Abstract
Pre-eclampsia is a life-threatening disease of pregnancy unique to humans and a leading cause of maternal and neonatal morbidity and mortality. Women who survive pre-eclampsia have reduced life expectancy, with increased risks of stroke, cardiovascular disease and diabetes, while babies from a pre-eclamptic pregnancy have increased risks of preterm birth, perinatal death and neurodevelopmental disability and cardiovascular and metabolic disease later in life. Pre-eclampsia is a complex multisystem disease, diagnosed by sudden-onset hypertension (>20 weeks of gestation) and at least one other associated complication, including proteinuria, maternal organ dysfunction or uteroplacental dysfunction. Pre-eclampsia is found only when a placenta is or was recently present and is classified as preterm (delivery <37 weeks of gestation), term (delivery ≥37 weeks of gestation) and postpartum pre-eclampsia. The maternal syndrome of pre-eclampsia is driven by a dysfunctional placenta, which releases factors into maternal blood causing systemic inflammation and widespread maternal endothelial dysfunction. Available treatments target maternal hypertension and seizures, but the only ‘cure’ for pre-eclampsia is delivery of the dysfunctional placenta and baby, often prematurely. Despite decades of research, the aetiology of pre-eclampsia, particularly of term and postpartum pre-eclampsia, remains poorly defined. Significant advances have been made in the prediction and prevention of preterm pre-eclampsia, which is predicted in early pregnancy through combined screening and is prevented with daily low-dose aspirin, starting before 16 weeks of gestation. By contrast, the prediction of term and postpartum pre-eclampsia is limited and there are no preventive treatments. Future research must investigate the pathogenesis of pre-eclampsia, in particular of term and postpartum pre-eclampsia, and evaluate new prognostic tests and treatments in adequately powered clinical trials.
Similar content being viewed by others
Introduction
Pre-eclampsia is a complex multisystem disease, diagnosed by sudden-onset hypertension (>20 weeks of gestation) and at least one other associated complication, including proteinuria, maternal organ dysfunction or uteroplacental dysfunction (for example, fetal growth restriction (FGR) or angiogenic imbalance). Pre-eclampsia is one of the most severe complications of pregnancy and a leading cause of maternal and perinatal morbidity and mortality1. Worldwide, an estimated 4 million women are diagnosed with pre-eclampsia (previously called toxaemia) each year, causing the deaths of >70,000 women and 500,000 babies1,2. Women who survive pre-eclampsia have reduced life expectancy, with increased risks of stroke, cardiovascular disease and diabetes1,3,4, while babies from a pre-eclamptic pregnancy have increased risks of preterm birth, perinatal death, neurodevelopmental delay, and cardiovascular and metabolic disease later in life1,3. Worldwide, >300 million women and children are estimated to be at increased risk of chronic health problems due to previous exposure to pre-eclampsia5.
Pre-eclampsia is classified based on the gestational age at clinical presentation (Box 1). The International Society for the Study of Hypertension in Pregnancy (ISSHP) classifies pre-eclampsia into preterm (delivery <37 weeks of gestation), term (delivery ≥37 weeks of gestation) and postpartum pre-eclampsia2. Classifications of early-onset (delivery at <34 weeks of gestation) pre-eclampsia and late-onset (delivery at ≥34 weeks of gestation) pre-eclampsia are also used, particularly for mechanistic studies, although these are not favoured clinically as they do not adequately reflect the maternal and fetal prognosis. The terms preterm, term, early-onset and late-onset are used throughout this Primer to precisely reflect the study populations from which the data was obtained. The time of pre-eclampsia onset is thought to reflect an underlying difference in the aetiology. This is supported by differences in the efficacy of early pregnancy pre-eclampsia prediction tests6 and preventive aspirin prophylaxis, which show utility for preterm but not term pre-eclampsia7. However, it is also clear that basing classification on the time of diagnosis carries inherent imprecision8, particularly associated with the variation in disease progression and the timing of presentation to hospital for diagnosis. A recent retrospective population-based study determined that classifying pre-eclampsia on delivery timing alone may underestimate early-onset pre-eclampsia incidence by up to 20%9. While the clinical impact of this underestimation may be negligible, correct classification based on time of onset in research studies may improve the development of new predictive tests and preventive treatments.
Whether pre-eclampsia can be classified based on symptoms is unresolved. Pre-eclampsia is associated with complications such as eclampsia (seizures), haemorrhagic stroke, haemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome, placental abruption, renal failure, and pulmonary oedema10,11 (Fig. 1). All women with pre-eclampsia are at risk of rapid deterioration and severe disease regardless of the timing of onset2,8,12; thus, ISSHP guidelines2,13 no longer support the classification of ‘severe’ or ‘mild’ pre-eclampsia in ongoing pregnancies. HELLP syndrome (Box 1) or eclampsia may be severe subtypes of pre-eclampsia with principally hepatic or neurological involvement, respectively8.
Pre-eclampsia affects the fetus and multiple maternal tissues. Pre-eclampsia is defined as new-onset hypertension after 20 weeks of gestation in a patient with previous normotension plus one other symptom of maternal organ dysfunction, which can include kidney, lung, brain, liver or placental dysfunction. Pre-eclampsia is associated with complications such as eclampsia, haemorrhagic stroke, haemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome, placental abruption, renal failure and pulmonary oedema.
In this Primer, we summarize current knowledge of the epidemiology, risk factors, pathophysiology, clinical presentation, diagnosis, prediction, management and outcomes of pre-eclampsia. We also discuss patient quality of life and the outstanding research questions aimed at improving clinical practice and understanding the aetiology of pre-eclampsia.
Epidemiology
Incidence and mortality
The global prevalence of all pre-eclampsia in the years 2002–2010 was estimated at 4.6% of deliveries but reported regional rates varied between 1% and 5.6%14. Where reported, the prevalence of preterm pre-eclampsia is <1%15,16,17,18. The prevalence of pre-eclampsia is generally reported as lower in low-income and middle-income countries (LMICs) (except sub-Saharan Africa) than in high-income countries (HICs)19,20; however, it is likely that differences in classification, access to prenatal care and under-reporting in LMICs affect prevalence data20,21. Furthermore, most pre-eclampsia research is performed in HICs, potentially leading to bias and generalizability concerns in the populations sampled and the research questions asked.
Hypertensive disorders of pregnancy (including pre-eclampsia) are the second most common cause (behind haemorrhage) of maternal deaths worldwide (14% of deaths, 95% CI 11.4–17.4), causing an estimated 62,000–77,000 deaths per year22,23. Maternal mortality is higher in a pre-eclamptic pregnancy than in a non-pre-eclamptic pregnancy (adjusted odds ratio (aOR) 3.73, 95% CI 2.15–6.47)20. The risk of fetal death in pre-eclamptic pregnancies is higher than that in non-pre-eclamptic pregnancies (aOR 3.12, 95% CI 2.77–3.51)20 as a result of FGR and placental abruption. High rates of medically indicated preterm birth also result in an increase in neonatal deaths, which are 2.7 times higher (aOR 2.7, 95% CI 2.28–3.21) than in pregnancies resulting in a term birth20.
Risk factors
There are many risk factors identified as associated with pre-eclampsia (Table 1); however, individually, none of these has strong power to predict pre-eclampsia risk and, even in combination, their predictive power is weak24. Recognized high-risk factors are broadly similar among ISSHP2, American College of Obstetricians and Gynecologists (ACOG)25 and National Institute for Health and Care Excellence (NICE)26 guidelines and include obstetric history (for example, previous pre-eclampsia, multiple pregnancy (ACOG only)) and maternal factors (for example, chronic renal disease, chronic hypertension, diabetes mellitus, systemic lupus erythematosus (SLE), antiphospholipid syndrome). Only ISSHP classifies BMI >30 kg/m2 and assisted reproductive technologies as high-risk factors. A recent comparison of clinical practice guidelines and evidence supporting these risk factors found that many guidelines had a lack of close alignment between the risk factors and the evidence supporting them24. Furthermore, these guidelines are mostly produced in HICs; thus, they do not include factors important to LMICs such as access to health-care providers/clinical instrumentation to perform tests and risk-factors particular to LMICs including adolescence, malaria or anaemia24,27. Moreover, few models are validated in low-resource settings24,27.
Genetic risk factors
Recognition that eclampsia was commonly reported in mothers, sisters and daughters suggests genetic involvment28; yet, to date, no single high-risk gene has been identified. Population-wide and large cohort studies confirm that maternal family history of pre-eclampsia increases pre-eclampsia risk by threefold to fourfold29,30,31,32,33. This association is stronger for preterm pre-eclampsia (risk ratio (RR) 2.15, 95% CI 1.69–2.73) than for term pre-eclampsia (RR 1.49, 95% CI 1.4–1.58)32. There are multiple maternal and fetal gene alleles and mutations associated with pre-eclampsia33,34,35,36,37,38,39, possibly reflecting the syndromic nature of this disease. Many of these identified genes are associated with thrombophilic factors34,35,36,37, angiogenic factors33,40 or immune responses38,39. Fetal trisomy 13 is also associated with increased risk of pre-eclampsia likely due to the extra copy of FLT1 (encoding Fms-related receptor tyrosine kinase 1; FLT1), which is located on chromosome 13 (ref. 41).
Women lacking the activating KIR (killer-cell immunoglobulin-like receptor; AA genotype) have a much greater risk of pre-eclampsia when the fetus expresses the HLA-C2 genotype, a major histocompatibility complex (MHC) class I factor expressed on the cell surface that has a dimorphism at position 80 (ref. 39). The KIR family are expressed on natural killer (NK) cells and interact with paternal HLA-C expressed on the trophoblast cell surface to control invasion and fetal vascular supply. Individuals with an AA genotype do not express activating receptors and may, therefore, have poor trophoblast invasion of the maternal uterine arteries and placental development.
A genome-wide association meta-analysis of European and Central Asian mothers and offspring33 identified three sequence variants associated with pre-eclampsia: one in the FLT1 gene of individuals born of pre-eclamptic pregnancies and two in the FTO (encoding α-ketoglutarate-dependent dioxygenase) and ZNF831 (encoding zinc finger protein 831) genes in women with pre-eclamptic pregnancies33. The soluble form of FLT1 (sFLT1) is a placental-released anti-angiogenic factor used in diagnostic tests for pre-eclampsia42. Variants in FTO and ZNF831 have previously been associated with blood pressure43,44, obesity, BMI and type 2 diabetes45. However, the causality of these associations and the clinical utility of identifying such changes are yet to be examined in larger studies.
Racial disparities
Caution is required when interpreting studies that investigate pre-eclampsia risk associated with racial background as many do not correctly account for possible mediators and confounding factors, including health-care disparities and differing cardiovascular profiles. In epidemiological studies, Black women and women of South Asian origin have a higher risk of developing pre-eclampsia than white women, even when accounting for social deprivation17,46,47,48 and increased risk of chronic hypertension and cardiovascular disease49,50,51. In a large cohort study involving >168,000 women with singleton pregnancies in the UK, Black women had twice the risk of developing pre-eclampsia at any stage of gestation than white women52. This association was strongest for early-onset (3.5-fold) and preterm (2.5-fold) pre-eclampsia52. Women of South Asian origin had a 1.5-fold higher risk of preterm pre-eclampsia than white women, but there was no association when all pre-eclampsia was measured52. In women of East Asian origin, there was no significant difference in risk of pre-eclampsia or hypertensive disorders52. This study adjusted for mediators and confounding factors in maternal characteristics and medical history. First-trimester screening following the Fetal Medicine Foundation algorithm improves perinatal outcomes in the non-white population by 60%53, suggesting that health disparity is avoidable with personalized risk assessment and correct care pathway allocation.
Maternal age
The relationship between pre-eclampsia risk and maternal age follows a J-shaped curve, with increased risk in adolescents and in women older than 35 years of age54,55,56. Advanced maternal age (≥35 years) is associated with an increased risk of pre-existing cardiometabolic dysfunction and medical disorders, multiple pregnancies, and use of artificial reproductive technologies, all of which increase the risk of pre-eclampsia. It has been reported that the risk of pre-eclampsia increases for every additional year after the age of 32 years56, even after adjustment for mediators, confounders and interactions55. Mothers <20 years old may be at increased risk due to a combination of obstetric, immunological and socioeconomic factors, including primiparity and access to prenatal care. Maternal age younger than 20 years is mainly associated with late-onset pre-eclampsia (≥34 weeks of gestation)57.
Pre-existing maternal medical conditions
Pre-existing medical conditions may increase the risk of developing hypertensive disease in pregnancy, including pre-eclampsia. Many of these risk factors can be identified before or during early pregnancy, enabling interventions to modify the risk of later developing pre-eclampsia.
Chronic hypertension (or hypertension diagnosed <20 weeks of gestation) is associated with a fivefold increase in the risk of pre-eclampsia compared with normotension58. Treatment of even mild chronic hypertension with antihypertensive medication from before or in early pregnancy reduces the risk of developing pre-eclampsia by 18% (adjusted RR 0.82, 95% CI 0.74–0.92)59.
Pre-pregnancy BMI >30 kg/m2 confers a twofold to fourfold increase in the risk of pre-eclampsia30,56,60 and there is a higher prevalence of late-onset pre-eclampsia among women with overweight and obesity61,62,63. This is likely, in part, due to the association of pre-eclampsia with obesity and cardiometabolic dysfunction64. While weight loss during pregnancy is not recommended, antenatal lifestyle modifications are important to minimize weight gain and reduce the risk of pre-eclampsia. A meta-analysis demonstrated that exercise-only interventions during pregnancy significantly reduced the odds of developing pre-eclampsia (OR 0.59, 95% CI 0.37–0.90)65.
