
van Aken, M. O. et al. Quality of life in patients after long-term biochemical cure of Cushing’s disease. J. Clin. Endocrinol. Metab. 90, 3279–3286 (2005).
Hamblin, R., Coulden, A., Fountas, A. & Karavitaki, N. The diagnosis and management of Cushing’s syndrome in pregnancy. J. Neuroendocrinol. 34, e13118 (2022).
Sridharan, K. et al. Diagnosis and treatment outcomes of Cushing’s disease during pregnancy. Pituitary 24, 670–680 (2021).
Hochman, C. et al. Pre-term birth in women exposed to Cushing’s disease: the baby-cush study. Eur. J. Endocrinol. 184, 469–476 (2021).
Luger, A. et al. ESE clinical practice guideline on functioning and nonfunctioning pituitary adenomas in pregnancy. Eur. J. Endocrinol. 185, G1–G33 (2021).
Valassi, E. et al. Worse health-related quality of life at long-term follow-up in patients with Cushing’s disease than patients with cortisol producing adenoma. Data from the ERCUSYN. Clin. Endocrinol. 88, 787–798 (2018).
Valassi, E. et al. High mortality within 90 days of diagnosis in patients with Cushing’s syndrome: results from the ERCUSYN registry. Eur. J. Endocrinol. 181, 461–472 (2019).
Lambert, J. K. et al. Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J. Clin. Endocrinol. Metab. 98, 1022–1030 (2013).
Boscaro, M. et al. Extended treatment of Cushing’s disease with pasireotide: results from a 2-year, phase II study. Pituitary 17, 320–326 (2014).
Schopohl, J. et al. Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing’s disease: results from an open-ended, open-label extension trial. Pituitary 18, 604–612 (2015).
Trementino, L. et al. A single-center 10-year experience with pasireotide in Cushing’s disease: patients’ characteristics and outcome. Horm. Metab. Res. 48, 290–298 (2016).
Petersenn, S. et al. Long-term treatment of Cushing’s disease with pasireotide: 5-year results from an open-label extension study of a phase III trial. Endocrine 57, 156–165 (2017).
Fleseriu, M. et al. Safety and efficacy of subcutaneous pasireotide in patients with Cushing’s disease: results from an open-label, multicenter, single-arm, multinational, expanded-access study. Front. Endocrinol. 10, 436 (2019).
Pivonello, R. et al. The medical treatment with pasireotide in Cushing’s disease: an Italian multicentre experience based on “real-world evidence”. Endocrine 64, 657–672 (2019).
Godbout, A. et al. Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur. J. Endocrinol. 163, 709–716 (2010).
Pivonello, R. et al. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J. Clin. Endocrinol. Metab. 94, 223–230 (2009).
Fleseriu, M. et al. Osilodrostat, a potent oral 11beta-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing’s disease. Pituitary 19, 138–148 (2016).
Bunevicius, A., Laws, E. R., Vance, M. L., Iuliano, S. & Sheehan, J. Surgical and radiosurgical treatment strategies for Cushing’s disease. J. Neurooncol. 145, 403–413 (2019).
Acknowledgements
The authors thank Shira Berman from Cedars-Sinai Medical Center for editorial assistance. Images of staining patterns (Fig. 3Ac–Af) are courtesy of Matthew Wood (Department of Pathology, Oregon Health & Science University).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.F. has received grants to the institution from Crinetics, Novartis, Recordati, Sparrow, and Xeris (formerly Strongbridge), consulting fees from Crinetics, HRA Pharma, Novartis, Recordati, Sparrow, and Xeris (formerly Strongbridge), and serves as a member of the Board of Directors (non-compensated) for the Pituitary Society. E.V.V. has received grants to the institution from Recordati. F.L. has served as an adviser to Novartis and Recordati, and has served as a member of the Continuing Medical Education Committee for AMEQ (Association des Médecins Endocrinologues du Québec). S.M. has received grants to the institution from the US Food and Drug Administration and Recordati and non-financial support from Cyclacel, and serves as Secretary (non-compensated) for the Pituitary Society. J.M.H.-A. declares no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Irina Bancos, who co-reviewed with Rashi Sandooja; Prashant Chittiboina; and Constantine Stratakis for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed for full-text articles published in English, French and Spanish from January 2000 through January 2023 using the terms “Cushing’s disease”, “ACTH-secreting adenoma”, “hypercortisolism”, “hypercortisolemia”, and “pituitary adenoma”, in combination with the terms “treatment”, “surgery” and “radiation therapy”. Inclusion preference was for articles published within the past 5 years. Some articles were not included in the review due to space limitations or low patient numbers.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fleseriu, M., Varlamov, E.V., Hinojosa-Amaya, J.M. et al. An individualized approach to the management of Cushing disease. Nat Rev Endocrinol 19, 581–599 (2023). https://doi.org/10.1038/s41574-023-00868-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00868-7