The risk of developing pre-eclampsia in women with pre-gestational diabetes mellitus is more than three times higher than in women without diabetes mellitus30,66. These women might have existing microvascular and macrovascular complications of diabetes, including renal disease, which contributes to this risk. Diabetes can also increase oxidative stress, inflammation and endothelial dysfunction, a shared pathway with the development of pre-eclampsia67.
Women with pre-eclampsia are three times more likely to have chronic kidney disease than the general population68, with some evidence that women with chronic kidney disease are more likely to develop late-onset than early-onset pre-eclampsia57. Glomerulonephritis, diabetic kidney disease and polycystic kidney disease are commonly associated with an increased risk68. The severity of kidney disease and degree of proteinuria are important predictors of the risk of developing pre-eclampsia, even in the absence of associated pathologies, including chronic hypertension.
Thyroid dysfunction before and during pregnancy is associated with an increased risk of pre-eclampsia69. Untreated overt hypothyroidism and hyperthyroidism have a high risk of pre-eclampsia70, which may be reduced by treatment with thyroxine replacement71 or antithyroid drugs, respectively72. Treating subclinical hypothyroidism is controversial and not associated with a reduction in pre-eclampsia73 and subclinical hyperthyroidism is not associated with pre-eclampsia69.
Pre-eclampsia risk is increased in SLE and antiphospholipid syndrome, particularly in the active disease state, indicated by the presence of lupus nephritis in SLE (OR 2.84, 95% CI 1.87–4.30) or of lupus anticoagulant (OR 2.45, 95% CI 1.18–4.64) or anticardiolipin antibodies (OR 1.52, 95% CI 1.05–2.20) in antiphospholipid syndrome2,74,75,76,77,78,79,80,81,82. Adequate treatment and conception in the absence of active disease are associated with a reduction in the risk of pre-eclampsia.
Disrupted gut microbiota profiles have been identified in women with pre-eclampsia, with changes persisting up to 6 weeks postpartum83,84. A disrupted gut microbiota has also been linked to other diseases that are risk factors for pre-eclampsia, including obesity and metabolic disorders84.
Obstetric history
Primiparity is associated with a threefold increase in the likelihood of developing pre-eclampsia30. It is proposed that one mechanism by which pre-eclampsia arises is due to immune maladaptation and a maternal alloimmune reaction triggered by rejection of paternal antigens on the fetal allograft85. This response is greatest in the first pregnancy; hence, primiparous mothers are more likely to develop pre-eclampsia57, whereas multiparity is protective and reduces the risk of pre-eclampsia86. This protective effect is lost when a subsequent pregnancy involves exposure to new paternally inherited antigens87. Epidemiological studies have shown an increase in the risk of pre-eclampsia with increasing inter-pregnancy interval, equivalent to that of primiparity, when the interval is >10 years58,88. This hypothesis is also consistent with the finding that women who conceived by in vitro fertilization (IVF) or intrauterine insemination using donor gametes are at significantly higher risk than those who undergo IVF with autologous egg or partner sperm89,90.
Multiple fetal pregnancies are associated with a significantly higher rate of pre-eclampsia (OR 2.93, 95% CI 2.04–4.21) than singleton pregnancies, with the rate increasing with the number of fetuses present30. Neither chorionicity nor zygosity alters the risk, although the rate may be underestimated in monochorionic pregnancies, which are likely to be electively delivered preterm for fetal indications, unlike dichorionic pregnancies, which are mostly delivered at term91.
A previous pre-eclamptic pregnancy increases the risk of recurrence in subsequent pregnancies by sevenfold to tenfold92,93,94,95. Recurrence risk is more strongly associated with a previous pregnancy complicated by early-onset pre-eclampsia rather than with previous pregnancy complicated by late-onset pre-eclampsia, with gestation time at recurrence often being weeks later in subsequent pregnancies57,62. A meta-analysis including data from 94 studies reported a risk of recurrence of 13.8%, inversely related to gestational age at delivery in the previous pregnancy affected by pre-eclampsia96,97.
Previous pregnancies complicated by FGR, placental abruption and stillbirth increase the risk of pre-eclampsia, especially when previously associated with early-onset pre-eclampsia or evidence of placental malperfusion57. There is limited evidence linking pre-eclampsia to previous ectopic pregnancy; however, one national cohort study in Scotland (1981–2000) identified a higher risk of developing pre-eclampsia in women who had an ectopic first pregnancy than in women who had a live birth98. There is no evidence suggesting that a previous history of early pregnancy loss or termination is associated with pre-eclampsia57,60,99,100.
Conception by IVF, intracytoplasmic sperm injection or egg donation increases the risk of pre-eclampsia compared with pregnancies conceived naturally or via intrauterine insemination; the risk is even higher with frozen-thawed embryo transfer cycles than with fresh cycles101. This may be because of impaired vascular health and maternal adaptation to pregnancy in women who lack a corpus luteum at conception102,103,104. Hormonal treatments are often given to women undergoing frozen-thawed embryo transfer, which suppress the pituitary–ovarian axis, resulting in the absence of corpora lutea102,103. Additionally, differences in the hormonal preparation of the endometrium before a frozen-thawed cycle may have a detrimental effect on maternal adaptation to pregnancy104, including abnormal decidualization (formation of the decidua)105. Single embryo transfer also reduces the risk of a multiple pregnancy, thus reducing the risk of pre-eclampsia.
Since the COVID-19 pandemic, there has been data to suggest a link between SARS-CoV-2 infection in pregnancy and an increased risk of developing pre-eclampsia. Some systematic reviews found an increase in risk when collating data from different cohorts106,107; however, other studies have reported that COVID-19 infection during pregnancy does not increase the risk of pre-eclampsia108,109,110. Pre-eclampsia is more likely to be associated with severe COVID-19, although whether one is causal of the other has not been definitively proven107,111. Both pre-eclampsia and COVID-19 are characterized by increased circulating pro-inflammatory cytokines and endothelial dysfunction, suggesting common mechanisms111. Given that there seems to be a dose–response relationship and similarity in the activation of many of the same molecular pathways such as angiogenesis and endothelial dysfunction, this finding warrants further investigation.
Environmental factors
Residence at high altitudes (>2,700 m) is associated with increased risk of pre-eclampsia (for example, Colorado, USA, 33% of pregnancies; Peru, 22% of pregnancies; Bolivia, 20% of pregnancies; worldwide prevalence, 4.6% of pregnancies)14,112,113,114. Maternal hypoxia affecting multiple physiological systems, including placenta/decidual vasculature, is thought to drive this increased rate of pre-eclampsia112, and it has been reported that multigenerational residents at high altitudes may be protected against pre-eclampsia compared with immigrants113.
Air quality and exposure to ambient pollutants are also risk factors for pre-eclampsia. Associations between exposure to ambient particulate matter with diameter <2.5 µm (PM2.5)115,116,117 and nitrogen dioxide116 during pregnancy and increased pre-eclampsia have been reported; for PM2.5 exposure, this association may be more pronounced in pre-eclampsia with FGR115.
Mechanisms/pathophysiology
Establishment of a healthy pregnancy
Placentation
The placenta is central to pre-eclampsia: pre-eclampsia is found only when a placenta is or was recently present. Healthy placental function depends on extensive placental villus branching and vascularization during early pregnancy, with the mature placenta largely formed by the end of the first trimester118.
Pregnancy is initiated following implantation of a competent blastocyst into a receptive endometrium119. The endometrium is transiently receptive to blastocyst implantation during the mid-luteal phase of the menstrual cycle119. Following implantation, the placenta is formed from extra-embryonic lineages in the blastocyst: trophectoderm cells differentiate into villus progenitor cytotrophoblasts, which fuse to form the syncytiotrophoblast or differentiate into invasive extravillous trophoblasts120, and extra-embryonic mesoderm differentiates into villus core stromal tissue and blood vessels120. The placental villus is lined by two layers of trophoblast: the multinucleated syncytiotrophoblast, which covers the entire placenta and is in direct contact with maternal blood, and the cytotrophoblast, which formed the extravillous trophoblast and anchors the placental villus to the maternal decidua via cell columns120 (Fig. 2a). Extravillous trophoblasts invade from cell columns into the upper third of the myometrium from as early as 14 days after implantation120 until 18 weeks of gestation, when placentation is largely completed121. Factors released by the extravillous trophoblasts (including progesterone) enhance decidualization122,123, creating the semi-permanent decidual tissue maintained throughout pregnancy.
a, Each placental villus has a mesodermal core surrounded by an inner layer of progenitor cytotrophoblasts and an outer syncytiotrophoblast layer. Cytotrophoblasts at the villous tip interrupt the syncytiotrophoblast to form a columnar structure, which anchors the placenta to the decidua. Extravillous trophoblasts (EVTs) differentiate from the columnar cytotrophoblasts and migrate into the decidua into the upper myometrium (interstitial EVTs) or plug maternal spiral arteries (endovascular EVTs), preventing maternal blood flow into the intervillous space until ~12 weeks of gestation, when the trophoblast plugs are lost. EVTs and uterine-resident immune cells, including uterine natural killer cells and regulatory T (Treg) cells, actively remodel the maternal spiral arteries into wide-bore, low-flow uterine arteries by removing vascular smooth muscle cells that surround the artery. b, There are multiple proposed drivers of syncytiotrophoblast stress associated with pre-eclampsia. Poor spiral artery remodelling, which is associated with shallow EVT invasion, is proposed to drive syncytiotrophoblast stress by causing an ischaemic blood supply to the placenta. Overcrowding and compression of the placental villus are proposed to cause reduced placental perfusion and a hypoxic placenta, driving syncytiotrophoblast stress. Syncytiotrophoblast senescence from premature placental ageing may also lead to syncytiotrophoblast stress. Syncytiotrophoblast stress manifests as endoplasmic reticulum stress, mitochondrial dysfunction, oxidative stress, and apoptosis and leads to abnormal syncytiotrophoblast release of factors, including cell-free DNA, reactive oxygen species, syncytial knots, exosomes/microvesicles, pro-inflammatory cytokines and anti-angiogenic factors, into the maternal circulation. sEng, soluble endoglin; sFLT1, soluble Fms-related receptor tyrosine kinase 1. Adapted from ref. 349, Springer Nature Limited.
Placental villi bathed in maternal blood facilitate all nutrient and gas exchange required to support the fetus during pregnancy. Blood vessels within the villus core transport nutrients and gases to and from the fetus via the umbilical blood vessels. During the first trimester, uterine spiral arteries are remodelled to create wide-bore, high-flow, low-resistance vessels124 capable of accommodating increased placental perfusion requirements in the later stages of pregnancy. Remodelling is initiated by uterine-resident innate immune cells, including uterine NK cells and T regulatory (Treg) cells, which cause the loss of vascular smooth muscle cells surrounding the spiral arteries and regulate extravillous trophoblast invasion through the decidua via the secretion of angiogenic growth factors and cytokines125. Endovascular extravillous trophoblasts invade the arteries, replacing vascular endothelial cells and temporarily plugging the maternal arteries, blocking blood flow to the developing placenta, and leading to embryo development under low oxygen conditions. At around 10–12 weeks of gestation, the trophoblast endovascular plugs dislodge126,127, enabling increasing volumes of maternal blood to perfuse the intervillous space and thus increasing fetoplacental oxygenation.
Vascular adaptations during pregnancy
In a healthy pregnancy, the maternal cardiovascular system undergoes significant expansion, including increased plasma volume and increased cardiac output from as early as 3–4 weeks of gestation, primarily driven by placental-released factors128. Resultant increases in blood pressure are prevented by concomitant decreases in systemic vascular (peripheral) resistance, increased arterial compliance, increased peripheral vasodilation, blunted contractility, enhanced endothelial release of vasodilatory factors and activation of the renal renin–angiotensin–aldosterone system129,130,131,132. There is little evidence for autonomic regulation driving changes in the cardiovascular system during pregnancy: the primary adaptations are endothelial and myogenic128. The endothelium is the interface between blood and vascular smooth muscle and is highly responsive to humoral factors and physical forces128.
Pathophysiology of pre-eclampsia
The underlying aetiology of pre-eclampsia, both preterm and term, remains uncertain. It is likely that maternal and placental factors are involved and that there is overlap in the pathogenesis of preterm and term pre-eclampsia133, but there is a remarkable gap in research to determine the pathogenesis of term pre-eclampsia, which is more common and often associated with maternal complications134.
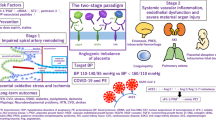
The two-stage model of pre-eclampsia proposes that pre-eclampsia results from placental dysfunction causing syncytiotrophoblast stress (stage 1), which leads to the maternal clinical manifestation of pre-eclampsia (stage 2)135,136. The cause and timing of the placental insult are proposed to differ between preterm and term disease136. Syncytiotrophoblast stress (stage 1) manifests as oxidative stress, endoplasmic reticulum stress, mitochondrial damage, dysregulated metabolism and apoptosis137,138. The stressed syncytiotrophoblast abnormally releases pro-inflammatory cytokines, reactive oxygen species, extracellular vesicles, anti-angiogenic agents (for example, soluble FLT1 (sFLT1) and cell-free fetal DNA) into the maternal circulation135,139,140,141. These factors promote maternal endothelial dysfunction and systemic multiorgan disorder that involves reduced vasodilation, systemic inflammation and thrombosis142,143,144 (stage 2). The effects include hypertension, liver and renal impairment, thrombocytopenia, and coagulopathy.
Placental dysfunction in preterm pre-eclampsia
Syncytiotrophoblast stress in preterm pre-eclampsia is thought to arise from abnormal placentation during early pregnancy, characterized by inadequate extravillous trophoblast invasion and spiral artery remodelling145,146 (Fig. 2b). This reduces blood flow to the placenta, resulting in placental hypoxia and placental ischaemia and reperfusion injury, causing syncytiotrophoblast stress. Inadequate trophoblast invasion of the spiral arteries and associated placental hypoperfusion also directly contribute to the FGR that often accompanies preterm pre-eclampsia.
Placental dysfunction in term pre-eclampsia
The abnormal placental histopathological findings common in preterm pre-eclampsia are relatively uncommon in term disease147,148. It is hypothesized that placental development is normal in term pre-eclampsia and that syncytiotrophoblast stress is initiated later in gestation. It is proposed that there are two mechanisms by which syncytiotrophoblast stress arises in term pre-eclampsia: compression of chronic villus when there is insufficient space for the larger placenta in late pregnancy and syncytiotrophoblast senescence associated with premature placental ageing135,149 (Fig. 2b). Syncytiotrophoblast stress increases as gestation proceeds, even during an uncomplicated pregnancy, driven by the increasing mismatch between normal maternal perfusion and the metabolic demands of the placenta and fetus135, leading to the hypothesis that pre-eclampsia is inevitable if gestation continues beyond the capacity of the placenta125.
It is hypothesized that syncytiotrophoblast stress is not present early in gestation of a pregnancy that is later complicated by term pre-eclampsia149, explaining why early pregnancy predictive models for pre-eclampsia, which often include risk factors and biomarkers of abnormal placentation, demonstrate high accuracy in the prediction of preterm pre-eclampsia (detection rates of 75–90%) but underperform in the prediction of term pre-eclampsia (detection rate below 50%)150. However, these hypotheses have been difficult to test experimentally due to the inherent problem of obtaining early pregnancy placenta from ongoing pregnancies.
Immune system dysfunction
Maternal immunological problems are associated with abnormalities at the fetal–maternal interface. Immunological tolerance to the fetus and placenta, whose genes are half-paternal, is facilitated, in part, by reduced placental expression of MHC and the human leukocyte antigen (HLA) system; this mechanism endeavours to avoid innate rejection of semi-allogeneic fetal cells151. Uterine NK cells and T lymphocytes are located in the decidua and have a critical role in promoting maternal immune tolerance to the fetus. In particular, Treg cells exert immune tolerance functions by mechanisms including antigen presentation, secretion of inhibitory cytokines and cytolysis of target cells152,153. Abnormal release of Treg cell factors, including cytokines and microRNA, is found in pre-eclampsia154. Epidemiological studies support experimental data reporting that the maternal immune response to paternally derived antigens on the trophoblast155 is decreased by previous exposure to seminal fluid156: a higher incidence of pre-eclampsia is found in primiparity, pregnancies in which the paternity has changed, pregnancies after a prolonged inter-pregnancy interval (>10 years), pregnancies conceived soon after first coitus, and pregnancies conceived with the use of donor egg157 and sperm90. Angiotensin II receptor type 1 auto-antibodies (AT1-AAs) are elevated in the serum of women with pre-eclampsia158. AT1-AAs have a sustained effect on vasoconstriction and can cause endothelial cell damage158.
Maternal metabolic and cardiovascular health
Accumulating evidence suggests that pre-eclampsia is associated with impaired maternal metabolic and cardiovascular function, leading to inadequate adaptation to the demands of pregnancy159,160,161,162,163. Altered metabolic and cardiovascular function is proposed to contribute to pre-eclampsia by causing reduced spiral artery remodelling in preterm pre-eclampsia and altered placental metabolic function in both preterm and term pre-eclampsia163. Metabolomic studies undertaken in serum of women at 11–13 weeks of gestation who later developed late-onset pre-eclampsia identified that insulin resistance and metabolic syndrome, mitochondrial dysfunction, disturbance of energy metabolism, oxidative stress, and lipid dysfunction are present in late-onset pre-eclampsia164, suggesting that disturbances can be identified early in the disease process.
Dysregulated placental gene expression
Two small studies using chorionic villus samples (CVS) collected from pregnancies that subsequently developed early-onset (<34 weeks of gestation) pre-eclampsia identified dysregulated placental and decidual gene expression at the end of the first trimester165,166,167. The placental tissue exhibited dysregulated expression of genes associated with angiogenesis and oxidative stress165 and, in decidual tissue, genes associated with inflammation/immunoregulation, cell motility, decidualization and NK cell function were altered166,167. Many of these factors have since been validated in preterm pre-eclampsia, including complement factor H and prothrombin35,36,37,168. No study to date has examined the gene expression of CVS collected from pregnancies that subsequently develop late preterm (35–36 weeks of gestation) or term pre-eclampsia (≥37 weeks of gestation).
Meta-analysis on placenta samples collected at delivery has identified dysregulated genes involved in carbohydrate and energy utilization, immune response, and developmental/pregnancy processes in all forms of pre-eclampsia169. Smaller studies that distinguished between early-onset and late-onset pre-eclampsia identified that early-onset placenta samples had increased gene expression for genes involved in metabolic processes, and late-onset placenta samples had increased expression of genes involved in immune processes170,171. These findings further suggest that the mechanisms involved in the three forms of pre-eclampsia are different.
Dysregulated placental release of factors
Alterations in placental secreted factors, including angiogenic proteins, pro-inflammatory cytokines and small extracellular vesicles, before the development of pre-eclampsia have been demonstrated in maternal blood139,172,173,174,175,176.
Placental-released angiogenic factors, including sFLT1 and placental growth factor (PGF), have been implicated in the development of pre-eclampsia177,178,179. There is a steep increase of serum sFLT1 levels and decreased PGF from approximately 5 weeks before the onset of pre-eclampsia178. The ratio of sFLT1 to PGF is therefore used as a helpful tool when diagnosing placental dysfunction in pre-eclampsia, with higher sensitivity and specificity being achieved for early-onset pre-eclampsia177,178. Soluble endoglin is another notable anti-angiogenic factor released by the pre-eclamptic placenta with a similar pattern in serum as sFLT1 (refs. 177,180). It is thought that elevated maternal serum levels of the cleaved soluble form of endoglin lead to disturbed angiogenesis and vasoconstriction, causing pre-eclampsia symptoms180. A recent meta-analysis suggests that maternal serum soluble endoglin may be useful as a predictive biomarker of pre-eclampsia but may not distinguish between early-onset and late-onset disease181.
Inflammasome activation and its associated pro-inflammatory cascade are elevated in pre-eclampsia182,183. Inflammasomes are innate immune system receptors and sensors comprised of multimeric proteins that regulate the activation of caspase 1 and induce inflammation in response to infectious microbes and molecules derived from host proteins (called sterile inflammation)184. The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) and NLRP7 and their associated adaptor protein PYCARD are elevated in placenta and blood of women with pre-eclampsia182,183. There are several strategies being developed to inhibit inflammasome activation185 that may be useful to treat inflammation-related pathways in pre-eclampsia.
Circulating IL-11 is increased in early pregnancy of women who subsequently develop pre-eclampsia186. However, it is important to determine whether IL-11 levels in the placenta are dysregulated during placental development in humans, which may be critical in driving the pathogenesis of pre-eclampsia.
Galectins are a family of β-galactoside-binding lectins with important roles in the development of pre-eclampsia187. Galectins 1, 2, 3, 7, 9, 13 and 14 have all been implicated in the pathogenesis of pre-eclampsia187, likely due to their functions in promoting maternal fetal tolerance169,187 or causing alterations to the renin–angiotensin–aldosterone system and oxidative stress188,189. A correlation between maternal serum levels and CVS expression of galectin 7 has been found in preterm pre-eclampsia188,190.
Increased syncytiotrophoblast release of extracellular vesicles (Fig. 2b) into the maternal circulation is found in pre-eclampsia191,192,193. These extracellular vesicles are enriched in anti-angiogenic factors, apoptosis-inducing ligands and active caspase 3, especially in early-onset pre-eclampsia192,193. Emerging evidence suggests that syncytiotrophoblast-released extracellular vesicles are internalized by endothelial cells, into which they release these factors and drive the maternal endothelial dysfunction and inflammation observed in pre-eclampsia176,194,195,196.
Systemic consequences of pre-eclampsia onset
Vascular involvement
The maternal endothelium is thought to be an important target of the placental-released factors hypothesized to drive pre-eclampsia197. Widespread endothelial dysfunction can also explain systemic organ damage in women with pre-eclampsia198. The endothelium controls smooth muscle tone and the production and release of vasoconstrictor and vasodilatory factors (including nitric oxide) as well as regulating anti-coagulation, anti-platelet and fibrinolysis functions197. Endothelial dysfunction can lead to reduced blood flow to organs such as the heart and kidney133 and reduced venous blood drainage and associated venous congestion. This contributes to organ dysfunction and can induce reflex constriction of arteries199. Altogether, it is hypothesized that the endothelial dysfunction driven by placental-released factors initiates and drives hypertension in pre-eclampsia.
Abnormal uterine artery Doppler findings (vessel blood flow) are more common in early-onset than in late-onset pre-eclampsia62, confirming the high impedance to blood flow associated with the failure of physiological remodelling of spiral arteries and the consequent poor placental perfusion, hypoxia and reperfusion injuries in early-onset pre-eclampsia133,134. By contrast, late-onset pre-eclampsia is often related to maternal endothelial cell dysfunction200 and is thought to be influenced by pre-existing maternal conditions that could affect endothelial integrity131. However, machine learning approaches using biochemical data retrieved from electronic medical records (for example, systolic blood pressure, serum blood urea nitrogen, potassium, calcium, and creatinine, platelet and white blood cell counts, and urinary protein) from 11,006 women at 14–17 to 34 weeks of gestation have been documented to predict late-onset pre-eclampsia early in the second trimester92, suggesting that early pregnancy placental vascular impairment also occurs in late-onset pre-eclampsia.
Pulmonary oedema
Characterized by excessive fluid accumulation in the lungs, pulmonary oedema is a rare (in 0.6–5% of women with pre-eclampsia), acute, life-threatening condition, primarily associated with severe pre-eclampsia201,202. Pulmonary oedema is the second most common cause of death in pregnancies complicated by hypertension203. There are multiple causes of pulmonary oedema, including decreased oncotic pressure, increased capillary permeability, increased hydrostatic pressure and diastolic dysfunction. Antihypertensive medications and excessive fluid administration are risk factors for pulmonary oedema204. Pulmonary oedema is most common (39%) postpartum204, when fluid sequestered in the extravascular space is mobilized into the vascular space, increasing central venous and pulmonary capillary wedge pressure.
Renal involvement
The kidney is the organ most likely to be affected by endothelial injury in pre-eclampsia198. Renal biopsies of women with pre-eclampsia show glomerular endotheliosis (swelling of endothelial cells, obliteration of fenestrations and invasion of the capillary space) that seems to be responsible for the decreased glomerular filtration rate noted in pre-eclampsia205. The characteristic proteinuria in pre-eclampsia is caused by high concentrations of sFLT1 inhibiting the expression of proteins of the podocyte slit diaphragm, such as synaptopodin and nephrin206, which increases inter-podocyte separation. In turn, the lack of vascular endothelial growth factor (VEGF) and PGF availability in the glomerular endothelium stimulates endothelin 1 expression that promotes podocyte detachment207.
Liver involvement
Liver damage in pre-eclampsia is characterized by periportal inflammation and hepatocellular damage (manifested as right-upper quadrant or epigastric pain and elevated transaminases), subcapsular haematoma and, in rare cases, hepatic failure or rupture208. Widespread microangiopathy causes vasospasm of hepatic sinusoids and promotes fibrin deposition in the microcirculation209, leading to ischaemia. Hepatic endothelial cells are highly dependent on VEGF, and its antagonism with sFLT1 significantly alters their function given the decreased availability of nitric oxide210. The resulting ischaemia causes oxidative stress and inflammation that affect hepatic acini, elevating the concentration of liver enzymes in blood and contributing to the onset of HELLP syndrome.
HELLP syndrome encompasses microangiopathic haemolysis, elevation of liver enzymes and thrombocytopenia. The most common symptoms in affected patients are right-upper quadrant pain, epigastralgia, nausea and vomiting, headache and visual changes. In hyperbilirubinaemia, indirect bilirubin predominates; thus, only in advanced cases can the patient present clinical jaundice211. Changes in the coagulation system may occur. The cause of thrombocytopenia is believed to be consumption due to exaggerated platelet activation caused by diffuse endothelial injury. Evolution, in severe cases, to disseminated intravascular coagulation corroborates the worsening of the case and is diagnosed by decreased levels of fibrinogen, antithrombin, and increased prothrombin time and fibrin212.
Neurological involvement
Neurological symptoms have been recognized as high-risk features of eclampsia for thousands of years213. Neurological complications are the direct cause of many maternal deaths due to pre-eclampsia, particularly in LMICs, and include eclampsia (seizures), visual scotomata, cortical blindness, arterial ischaemic stroke, cerebral venous sinus thrombosis, subarachnoid and intracerebral haemorrhage, reversible cerebral vasoconstriction syndrome, and posterior reversible encephalopathy syndrome213,214. Cerebral venous sinus thrombosis, reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome occur most commonly in the postpartum period and often with little warning215. The mechanisms leading to neurological complications are being uncovered; the maternal cerebral vasculature is highly sensitive to pre-eclampsia215. Neurovascular dysfunction is clear in pre-eclampsia, with studies showing increased sympathetic activity of the autonomic nervous system213,216 (arterial stiffness and endothelial dysfunction are, in part, under sympathetic control216), impaired cerebral autoregulation (which prevents hyperperfusion injury to the brain213,217), increased blood–brain barrier permeability132, and vasogenic oedema, with cerebral markers, including neurofilament light chain, being dysregulated in pre-eclamptic cerebrospinal fluid, serum and plasma218.
Fetal growth restriction
FGR occurs mainly due to placental dysfunction and is therefore highly associated with preterm pre-eclampsia219,220,221,222. Abnormalities in trophoblast invasion during early pregnancy lead to inadequate uterine spiral artery remodelling and can lead to hypoxia and nutritional deficiency, eventually causing FGR220. Late-onset pre-eclampsia complicated by FGR is generally accompanied by lower placental weight and more vascular and villous abnormalities than late-onset pre-eclampsia alone223. In any pregnancies with suspected FGR, ISSHP recommends fetal growth velocity, amniotic fluid volume and umbilical artery Doppler be assessed by ultrasonography every 2 weeks, although the utility of umbilical artery Doppler close to term may be limited2. In low-resource settings, ISSHP recommends that monitoring fetal heart rate, cardiotocography performed 6-hourly and maternal characteristics plus proteinuria can be used to estimate perinatal risk at ≥32 weeks of gestation as, before this, low gestational age drives most risk2.
Diagnosis, screening and prevention
Diagnosis
The ISSHP guidelines2,13 specify that pre-eclampsia can be diagnosed after 20 weeks of gestation by new-onset hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg; average of two measurements) in a patient previously with normotension plus one other pre-eclampsia-related symptom or sign. These can include proteinuria (protein/creatinine ≥30 mg/mmol in a spot urine sample or ≥300 mg/mmol in >0.3 g/day), acute kidney injury (creatinine ≥90 μmol/l), liver involvement (elevated transaminases, for example, ALT or AST >40 IU/l), neurological symptoms (eclampsia, altered mental status, blindness, stroke, clonus, severe headaches, persistent visual scotomata), haematological abnormalities (thrombocytopenia (platelet count below 150,000/μl), disseminated intravascular coagulation, haemolysis), cardiorespiratory complications (pulmonary oedema, myocardial ischaemia or infarction, oxygen saturation <90%, ≥50% inspired oxygen for more than 1 h, intubation other than for caesarean) or uteroplacental dysfunction (FGR, angiogenic imbalance, placental abruption). This new set of diagnostic criteria published in 2014 and revised in 2018 and 2021, represents a significant change compared with the previous recommendations published in 2001 (ref. 224), which required the presence of proteinuria and new-onset hypertension in a patient with previous normotension. Use of the ISSHP guidelines increases pre-eclampsia diagnoses when compared with guidelines published before 2014, improving the identification of women and neonates at risk of adverse outcomes225, although most women newly identified have only mild disease and low risk of adverse outcomes226. ACOG25 and NICE26 both updated their guidelines for the diagnosis of pre-eclampsia in 2019 to be broadly similar to the ISSHP guidelines227.
For suspected pre-eclampsia, ISSHP recommends evaluation of angiogenic imbalance as a marker of uteroplacental dysfunction, whereas normal angiogenic balance would strengthen a diagnosis of gestational hypertension2. NICE guidelines and meta-analyses support the measurement of PGF alone or in combination with sFLT1 to rule out suspected pre-eclampsia within the 14 days following measurement26,42,228,229,230,231,232,233,234. A stepped-wedge cluster trial demonstrated that measurement of PGF in women with suspected pre-eclampsia reduces the time to clinical confirmation and may reduce the incidence of adverse maternal outcomes235. Similarly, a ratio of sFLT1 to PGF below a certain cut-off (commonly <38) can reliably rule out pre-eclampsia among women with suspected disease42.
Small, retrospective trials have identified total thiols as increased in late-onset pre-eclampsia236, and serum ADT-ProteoStat protein aggregates237 and podocalyxin238 as elevated in both early-onset and late-onset pre-eclampsia. These small studies require validation in larger cohorts.
Disease progression
All women with pre-eclampsia are at risk of rapid progression and severe disease regardless of the timing of onset8,12, with up to 18% of HELLP syndrome and 55% of eclampsia occuring in term (≥37 weeks of gestation) pre-eclampsia239,240. A history of chronic hypertension and elevated systolic blood pressure or serum creatinine on admission can be associated with increased risk of progressing to severe disease in late preterm (34–36 (+6 days) weeks of gestation)241 or term12 pre-eclampsia. However, the best-performing model to predict risk of severe outcomes in early-onset pre-eclampsia (gestational age, chest pain or dyspnoea, oxygen saturation, platelet count, and creatinine and aspartate transaminase concentrations) does not include chronic hypertension or blood pressure at admission242, possibly reflecting differences in the underlying aetiology of disease. Larger, multicentre studies are required to confirm this243,244.
The ratio of sFLT1 to PGF has been investigated in the prediction of adverse pregnancy outcomes. In a study in Asia, an sFLT1 to PGF ratio of ≤38 had a negative predictive value of 98.9% (95% CI 97.6–99.6%) and a ratio of >38 had a positive predictive value of 53.5% (95% CI 45.0–61.8%) for a composite of adverse maternal outcomes (including death, pulmonary oedema, acute renal failure, cerebral haemorrhage, cerebral thrombosis and disseminated intravascular coagulation)245. A recent study demonstrated that the ratio of sFLT1 to PGF performs better in the prediction of adverse perinatal outcomes (area under the receiver-operating characteristics curve (AUROC) 0.87, 95% CI 0.81–0.93) than in the prediction of adverse maternal outcomes (AUROC 0.69, 95% CI 0.59–0.78)246. Conversely, multivariable predictive models, such as the fullPIERS model, seem to predict adverse maternal outcomes reasonably well (AUROC 0.88, 95% CI 0.84–0.92) in both early-onset and late-onset pre-eclampsia242,247.
Screening
NICE and ACOG have published guidelines for risk assessment based on elements from maternal characteristics and medical history such as a history of chronic hypertension (Table 1). The Fetal Medicine Foundation (FMF) competing-risks model248,249 incorporates maternal age, ethnic background, weight and height, medical and obstetric history, mean arterial blood pressure, uterine artery pulsatility index on ultrasonography, and maternal circulating PGF levels at 11–13 weeks of gestation to estimate the individual risk of developing pre-eclampsia. These two approaches to screening were assessed in the UK NHS Screening Programme for Pre-eclampsia (SPREE) study involving 16,747 participants14. At a screen-positive rate of 10% (where 10% of the study population was considered to be at high risk using the NICE criteria), the detection rate of preterm pre-eclampsia was 41% with the risk scoring system recommended by NICE compared to 82% when screening was based on the FMF competing-risks model15. FMF screening is particularly effective for preterm pre-eclampsia, identifying ~90% of women who will develop pre-eclampsia at <34 weeks of gestation and ~80% of women who will develop pre-eclampsia at <37 weeks of gestation250 but only 44% of women who will develop pre-eclampsia at ≥37 weeks of gestation15. ISSHP and the International Federation of Gynecology and Obstetrics (FIGO) now recommend combined screening with the FMF algorithm where possible2,251; however, uterine artery ultrasonography and PGF assays are not routinely performed worldwide. It has been found that a step-wise screening protocol with an initial screen of maternal risk factors (maternal characteristics, medical history and blood pressure) followed by either uterine artery ultrasonography or PGF assays only on women with positive risk has a similar detection rate250 (Table 2).
The FMF first-trimester screening tool for preterm pre-eclampsia has been extensively validated in several different communities across the world. Implementation studies showed significant reductions in the rates of preterm pre-eclampsia and improvement in maternal and perinatal outcomes in the UK15 and Australia252, and the screen-and-treat approach based on the FMF algorithm seems highly cost-effective253, now being the recommended approach by various institutions13,251,254.
Screening tests under development
Using a similar prediction tool published by the FMF, screening at 19–24 weeks of gestation of women identified as being at low risk in the first trimester or who missed screening in the first trimester enables the identification of almost all women who will develop pre-eclampsia by 32 weeks and of up to 90% of women who will develop pre-eclampsia between 32 and 35 (+6 days) weeks of gestation255,256. This can be used to stratify women needing intensive monitoring at 24–31 (+6 days) weeks of gestation and women that require reassessment at 35–37 weeks of gestation255 (Fig. 3).
All pregnant women should be screened at 11–13 weeks of gestation with the Fetal Medicine Foundation competing-risks model248,249 to determine their risk of pre-eclampsia at <37 weeks of gestation. Women identified as being at high risk of pre-eclampsia at <37 weeks of gestation should be prescribed aspirin from before 16 weeks of gestation and cease taking aspirin at 36 weeks of gestation. Screening in the second and third trimesters is showing promise as a tool to identify risk of all pre-eclampsia but should be considered in the context of resource availability. Any woman identified as being at low risk in the screen at 11–13 weeks of gestation should be re-screened at 19–24 weeks of gestation to determine risk of pre-eclampsia at <32 weeks of gestation. Women identified as being at high risk should have increased monitoring from 24–31 (+6 days) weeks of gestation and, if they are still pregnant at 32 weeks of gestation, they should be screened again then to determine their risk of pre-eclampsia at <36 weeks of gestation. Women at high risk of pre-eclampsia at <36 weeks of gestation should continue to have increased monitoring from 32–35 (+6 days) weeks of gestation and induction of labour should be considered at 37/38 weeks of gestation. All women still pregnant at 35–37 weeks of gestation should be re-screened to assess their risk of pre-eclampsia >37 weeks of gestation. Women at high risk of pre-eclampsia at >37 weeks of gestation should have increased monitoring and induction of labour should be considered at >37 weeks of gestation. aMaternal characteristics: age, BMI, smoking, mother of pregnant woman had pre-eclampsia, conception method, comorbidities (for example, chronic hypertension, diabetes types 1 or 2, systemic lupus erythematosus, antiphospholipid syndrome) and obstetric history. PGF, placental growth factor; sFLT1, soluble Fms-related receptor tyrosine kinase 1.
Prediction of term pre-eclampsia at 35–37 weeks of gestation (Fig. 3) can identify up to 85% of all women who will develop pre-eclampsia at >36 weeks of gestation250,257. When ethnicity is included in the predictive algorithm, this screening was found to have stronger predictive power for Afro-Caribbean women (88%) than for white women (66%) in London258. The screening at 35–37 weeks of gestation includes maternal circulating sFLT1 but not the uterine artery pulsatility index as it is not useful for identifying women at high risk of developing pre-eclampsia at >36 weeks of gestation258.
As the prediction of preterm pre-eclampsia is so effective, new methods to predict risk of term pre-eclampsia are now urgently required. A small study using machine learning to aggregate maternal characteristics and laboratory parameters from the second and third trimesters identified 77.1% of late-onset pre-eclampsia (false-positive rate 0.9%)92. The most influential variables were systolic blood pressure, serum urea, nitrogen, potassium, calcium, and creatinine, platelet and white blood cell counts and urinary protein92. However, this study has not been validated in other cohorts. Other potential factors that may improve the prediction of late-onset pre-eclampsia but which require validation in large cohorts or meta-analyses include second-trimester circulating HtrA3 (ref. 259), cell-free RNA260,261, and third-trimester circulating ELABELA262 and progranulin263.
Prevention
The severe short-term and lifelong health risks of being exposed to a pre-eclamptic pregnancy for both the mother and child emphasize the need for new treatments to prevent pre-eclampsia. Adequately powered, multi-centre studies are required to identify patient populations who may benefit from the different preventive treatments under development.
Preventive treatments with good evidence
The use of aspirin to prevent pre-eclampsia has long been proposed. Despite a randomized trial published in 1985 showing that prophylactic aspirin leads to a large reduction in pre-eclampsia, FGR and stillbirth in women at high risk264, further trials were highly heterogeneous regarding aspirin dose, time of initiation and, importantly, the method used to select women at increased risk265,266,267. An individual-participant data meta-analysis concluded that aspirin provides a statistically significant but clinically modest 10% reduction in pre-eclampsia risk268. Further meta-analysis suggested that aspirin is highly effective in preventing preterm pre-eclampsia when given to women at high risk from before 16 weeks of gestation268 (Fig. 3). The Aspirin for Evidence-Based PREeclampsia (ASPRE) prevention trial, a multicentre, randomized, double-blind placebo-controlled trial of 1,776 women at high risk identified by means of combined screening with the FMF algorithm, provided further convincing evidence that daily aspirin from the first trimester reduces the risk of preterm pre-eclampsia by 62% (95% CI 20–80%), with no significant effect on the rate of term disease7.
A meta-analysis of 30 trials identified that low-dose calcium supplementation halves the risk of pre-eclampsia (both for early and late onset) in women at high risk of developing pre-eclampsia and with low dietary calcium intake269, and is therefore recommended by ISSHP guidelines.
Furthermore, the ISSHP guidelines recommend exercise to reduce the likelihood of gestational hypertension and pre-eclampsia2. A meta-analysis of 27 trials found that exercise of at least 260 metabolic equivalents of task minutes/week reduced the odds of developing pre-eclampsia by 25%65.
Preventive treatments under development
A multicentre trial of 6,000 nulliparous women at low risk randomly assigned to induction at 39 weeks of gestation or expectant monitoring showed labour induction reduced risks of adverse outcomes, including hypertensive disorders of pregnancy270. Further studies are required to determine whether the incidence of pre-eclampsia itself and the long-term, poor outcomes for mother and baby are improved by routine induction.
Pravastatin is an oral statin used to lower LDL cholesterol and triglycerides, which also has anti-inflammatory actions. A trial in 173 women at high risk of developing pre-eclampsia reported that daily pravastatin from the second trimester (14–20 weeks of gestation) until delivery significantly reduced the rate of preterm pre-eclampsia (13.8% versus 26.7% in the control group) and preterm birth271. Pravastatin may not be effective at preventing term pre-eclampsia: there was no reduction in the incidence of term pre-eclampsia in this271 or another trial of 1,120 women at high risk of developing term pre-eclampsia given daily pravastatin from 35–37 weeks of gestation to delivery272.
Metformin is an oral, insulin-sensitizing and glucose-lowering drug widely prescribed during pregnancy for gestational diabetes mellitus. A meta-analysis taking advantage of trials in which metformin was prescribed for other conditions and where participants fell pregnant identified a reduction in the likelihood of pre-eclampsia273. In a randomized control trial of singleton pregnancies in which women with a BMI >35 kg/m2 were given metformin daily from 12–18 weeks until delivery, a significant reduction in pre-eclampsia (OR 0.25, 95% CI 0.1–0.61) and a significant reduction in gestational weight gain were reported274.
A meta-analysis including 313 women from three randomized controlled trials found that daily vitamin D supplementation significantly reduced pre-eclampsia risk (RR 0.29, 95% CI 0.09–0.95)275. Another meta-analysis including 2,464 women from 13 studies found a significantly reduced incidence of pre-eclampsia with prophylactic, low-molecular-weight heparin started before 16 weeks of gestation276; however, the ISSHP guidelines currently do not recommend heparin treatment2.
Management
In a pre-eclamptic pregnancy, the mother and fetus have competing interests. For the mother, delivery of the placenta will alleviate symptoms (Box 2); however, this may cause preterm birth and the resulting complications of prematurity for the neonate. Preterm management of pre-eclampsia (Fig. 4) requires treatment of maternal high blood pressure with the goal of preventing severe maternal outcomes and prolonging pregnancy with surveillance of fetal health and timing of delivery to provide the best outcome for both mother and neonate.
Antihypertensive medications should be given to all women with hypertension (blood pressure (BP) >140/90 mmHg) trying to become pregnant or who are pregnant. Labetalol, nifedipine and methyldopa are recommended. Women who have hypertension before pregnancy or during early pregnancy should be screened using the Fetal Medicine Foundation (FMF) competing-risks model248,249 in the first trimester. If this screening is unavailable, women with hypertension in early pregnancy should be started on aspirin. When pre-eclampsia is diagnosed preterm, corticosteroids and MgSO4 should be given to mature fetal lungs and for fetal neuroprotection, respectively. When pre-eclampsia is diagnosed at ≥37 weeks of gestation, induction should be considered. Women should be weaned from antihypertensive medications at postpartum or they should be changed to antihypertensive medications valid for use while breastfeeding. Support for renal/hepatic/haematological dysfunction should be given. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; IV, intravenous.
Treatment common to all disease subtypes
Hypertension is the predominant diagnostic feature of pre-eclampsia and typically becomes progressively worse with advancing gestation. Severe hypertension (≥160/110 mmHg) may lead to intracerebral haemorrhage, eclampsia and placental abruption and should be treated in all circumstances277. The aim of treatment is to reduce blood pressure to a target range of 140–155/90–105 mmHg (ref. 277).
Oral antihypertensive medications
Although intravenous antihypertensive agents may be indicated in an acute situation, oral medications that are commonly used to manage hypertension in pregnancy include methyldopa, labetalol and nifedipine. These agents are typically available even in low-resource settings. A recent multicentre, parallel-group, open-label, randomized controlled trial compared these three agents (hourly doses of either nifedipine or labetalol or a daily dose of methyldopa) and reported that, although as a single drug, nifedipine resulted in a greater frequency of blood pressure control (120–150 mmHg systolic and 70–100 mmHg diastolic blood pressure) within 6 h, all three agents are potentially viable initial options for the treatment of severe hypertension in pregnancy278.
Nifedipine is a calcium channel antagonist and causes peripheral vasodilatation. It has a rapid onset of action279 and women may complain of severe headaches (particularly in the first 24 h), dizziness, flushing, palpitations and increasing ankle oedema. Initially, sublingual dosing was supported for rapid correction of severe hypertension in pregnancy but there have been significant adverse outcomes in non-pregnant adults, and this can also cause acute fetal distress due to reduced placental perfusion pressure280.
Labetalol blocks β1, β2 and α1 adrenergic receptors and reduces peripheral vascular resistance281. It is a negative inotrope and may promote pulmonary oedema and cardiac failure. It may cause bronchospasm and should be avoided in women with a history of asthma. Women may report headaches and nausea, particularly within the first 24 h of use. Peak plasma doses are reached within 2 h of oral dosing and the peak effect is reached within 48 h of treatment.
Methyldopa is a centrally acting sympathomimetic acting as an α2 adrenergic receptor agonist. Women commonly report feeling lethargic and drowsy, particularly within the first 72 h of use, and methyldopa may worsen depression282. Peak plasma doses are reached 6 h after oral dosing with the peak effect reached following 72 h of treatment, potentially making other treatment strategies more useful at acute presentation. Abrupt cessation (following long-term use) may cause rebound hypertension.
Interventions to manage mild hypertension
Previously, there has been concern that aggressive treatment of mild hypertension in pregnancy (defined in most jurisdictions as blood pressure ≥140/90 mmHg) may affect placental perfusion, leading to an increased prevalence of adverse perinatal outcomes. Two randomized controlled trials that addressed these concerns have demonstrated that this is not the case, and that maintaining blood pressure <140/90 mmHg improves maternal and potentially perinatal outcomes59,283 (Table 3). The Control of Hypertension In Pregnancy Study (CHIPS; including women with chronic hypertension and gestational hypertension) and Control of Hypertension And Pregnancy (CHAP; including women with chronic hypertension) trials have some differences but both reached similar conclusions. CHIPS predominantly recruited women with hypertension >140/90 mmHg at 14–33 weeks of gestation (75% with chronic hypertension; 25% with gestational hypertension)283. The intervention involved assigning women to two different diastolic blood pressure target groups (<85 mmHg versus <100 mmHg) to determine the effect of acceptance of higher diastolic blood pressure on pregnancy outcomes. The primary outcome measure was a composite of perinatal death and significant neonatal admission, with no significant differences between groups. The main finding was a reduction in severe hypertension in the <85 mmHg group, supporting the conclusion that there was no apparent benefit to more relaxed control given the known association between severe hypertension and severe maternal morbidity and mortality.
By contrast, the CHAP trial was restricted to women with chronic hypertension presenting at <23 weeks of gestation59. The intervention involved treatment of hypertension >140/90 mmHg compared with the control group, who only received antihypertensive medication if their blood pressure was ≥160/105 mmHg. The incidence of a primary outcome event (small-for-gestational-age birthweight, serious maternal complications, severe neonatal complications, pre-eclampsia and preterm birth) was significantly lower in the active-treatment group than in the control group.
The findings of these two trials are complementary and suggest that an aggressive approach to managing mild hypertension in pregnancy results in a reduction in maternal morbidity without affecting fetal safety. The potential reduction in preterm delivery may in fact be beneficial from a fetal perspective. These trials support a meta-analysis that reviewed the use of antihypertensive medications in pregnancy284. This meta-analysis also concluded that labetalol or nifedipine may be preferred when compared with methyldopa in women with mild to moderate hypertension during pregnancy284.
Planned delivery
The Hypertension and Preeclampsia Intervention Trial At Near Term-I (HYPITAT-I) trial showed a significant decrease in adverse maternal outcomes with active management (induction of labour) than expectant monitoring of women with gestational hypertension or mild pre-eclampsia at >36 weeks of gestation, and concluded that induction of labour should be advised for these women243. However, the HYPITAT-II trial showed a significant increase in the risk of neonatal respiratory distress syndrome with immediate delivery (induction of labour or caesarean section within 24 h of randomization into trial groups) than with expectant monitoring (prolonging pregnancy until 37 weeks of gestation) in women with non-severe pre-eclampsia at 34–37 weeks of gestation, and concluded that it is generally safe and beneficial to prolong pregnancy until 37 weeks of gestation244. By contrast, the Planned early delivery or expectant monitoring for late preterm pre-eclampsia (PHOENIX)285 study showed a significantly lower incidence of the co-primary maternal outcome, a composite of maternal morbidity or recorded systolic blood pressure of >160 mmHg, and a significantly higher incidence of the co-primary perinatal outcome (composite of perinatal deaths or neonatal unit admission) in the planned delivery group than in the expectant monitoring group in women with late preterm pre-eclampsia at 34–37 weeks of gestation. The authors recommended that this trade-off between the maternal and neonatal prognosis should be discussed with patients to enable shared decision-making on the timing of delivery285,286.
Treatments for specific patient populations
Some signs and symptoms in pre-eclampsia deserve special attention, including several continuous or recurrent headaches, visual scotomas (blind spots), nausea/vomiting, epigastric pain and severe hypertension as well as changes in laboratory tests, such as increased creatinine or liver transaminases, thrombocytopenia, and altered fetal growth and fetal wellbeing tests. All of these are signs and symptoms that manifest when special conditions, such as eclampsia and HELLP syndrome, occur in pre-eclampsia. If a woman has had a chronic disease before pregnancy, especially hypertension, problems often occur during pregnancy, requiring close blood pressure control, including drug switching, from early pregnancy.
Chronic hypertension
Factors such as increasing maternal age and obesity are associated with chronic hypertension, which affects ~2% of pregnant women59. Aspirin prophylaxis against pre-eclampsia may be less effective in these women, who have a significant risk of a range of adverse maternal and perinatal outcomes, including a higher prevalence of ‘superimposed’ pre-eclampsia (pre-eclampsia complicating hypertension of another cause), FGR and placental abruption287.
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are commonly used to manage hypertension in a non-pregnant population but have been associated with adverse outcomes, including FGR, oligohydramnios, fetal renal failure and stillbirth288. Angiotensin-converting enzyme inhibitors may also be teratogenic, with increased rates of cardiac anomalies, although it is not clear whether this is independent of other risk factors (such as diabetes). Ideally, women taking these medications should be transferred to oral antihypertensive medications that have a recognized safety profile, such as methyldopa, labetalol and nifedipine, before pregnancy or by 12 weeks of gestation2.
Eclampsia
The initial management in patients with eclampsia should be the use of measures for clinical stabilization of critically ill patients (fasting, oxygenation, tongue protection with Guedel cannula, venous access, bed with raised guardrails and in a semi-sitting position). The patient should preferably be kept in a calm environment but under intense monitoring. MgSO4 is used worldwide as an anticonvulsant to stop and prevent seizures289; however, due to its increased risk of caesarean delivery and maternal adverse effects, intense clinical monitoring is required. It is reasonable therefore to restrict the use of MgSO4 to patients who have presented with eclampsia or ‘severe’ pre-eclampsia as defined by the Magpie trial (160/110 mmHg, 3 + proteinuria) or a slightly lower threshold (150/100 mmHg, 2 + proteinuria) if accompanied by two or more signs of imminent eclampsia (headache, visual symptoms, clonus)2,290. Medication administration schedules and clinical signs of magnesium intoxication should be monitored. Delivery is generally indicated when eclampsia is present, especially if the gestational age is above viability in the given clinical care setting291. If gestational viability is not yet reached, expectant monitoring can be used in cases close to viability, provided there is rigorous monitoring291.
HELLP syndrome
HELLP syndrome cases are very serious and delivery is usually indicated. In patients with uncontrolled blood pressure and without prior treatment, treatment optimization can be attempted; early reassessment of laboratory tests (within a maximum of 6 h after admission) and if there is laboratory and clinical improvement, expectant monitoring is possible291.
Postpartum management
The American Heart Association (AHA)292 considers pre-eclampsia as a significant risk factor for future cardiovascular disease. The Health after Preeclampsia Patient and Provider Engagement Network (HAPPEN) makes recommendations for awareness campaigns (for patients and primary care physicians) and yearly physical exams and laboratory evaluations for all women with prior pre-eclampsia293, including blood pressure monitoring, BMI calculation, and fasting glucose or haemoglobin A1C to assess the risk of glucose intolerance/diabetes. These recommendations are broadly in line with the AHA and ACOG recommendations25,292. A randomized control trial of women with gestational hypertension or pre-eclampsia showed that self-measurement of blood pressure for 6 months postpartum significantly reduced 24-h diastolic blood pressure at 6 months (–4.5 mmHg, 95% CI –8.1 to –0.8)294 and 3.6 years postpartum (–7.4 mmHg, 95% CI –10.7 to –4.2)295.
Quality of life
Long-term health outcomes
Effect on women with a pre-eclamptic pregnancy
It is well established that pre-eclampsia is a risk factor for long-term adverse cardiovascular, renal and cerebrovascular events. Consequently, women with a history of early-onset and late-onset pre-eclampsia have a 5-fold and 1.65-fold increased risk from cardiovascular disease-related death, respectively, compared with women without a previous history of pre-eclampsia296. Compared with women who had late-onset pre-eclampsia, women who have had early-onset pre-eclampsia are at increased risk of cardiovascular and cerebrovascular disease133,297. To improve this, a clinical programme, Heart Health 4 Moms from Brigham and Women’s Hospital (Boston, MA, USA), has recently been developed in an effort to increase awareness of preventing future cardiovascular disease293,298. Observational studies have also recorded a 5–12-fold increased risk of having end-stage renal disease for women with a previous history of pre-eclampsia299,300. Within pre-eclampsia groups, women who have had early-onset pre-eclampsia have a higher burden of kidney disease than women with late-onset pre-eclampsia297. Pre-eclampsia also leads to long-term cognitive dysfunction301,302 and neurological complications, including stroke303, cerebral white matter lesions304,305 and posterior reversible encephalopathy syndrome306. Compared with late-onset pre-eclampsia, some of the neurological symptoms are more common in early-onset pre-eclampsia304. For cognitive outcomes, it remains uncertain if there is a difference between early-onset and late-onset pre-eclampsia302. An effect of pre-eclampsia on malignancies has recently been identified307. In a meta-analysis of >5 million women, a history of pre-eclampsia was associated with a lower risk of breast cancer (RR 0.88, 95% CI 0.83–0.93) and increased risk of ovarian cancer (RR 1.82, 95% CI 1.16–2.85)307.
Effect on the child of a pre-eclamptic pregnancy
The long-term effect of pre-eclampsia on the child’s health is mainly caused by FGR and medically indicated preterm birth308; therefore, these effects are most often associated with preterm pre-eclampsia309. It has been shown that children with fetal exposure to early-onset pre-eclampsia are more susceptible to cardiovascular dysfunction310 and hypertension311. In addition, children born from severe early-onset pre-eclamptic pregnancies are at higher risk of impaired cognitive ability and neurodevelopmental outcomes312.
Psychosocial effects
Pre-eclampsia has severe negative effects on the psychosocial profile of women. Such effects are associated with the severity of pre-eclampsia, timing of diagnosis and pregnancy outcomes. Independent observational studies report that a diagnosis of severe pre-eclampsia has a worse impact on mental health and quality of life than a diagnosis of mild pre-eclampsia313,314,315,316,317. In this case, within the preterm group, diagnosis of severe pre-eclampsia before 30 weeks has worse psychosocial consequences than diagnosis at 30–34 weeks of gestation315. Three independent time-course studies have investigated psychological effects, including depression and post-traumatic stress disorder, between 6 and 12 weeks postpartum, and recorded an overall decline of effect over time316,317,318; however, the psychosocial impact of pre-eclampsia can be long lasting. For women with early-onset pre-eclampsia with preterm birth, post-traumatic stress symptoms have been recorded 7 years post pregnancy319. The psychosocial impact of pre-eclampsia is also highly associated with pregnancy outcomes. Adverse outcomes, such as perinatal death and admission to the neonatal intensive care unit, have a significant impact on the psychological wellbeing of mothers315,316.
Outlook
There are still many outstanding questions and opportunities to improve the prevention and treatment of pre-eclampsia, particularly term pre-eclampsia. A major obstacle in the field is how to detect placental dysfunction well before diagnosis so that preventive strategies to reverse placental dysfunction can be developed. Predictive biomarkers and causative pathways caused by abnormal placental development/function likely precede diagnosis and, to date, have been difficult to define even though some inroads have been made to predict and prevent preterm pre-eclampsia. One major consideration is that subtypes of pre-eclampsia, particularly preterm versus term pre-eclampsia, most likely have a different pathophysiology, yet much research does not distinguish between the two subtypes. Indeed, whether abnormal placental development and function drive term pre-eclampsia is yet to be experimentally proven. Examining placental-specific products directly through CVS or indirectly via liquid biopsies, such as microparticles, extracellular non-coding RNA and cell-free DNA, in maternal blood can provide a clue of the placental dysfunction that may occur in term pre-eclampsia.
The development of new in vitro and in vivo systems to model pre-eclampsia is required to substantially progress knowledge on pathogenesis.
Animal models
There is no ideal animal in vivo model that can completely cover all pathophysiological changes recorded in pre-eclampsia320,321 although models that mimic preterm versus term pre-eclampsia subtypes are available321. These models serve as useful strategies to identify the mechanistic basis of pre-eclampsia subtypes and test novel therapies. Existing models include spontaneous genetic models (for example, BPH/5 mice322), transgenic animals, including placental-specific transgenic rodents (for example, sFLT1 (ref. 323), human renin/angiotensin324,325, human STOX1 (ref. 326), C1q–/– (ref. 327)), surgically induced animal models (for example, rodent reduced uterine perfusion pressure328 and uterine artery ligation in baboon329), pharmacologically induced animal models (for example, Nω-nitro-L-arginine methyl ester (L-NAME)330, lipopolysaccharide331), human pre-eclamptic serum/extracellular vesicle-induced animal models332,333, or animal models induced by exogenous administration of placental-released factors (for example, sFLT1 (ref. 179)). Notably, a time frame modification of treatment enables the pharmacologically induced L-NAME model of pre-eclampsia to mimic both preterm (treatment from gestational day 4–8) and term (treatment from gestational day 8–14) pre-eclampsia334. This model has been used to determine the effects of sildenafil citrate335,336, sodium hydrosulfide337, L-arginine338, omega 3 fatty acids and vitamin E339.
Ex vivo human models
Despite substantial progress in establishing new technologies, challenges in identifying suitable therapeutic treatments remain. Maternal hypertension and proteinuria observed in pre-eclampsia are caused by increased vascular tone and resistance340. Wire myography on arteries collected from patients both with or without pre-eclampsia is used as an ex vivo approach to investigate vascular reactivity and test potential therapeutic options341, including sulforaphane342. Dual placental perfusion is another ex vivo approach commonly used to investigate pre-eclampsia within placental tissue343. Generally, term placentas are collected and fetal and maternal circulations are re-established via cannulae, with oxygen levels controlled via connection with gas exchange devices344,345. Modification of oxygen levels enables the investigation of pre-eclampsia under both normoxia and hypoxic conditions. Perfusion medium can also be modified, for instance, with the addition of haemoglobin to introduce pre-eclampsia-like injuries and supplementing with potential reagents to test therapeutic potentials345. In addition, dual placental perfusion also enables the collection of maternal perfusates, which can be used to determine released factors and their implications in pre-eclampsia346,347. Such technologies will enable insight into vascular damage that leads to pre-eclampsia.
Future directions
Single-cell sequencing is an exciting opportunity to understand the heterogeneity of cell responses during placental development. However, presently, it is difficult to determine how placental single-cell RNA sequencing data can be interpreted with respect to the syncytium as it is a multinucleated single cell that covers the whole of the placenta. Combining single-cell sequencing with spatial transcriptomics and/or proteomics may better define the molecular interactions during placental development. Multiomic analyses are also now possible with the development of new bioinformatic approaches to identify the molecular pathways and cellular interactions that are critical for both placental and pre-eclampsia development. However, what is lacking is the ability to spatially map the placental dysfunction that contributes to disease development. One way to circumvent this is to use placental organoids that have recently been developed348. Organoids can enable the modelling of disease states and may be useful in testing responses of potential therapeutics targeting the placenta. This can be further expanded in developing organoid models that incorporate immune cells, vascular cells and immune cells that are already being developed in other pathologies such as cancer.
While strides have been made in understanding the aetiology and pathogenesis of pre-eclampsia, there are still many gaps in knowledge that require collaborative efforts worldwide to make inroads. Prevention must form part of the approach to combat pre-eclampsia and its significant lifelong effects for both mother and baby. In particular, the sharing of well-characterized clinical samples and undertaking robust and large multicentre clinical trials examining potential therapeutics are required to make a significant impact in the prevention and treatment of pre-eclampsia.
Change history
03 July 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41572-023-00451-4
References
Poon, L. et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia. Int. J. Gynaecol. Obstet. 145, 1 (2019).
Magee, L. A. et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 27, 148–169 (2022). This paper outlines best-practice guidelines for diagnosis and management of pre-eclampsia.
Pittara, T., Vyrides, A., Lamnisos, D. & Giannakou, K. Pre-eclampsia and long-term health outcomes for mother and infant: an umbrella review. BJOG https://doi.org/10.1111/1471-0528.16683 (2021).
Kvalvik, L., Wilcox, A. J., Skjærven, R., Østbye, T. & Harmon, Q. E. Term complications and subsequent risk of preterm birth. BMJ 369, m1007 (2020).
Davis, E. et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129, e1552 (2012).
O’Gorman, N. et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet. Gynecol. 49, 756 (2017).
Rolnik, D. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 377, 613 (2017). A convincing large trial of aspirin to prevent pre-eclampsia in women at high risk for preterm pre-eclampsia that sparked many follow-up trials and resulted in the worldwide clinical use of aspirin to prevent preterm pre-eclampsia.
Roberts, J. M., Rich-Edwards, J. W., McElrath, T. F. & Garmire, L. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension https://doi.org/10.1161/hypertensionaha.120.14781 (2021).
Simard, J. F. et al. Evidence of under-reporting of early-onset preeclampsia using register data. Paediatr. Perinat. Epidemiol. 35, 596–600 (2021).
Tomimatsu, T. et al. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20174246 (2019).
Amaral, L. M., Cunningham, M. W. Jr, Cornelius, D. C. & LaMarca, B. Preeclampsia: long-term consequences for vascular health. Vasc. Health Risk Manag. 11, 403–415 (2015).
Paul, T. et al. Prediction of adverse maternal outcomes in preeclampsia at term. Pregnancy Hypertens. 18, 75 (2019).
Brown, M. A. et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 13, 291–310 (2018).
Abalos, E., Cuesta, C., Grosso, A. L., Chou, D. & Say, L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 1–7 (2013).
Tan, M. Y. et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet. Gynecol. 51, 743–750 (2018).
Poon, L. C. et al. ASPRE trial: incidence of preterm pre-eclampsia in patients fulfilling ACOG and NICE criteria according to risk by FMF algorithm. Ultrasound Obstet. Gynecol. 51, 738–742 (2018).
Park, F. J. et al. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust. NZ J. Obstet. Gynaecol. 53, 532–539 (2013).
Wertaschnigg, D. et al. Hypertensive disorders in pregnancy–trends over eight years: a population-based cohort study. Pregnancy Hypertens. 28, 60–65 (2022).
Lokken, E. M. et al. Pooled prevalence of adverse pregnancy and neonatal outcomes in Malawi, South Africa, Uganda, and Zimbabwe: results from a systematic review and meta-analyses to inform trials of novel HIV prevention interventions during pregnancy. Front. Reprod. Health 3, 672446 (2021).
Abalos, E. et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization multicountry survey on maternal and newborn health. BJOG 121, 14–24 (2014).
Hodgins, S. et al. A new look at care in pregnancy: simple, effective interventions for neglected populations. PLoS ONE 11, e0160562 (2016).
Khan, K. S., Wojdyla, D., Say, L., Gülmezoglu, A. M. & Van Look, P. F. WHO analysis of causes of maternal death: a systematic review. Lancet 367, 1066–1074 (2006).
Say, L. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health 2, e323–e333 (2014).
Elawad, T. et al. Risk factors for pre-eclampsia in clinical practice guidelines: comparison with the evidence. BJOG https://doi.org/10.1111/1471-0528.17320 (2022).
[No authors listed.] ACOG practice bulletin No 202: gestational hypertension and preeclampsia. Obstet. Gynecol. 133, e1–e25 (2019).
Webster, K., Fishburn, S., Maresh, M., Findlay, S. C. & Chappell, L. C. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ 366, l5119 (2019).
Heestermans, T. et al. Prognostic models for adverse pregnancy outcomes in low-income and middle-income countries: a systematic review. BMJ Glob. Health 4, e001759 (2019).
Chesley, L. C. & Cooper, D. W. Genetics of hypertension in pregnancy: possible single gene control of pre-eclampsia and eclampsia in the descendants of eclamptic women. BJOG 93, 898–908 (1986).
Arngrimsson, R. et al. Genetic and familial predisposition to eclampsia and pre-eclampsia in a defined population. Br. J. Obstet. Gynaecol. 97, 762–769 (1990).
Duckitt, K. & Harrington, D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330, 565 (2005).
Cincotta, R. B. & Brennecke, S. P. Family history of pre-eclampsia as a predictor for pre-eclampsia in primigravidas. Int. J. Gynaecol. Obstet. 60, 23–27 (1998).
Boyd, H. A., Tahir, H., Wohlfahrt, J. & Melbye, M. Associations of personal and family preeclampsia history with the risk of early-, intermediate- and late-onset preeclampsia. Am. J. Epidemiol. 178, 1611–1619 (2013).
Steinthorsdottir, V. et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat. Commun. 11, 5976–5976 (2020).
Fong, F. M. et al. Maternal genotype and severe preeclampsia: a HuGE review. Am. J. Epidemiol. 180, 335–345 (2014).
Benedetto, C. et al. Factor V Leiden and factor II G20210A in preeclampsia and HELLP syndrome. Acta Obstet. Gynecol. Scand. 81, 1095–1100 (2002).
Wang, X., Bai, T., Liu, S., Pan, H. & Wang, B. Association between thrombophilia gene polymorphisms and preeclampsia: a meta-analysis. PLoS ONE 9, e100789 (2014).
Ahmed, N. A., Hamdan, H. Z., Kamis, A. H. & Adam, I. The association of the prothrombin G20210A single-nucleotide polymorphism and the risk of preeclampsia: systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 162–169 (2020).
Nasri, F. et al. Are genetic variations in IL-1β and IL-6 cytokines associated with the risk of pre-eclampsia? Evidence from a systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. https://doi.org/10.1080/14767058.2021.1918092 (2021).
Hiby, S. E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Liu, J., Song, G., Zhao, G. & Meng, T. Gene polymorphism associated with TGF-β1 and susceptibility to preeclampsia: a meta-analysis and trial sequential analysis. J. Obstet. Gynaecol. Res. 47, 2031–2041 (2021).
Dotters-Katz, S. K. et al. Trisomy 13 and the risk of gestational hypertensive disorders: a population-based study. J. Matern. Fetal Neonatal Med. 31, 1951–1955 (2018).
Zeisler, H. et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 374, 13–22 (2016).
Feitosa, M. F. et al. Novel genetic associations for blood pressure identified via gene-alcohol interaction in up to 570K individuals across multiple ancestries. PLoS ONE 13, e0198166 (2018).
Sung, Y. J. et al. A large-scale multi-ancestry genome-wide study accounting for smoking behavior identifies multiple significant loci for blood pressure. Am. J. Hum. Genet. 102, 375–400 (2018).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Gong, J., Savitz, D. A., Stein, C. R. & Engel, S. M. Maternal ethnicity and pre-eclampsia in New York City, 1995-2003. Paediatr. Perinat. Epidemiol. 26, 45–52 (2012).
Zhang, S. et al. Racial disparities in economic and clinical outcomes of pregnancy among Medicaid recipients. Matern. Child Health J. 17, 1518–1525 (2013).
Anderson, N. H., Sadler, L. C., Stewart, A. W., Fyfe, E. M. & McCowan, L. M. Ethnicity, body mass index and risk of pre-eclampsia in a multiethnic New Zealand population. Aust. NZ J. Obstet. Gynaecol. 52, 552–558 (2012).
Ramaraj, R. & Chellappa, P. Cardiovascular risk in South Asians. Postgrad. Med. J. 84, 518–523 (2008).
Cappuccio, F. P. Ethnicity and cardiovascular risk: variations in people of African ancestry and South Asian origin. J. Hum. Hypertens. 11, 571–576 (1997).
Cappuccio, F. P., Cook, D. G., Atkinson, R. W. & Strazzullo, P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart 78, 555–563 (1997).
Arechvo, A. et al. Maternal race and preeclampsia: cohort study and systematic review with meta-analysis. BJOG https://doi.org/10.1111/1471-0528.17240 (2022).
Liu, B. et al. Reducing health inequality in Black, Asian and other minority ethnic pregnant women: impact of first trimester combined screening for placental dysfunction on perinatal mortality. BJOG 129, 1750–1756 (2022).
Bianco, A. et al. Pregnancy outcome at age 40 and older. Obstet. Gynecol. 87, 917–922 (1996).
Poon, L. C., Kametas, N. A., Chelemen, T., Leal, A. & Nicolaides, K. H. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J. Hum. Hypertens. 24, 104–110 (2010).
Mittendorf, R., Lain, K. Y., Williams, M. A. & Walker, C. K. Preeclampsia. A nested, case-control study of risk factors and their interactions. J. Reprod. Med. 41, 491–496 (1996).
Robillard, P.-Y. et al. The blurring boundaries between placental and maternal preeclampsia: a critical appraisal of 1800 consecutive preeclamptic cases. J. Matern. Fetal Neonatal Med. https://doi.org/10.1080/14767058.2020.1786516 (2020).
Conde-Agudelo, A. & Belizán, J. M. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG 107, 75–83 (2000).
Tita, A. T. et al. Treatment for mild chronic hypertension during pregnancy. N. Engl. J. Med. 386, 1781–1792 (2022).
Robillard, P. Y. et al. Increased BMI has a linear association with late-onset preeclampsia: a population-based study. PLoS ONE 14, e0223888 (2019).
Syngelaki, A., Bredaki, F. E., Vaikousi, E., Maiz, N. & Nicolaides, K. H. Body mass index at 11-13 weeks’ gestation and pregnancy complications. Fetal Diagn. Ther. 30, 250–265 (2011).
Wadhwani, P., Saha, P. K., Kalra, J. K., Gainder, S. & Sundaram, V. A study to compare maternal and perinatal outcome in early vs. late onset preeclampsia. Obstet. Gynecol. Sci. 63, 270–277 (2020).
Durst, J. K., Tuuli, M. G., Stout, M. J., Macones, G. A. & Cahill, A. G. Degree of obesity at delivery and risk of preeclampsia with severe features. Am. J. Obstet. Gynecol. 214, 651.e1–651.e5 (2016).
Martínez-Hortelano, J. A. et al. Interpregnancy weight change and hypertension during pregnancy: a systematic review and meta-analysis. Obstet. Gynecol. 135, 68–79 (2020).
Davenport, M. H. et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br. J. Sports Med. 52, 1367 (2018).
Garner, P. R., D’Alton, M. E., Dudley, D. K., Huard, P. & Hardie, M. Preeclampsia in diabetic pregnancies. Am. J. Obstet. Gynecol. 163, 505–508 (1990).
Yang, Y. & Wu, N. Gestational diabetes mellitus and preeclampsia: correlation and influencing factors. Front. Cardiovasc. Med. 9, 831297 (2022).
Al Khalaf, S. et al. Chronic kidney disease and adverse pregnancy outcomes: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 226, 656–670.e32 (2022).
Toloza, F. J. K. et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 10, 243–252 (2022).
Shinohara, D. R. et al. Pregnancy complications associated with maternal hypothyroidism: a systematic review. Obstet. Gynecol. Surv. 73, 219–230 (2018).
Vissenberg, R. et al. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Hum. Reprod. Update 18, 360–373 (2012).
Alves Junior, J. M., Bernardo, W. M., Ward, L. S. & Villagelin, D. Effect of hyperthyroidism control during pregnancy on maternal and fetal outcome: a systematic review and meta-analysis. Front. Endocrinol. 13, 800257 (2022).
Jiao, X.-F. et al. The impact of levothyroxine therapy on the pregnancy, neonatal and childhood outcomes of subclinical hypothyroidism during pregnancy: an updated systematic review, meta-analysis and trial sequential analysis. Front. Endocrinol. 13, 964084 (2022).
Stamilio, D. M., Sehdev, H. M., Morgan, M. A., Propert, K. & Macones, G. A. Can antenatal clinical and biochemical markers predict the development of severe preeclampsia. Am. J. Obstet. Gynecol. 182, 589–594 (2000).
Clowse, M. E. B., Jamison, M., Myers, E. & James, A. H. A national study of the complications of lupus in pregnancy. Am. J. Obstet. Gynecol. 199, 127.e1–127.e6 (2008).
Abou-Nassar, K., Carrier, M., Ramsay, T. & Rodger, M. A. The association between antiphospholipid antibodies and placenta mediated complications: a systematic review and meta-analysis. Thromb. Res. 128, 77–85 (2011).
Wu, J., Ma, J., Zhang, W.-H. & Di, W. Management and outcomes of pregnancy with or without lupus nephritis: a systematic review and meta-analysis. Ther. Clin. Risk Manag. 14, 885–901 (2018).
Walter, I. J. et al. Pregnancy outcome predictors in antiphospholipid syndrome: a systematic review and meta-analysis. Autoimmun. Rev. 20, 102901 (2021).
Branch, D. W., Andres, R., Digre, K. B., Rote, N. S. & Scott, J. R. The association of antiphospholipid antibodies with severe preeclampsia. Obstet. Gynecol. 73, 541–545 (1989).
Pattison, N. S., Chamley, L. W., McKay, E. J., Liggins, G. C. & Butler, W. S. Antiphospholipid antibodies in pregnancy: prevalence and clinical associations. Br. J. Obstet. Gynaecol. 100, 909–913 (1993).
Dreyfus, M. et al. Antiphospholipid antibodies and preeclampsia: a case-control study. Obstet. Gynecol. 97, 29–34 (2001).
Sletnes, K. E., Wisløff, F., Moe, N. & Dale, P. O. Antiphospholipid antibodies in pre-eclamptic women: relation to growth retardation and neonatal outcome. Acta Obstet. Gynecol. Scand. 71, 112–117 (1992).
Chen, X. et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 69, 513 (2020).
Lv, L.-J. et al. Early-onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front. Cell. Infect. Microbiol. 9, 224 (2019).
Collier, A.-R. Y., Smith, L. A. & Karumanchi, S. A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum. Immunol. 82, 362–370 (2021).
Dekker, G. A. & Sibai, B. M. Etiology and pathogenesis of preeclampsia: current concepts. Am. J. Obstet. Gynecol. 179, 1359–1375 (1998).
Robillard, P. Y. et al. Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J. Reprod. Immunol. 24, 1–12 (1993).
Skjaerven, R., Wilcox, A. J. & Lie, R. T. The interval between pregnancies and the risk of preeclampsia. N. Engl. J. Med. 346, 33–38 (2002).
Keukens, A., van Wely, M., van der Meulen, C. & Mochtar, M. H. Pre-eclampsia in pregnancies resulting from oocyte donation, natural conception or IVF: a systematic review and meta-analysis. Hum. Reprod. 37, 586–599 (2022).
Pohjonen, E. M. et al. Obstetric and perinatal risks after the use of donor sperm: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 274, 210–228 (2022).
Narang, K. & Szymanski, L. M. Multiple gestations and hypertensive disorders of pregnancy: what do we know? Curr. Hypertens. Rep. 23, 1 (2020).
Jhee, J. H. et al. Prediction model development of late-onset preeclampsia using machine learning-based methods. PLoS ONE 14, e0221202 (2019).
Campbell, D. M., MacGillivray, I. & Carr-Hill, R. Pre-eclampsia in second pregnancy. Br. J. Obstet. Gynaecol. 92, 131–140 (1985).
Lee, C. J. et al. Risk factors for pre-eclampsia in an Asian population. Int. J. Gynaecol. Obstet. 70, 327–333 (2000).
Mostello, D., Catlin, T. K., Roman, L., Holcomb, W. L. Jr & Leet, T. Preeclampsia in the parous woman: who is at risk? Am. J. Obstet. Gynecol. 187, 425–429 (2002).
Robillard, P. Y. et al. Validation of the 34-week gestation as definition of late onset preeclampsia: testing different cutoffs from 30 to 37 weeks on a population-based cohort of 1700 preeclamptics. Acta Obstet. Gynecol. Scand. 99, 1181–1190 (2020).
van Oostwaard, M. F. et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am. J. Obstet. Gynecol. 212, 624.e1–624.e17 (2015).
Bhattacharya, S., McLernon, D. J., Lee, A. J. & Bhattacharya, S. Reproductive outcomes following ectopic pregnancy: register-based retrospective cohort study. PLoS Med. 9, e1001243 (2012).
Sibai, B. M. et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am. J. Obstet. Gynecol. 177, 1003–1010 (1997).
Aksornphusitaphong, A. & Phupong, V. Risk factors of early and late onset pre-eclampsia. J. Obstet. Gynaecol. Res. 39, 627–631 (2013).
Opdahl, S. et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum. Reprod. 30, 1724–1731 (2015).
von Versen-Höynck, F. et al. Absent or excessive corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertension 73, 680–690 (2019).
Shi, Y. et al. Transfer of fresh versus frozen embryos in ovulatory women. N. Engl. J. Med. 378, 126–136 (2018).
Saito, K. et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum. Reprod. 34, 1567–1575 (2019).
Garrido-Gómez, T., Castillo-Marco, N., Cordero, T. & Simón, C. Decidualization resistance in the origin of preeclampsia. Am. J. Obstet. Gynecol. 226, S886–S894 (2022).
Conde-Agudelo, A. & Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 226, 68–89.e63 (2022).
Yaghoobpoor, S. et al. Cardiovascular complications of COVID-19 among pregnant women and their fetuses: a systematic review. J. Clin. Med. 11, 6194 (2022).
Mullins, E. et al. Pregnancy and neonatal outcomes of COVID-19: the PAN-COVID study. Eur. J. Obstetr. Gynecol. Reprod. Biol. 276, 161–167 (2022).
Getahun, D. et al. Association between SARS-CoV-2 infection and adverse perinatal outcomes in a large health maintenance organization. Am. J. Perinatol. https://doi.org/10.1055/s-0042-1749666 (2022).
Snelgrove, J. W. et al. Preeclampsia and severe maternal morbidity during the COVID-19 pandemic: a population-based cohort study in Ontario, Canada. J. Obstet. Gynaecol. Can. 44, 777–784 (2022).
Tossetta, G. et al. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: a systematic review. J. Hypertens. 40, 1629–1638 (2022).
Zamudio, S. High-altitude hypoxia and preeclampsia. Front. Biosci. 12, 2967–2977 (2007).
Moore, L. G. Hypoxia and reproductive health: reproductive challenges at high altitude: fertility, pregnancy and neonatal well-being. Reproduction 161, F81–F90 (2021).
Nieves-Colón, M. A. et al. Clotting factor genes are associated with preeclampsia in high-altitude pregnant women in the Peruvian Andes. Am. J. Hum. Genet. 109, 1117–1139 (2022).
Mandakh, Y. et al. Maternal exposure to ambient air pollution and risk of preeclampsia: a population-based cohort study in Scania, Sweden. Int. J. Env. Res. Public Health 17, 1744 (2020).
Bearblock, E., Aiken, C. E. & Burton, G. J. Air pollution and pre-eclampsia; associations and potential mechanisms. Placenta 104, 188–194 (2021).
Gogna, P., Villeneuve, P. J., Borghese, M. M. & King, W. D. An exposure-response meta-analysis of ambient PM2.5 during pregnancy and preeclampsia. Environ. Res. 210, 112934 (2022).
Pijnenborg, R., Dixon, G., Robertson, W. B. & Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1, 3–19 (1980).
Evans, J. et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 12, 654–667 (2016).
Lunghi, L., Ferretti, M., Medici, S., Biondi, C. & Vesce, F. Control of human trophoblast function. Reprod. Biol. Endocrinol. 5, 6 (2007).
Pijnenborg, R., Bland, J. M., Robertson, W. B. & Brosens, I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4, 397–413 (1983).
Menkhorst, E. et al. Invasive trophoblast promote stromal fibroblast decidualization via Profilin 1 and ALOX5. Sci. Rep. 7, 8690 (2017).
Deryabin, P., Griukova, A., Nikolsky, N. & Borodkina, A. The link between endometrial stromal cell senescence and decidualization in female fertility: the art of balance. Cell. Mol. Life Sci. 77, 1357–1370 (2020).
Burton, G. J., Jauniaux, E. & Charnock-Jones, D. S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54, 303–312 (2010).
Lash, G. E. et al. Interaction between uterine natural killer cells and extravillous trophoblast cells: effect on cytokine and angiogenic growth factor production. Hum. Reprod. 26, 2289–2295 (2011).
Burton, G. J. & Jauniaux, E. The cytotrophoblastic shell and complications of pregnancy. Placenta 60, 134–139 (2017).
Aplin, J. D., Myers, J. E., Timms, K. & Westwood, M. Tracking placental development in health and disease. Nat. Rev. Endocrinol. 16, 479–494 (2020).
Osol, G., Ko, N. L. & Mandalà, M. Plasticity of the maternal vasculature during pregnancy. Annu. Rev. Physiol. 81, 89–111 (2019).
Kumar, S., Gordon, G. H., Abbott, D. H. & Mishra, J. S. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction 156, R155–R167 (2018).
Lumbers, E. R., Delforce, S. J., Arthurs, A. L. & Pringle, K. G. Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front. Endocrinol. 10, 563 (2019).
Pereira, M. M., Torrado, J., Sosa, C., Zócalo, Y. & Bia, D. Role of arterial impairment in preeclampsia: should the paradigm shift? Am. J. Physiol. Heart Circ. Physiol. 320, H2011–H2030 (2021).
Johnson, A. C. in Handbook of Clinical Neurology Vol. 171 (eds Steegers, E. A. P., Cipolla, M. J. & Miller, E. C.) 85–96 (Elsevier, 2020).
Phipps, E., Prasanna, D., Brima, W. & Jim, B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin. J. Am. Soc. Nephrol. 11, 1102–1113 (2016).
Aneman, I. et al. Mechanisms of key innate immune cells in early- and late-onset preeclampsia. Front. Immunol. 11, 1864 (2020).
Redman, C. W. G., Staff, A. C. & Roberts, J. M. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2020.09.047 (2020). A comprehensive review of our understanding of the aetiology of pre-eclampsia that encompasses both preterm and term physiology.
Staff, A. C. The two-stage placental model of preeclampsia: an update. J. Reprod. Immunol. 134–135, 1–10 (2019).
Redman, C. W., Sargent, I. L. & Staff, A. C. IFPA senior award lecture: making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta 35 (Suppl.), S20–S25 (2014).
Hu, X. Q. & Zhang, L. Mitochondrial dysfunction in the pathogenesis of preeclampsia. Curr. Hypertens. Rep. 24, 157–172 (2022).
Pankiewicz, K., Fijałkowska, A., Issat, T. & Maciejewski, T. M. Insight into the key points of preeclampsia pathophysiology: uterine artery remodeling and the role of microRNAs. Int. J. Mol. Sci. 22, 3132 (2021).
Burton, G. J., Yung, H. W., Cindrova-Davies, T. & Charnock-Jones, D. S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 30 (Suppl. A), S43–S48 (2009).
Staff, A. C. et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2020.09.026 (2020).
Kroll, J. & Waltenberger, J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem. Biophys. Res. Commun. 252, 743–746 (1998).
Mills, J. L. et al. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA 282, 356–362 (1999).
Burton, G. J., Redman, C. W., Roberts, J. M. & Moffett, A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 366, l2381 (2019).
Lyall, F., Robson, S. C. & Bulmer, J. N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 62, 1046–1054 (2013).
Gerretsen, G., Huisjes, H. J. & Elema, J. D. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br. J. Obstet. Gynaecol. 88, 876–881 (1981).
Robertson, W. B., Brosens, I. & Dixon, G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect. Nephrol. Hypertens. 5, 115–127 (1976). This publication identified that spiral artery remodelling was impaired in preterm pre-eclampsia.
Brosens, I., Dixon, H. G. & Robertson, W. B. Fetal growth retardation and the arteries of the placental bed. Br. J. Obstet. Gynaecol. 84, 656–663 (1977).
Redman, C. W. & Staff, A. C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obstet. Gynecol. 213 (Suppl. 4), S9.e1–S9.e4 (2015).
O’Gorman, N. et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet. Gynecol. 49, 751–755 (2017).
Saito, S., Sakai, M., Sasaki, Y., Nakashima, A. & Shiozaki, A. Inadequate tolerance induction may induce pre-eclampsia. J. Reprod. Immunol. 76, 30–39 (2007).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Hosseini, A., Dolati, S., Hashemi, V., Abdollahpour-Alitappeh, M. & Yousefi, M. Regulatory T and T helper 17 cells: their roles in preeclampsia. J. Cell. Physiol. 233, 6561–6573 (2018).
Zolfaghari, M. A. et al. T lymphocytes and preeclampsia: The potential role of T-cell subsets and related MicroRNAs in the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 86, e13475 (2021).
Sargent, I. L., Borzychowski, A. M. & Redman, C. W. NK cells and human pregnancy — an inflammatory view. Trends Immunol. 27, 399–404 (2006).
Robertson, S. A. & Sharkey, D. J. Seminal fluid and fertility in women. Fertil. Steril. 106, 511–519 (2016).
Martínez-Varea, A., Pellicer, B., Perales-Marín, A. & Pellicer, A. Relationship between maternal immunological response during pregnancy and onset of preeclampsia. J. Immunol. Res. 2014, 210241 (2014).
Lei, J. et al. The prognostic role of angiotensin II type 1 receptor autoantibody in non-gravid hypertension and pre-eclampsia: a meta-analysis and our studies. Medicine 95, e3494 (2016).
Melchiorre, K., Sutherland, G. R., Baltabaeva, A., Liberati, M. & Thilaganathan, B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 57, 85–93 (2011).
Melchiorre, K., Giorgione, V. & Thilaganathan, B. The placenta and preeclampsia: villain or victim? Am. J. Obstet. Gynecol. 226, S954–S962 (2022).
Thilaganathan, B. & Kalafat, E. Cardiovascular system in preeclampsia and beyond. Hypertension 73, 522–531 (2019).
Thilaganathan, B. Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet. Gynecol. 51, 714–717 (2018).
Hu, M., Li, J., Baker, P. N. & Tong, C. Revisiting preeclampsia: a metabolic disorder of the placenta. FEBS J. 289, 336–354 (2022).
Bahado-Singh, R. O. et al. Metabolomic determination of pathogenesis of late-onset preeclampsia. J. Matern. Fetal Neonatal Med. 30, 658–664 (2017).
Farina, A. et al. Gene expression in chorionic villous samples at 11 weeks’ gestation from women destined to develop preeclampsia. Prenat. Diagn. 28, 956–961 (2008).
Founds, S. A. et al. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 30, 15–24 (2009).
Rabaglino, M. B. & Conrad, K. P. Evidence for shared molecular pathways of dysregulated decidualization in preeclampsia and endometrial disorders revealed by microarray data integration. FASEB J. 33, 11682–11695 (2019).
Jia, K., Ma, L., Wu, S. & Yang, W. Serum levels of complement factors C1q, Bb, and H in normal pregnancy and severe pre-eclampsia. Med. Sci. Monit. 25, 7087–7093 (2019).
Vennou, K. E., Kontou, P. I., Braliou, G. G. & Bagos, P. G. Meta-analysis of gene expression profiles in preeclampsia. Pregnancy Hypertens. 19, 52–60 (2020).
Ren, Z. et al. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics 11, 5028–5044 (2021).
Saei, H., Govahi, A., Abiri, A., Eghbali, M. & Abiri, M. Comprehensive transcriptome mining identified the gene expression signature and differentially regulated pathways of the late-onset preeclampsia. Pregnancy Hypertens. 25, 91–102 (2021).
Than, N. G. et al. Early pathways, biomarkers, and four distinct molecular subclasses of preeclampsia: the intersection of clinical, pathological, and high-dimensional biology studies. Placenta 125, 10–19 (2022).
Michalczyk, M., Celewicz, A., Celewicz, M., Woźniakowska-Gondek, P. & Rzepka, R. The role of inflammation in the pathogenesis of preeclampsia. Mediators Inflamm. 2020, 3864941 (2020).
George, E. M. & Granger, J. P. Recent insights into the pathophysiology of preeclampsia. Expert Rev. Obstet. Gynecol. 5, 557–566 (2010).
Matsubara, K., Matsubara, Y., Uchikura, Y. & Sugiyama, T. Pathophysiology of preeclampsia: the role of exosomes. Int. J. Mol. Sci. 22, 2572 (2021).
Wang, Z., Zhao, G., Zeng, M., Feng, W. & Liu, J. Overview of extracellular vesicles in the pathogenesis of preeclampsia. Biol. Reprod. 105, 32–39 (2021).
Flint, E. J., Cerdeira, A. S., Redman, C. W. & Vatish, M. The role of angiogenic factors in the management of preeclampsia. Acta Obstet. Gynecol. Scand. 98, 700–707 (2019).
Verlohren, S. et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 202, 161.e1–161.e11 (2010).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003). Publication demonstrating the importance of sFLT1 in the aetiology of pre-eclampsia.
Venkatesha, S. et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649 (2006).
Margioula-Siarkou, G. et al. Soluble endoglin concentration in maternal blood as a diagnostic biomarker of preeclampsia: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 258, 366–381 (2021).
Murthi, P., Pinar, A. A., Dimitriadis, E. & Samuel, C. S. Inflammasomes-a molecular link for altered immunoregulation and inflammation mediated vascular dysfunction in preeclampsia. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21041406 (2020).
Silber, M. et al. Inflammasome activation in preeclampsia and intrauterine growth restriction. Am. J. Reprod. Immunol. 88, e13598 (2022).
Guo, H., Callaway, J. B. & Ting, J. P. Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Nunes, P. R., Mattioli, S. V. & Sandrim, V. C. NLRP3 Activation and its relationship to endothelial dysfunction and oxidative stress: implications for preeclampsia and pharmacological interventions. Cells https://doi.org/10.3390/cells10112828 (2021).
Winship, A. L. et al. Interleukin-11 alters placentation and causes preeclampsia features in mice. Proc. Natl Acad. Sci. USA 112, 15928–15933 (2015).
Menkhorst, E. et al. Medawar’s PostEra: galectins emerged as key players during fetal-maternal glycoimmune adaptation. Front. Immunol. 12, 784473 (2021).
Menkhorst, E. et al. Galectin-7 impairs placentation and causes preeclampsia features in mice. Hypertension 76, 1185–1194 (2020).
Menkhorst, E. et al. Galectin-7 dysregulates renin-angiotensin-aldosterone and NADPH oxide synthase pathways in preeclampsia. Pregnancy Hypertens. https://doi.org/10.1016/j.preghy.2022.09.008 (2022).
Menkhorst, E., Koga, K., Van Sinderen, M. & Dimitriadis, E. Galectin-7 serum levels are altered prior to the onset of pre-eclampsia. Placenta 35, 281–285 (2014).
Pillay, P., Maharaj, N., Moodley, J. & Mackraj, I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 46, 18–25 (2016).
Ayala-Ramírez, P. et al. Assessment of placental extracellular vesicles-associated fas ligand and TNF-related apoptosis-inducing ligand in pregnancies complicated by early and late onset preeclampsia. Front. Physiol. 12, 708824 (2021).
Chen, Y., Huang, Y., Jiang, R. & Teng, Y. Syncytiotrophoblast-derived microparticle shedding in early-onset and late-onset severe pre-eclampsia. Int. J. Gynaecol. Obstet. 119, 234–238 (2012).
Feng, Y. et al. The blocking of integrin-mediated interactions with maternal endothelial cells reversed the endothelial cell dysfunction induced by EVs, derived from preeclamptic placentae. Int. J. Mol. Sci. https://doi.org/10.3390/ijms232113115 (2022).
Murugesan, S. et al. Extracellular vesicles from women with severe preeclampsia impair vascular endothelial function. Anesth. Analg. 134, 713–723 (2022).
Cronqvist, T. et al. Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells. Sci. Rep. 7, 4558–4558 (2017).
Palei, A. C., Spradley, F. T., Warrington, J. P., George, E. M. & Granger, J. P. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol. 208, 224–233 (2013).
Armaly, Z., Jadaon, J. E., Jabbour, A. & Abassi, Z. A. Preeclampsia: novel mechanisms and potential therapeutic approaches. Front. Physiol. 9, 973 (2018).
Gyselaers, W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 226, S988–S1005 (2022).
Örgül, G. et al. First trimester complete blood cell indices in early and late onset preeclampsia. Turk. J. Obstet. Gynecol. 16, 112–117 (2019).
Sciscione, A. C. et al. Acute pulmonary edema in pregnancy. Obstet. Gynecol. 101, 511–515 (2003).
Pordeus, A. C. B., Katz, L., Soares, M. C., Maia, S. B. & Amorim, M. M. R. Acute pulmonary edema in an obstetric intensive care unit: a case series study. Medicine 97, e11508 (2018).
Pretorius, T., van Rensburg, G., Dyer, R. A. & Biccard, B. M. The influence of fluid management on outcomes in preeclampsia: a systematic review and meta-analysis. Int. J. Obstet. Anesth. 34, 85–95 (2018).
Cypher, R. L. Pulmonary edema in obstetrics: essential facts for critical care nurses. AACN Adv. Crit. Care 29, 327–335 (2018).
Stillman, I. E. & Karumanchi, S. A. The glomerular injury of preeclampsia. J. Am. Soc. Nephrol. 18, 2281–2284 (2007).
Garovic, V. D. et al. Urinary podocyte excretion as a marker for preeclampsia. Am. J. Obstet. Gynecol. 196, 320.e1–320.e7 (2007).
Collino, F. et al. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am. J. Physiol. Ren. Physiol. 294, F1185–F1194 (2008).
Magee, L. A., Nicolaides, K. H. & von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 386, 1817–1832 (2022).
Steingrub, J. S. Pregnancy-associated severe liver dysfunction. Crit. Care Clin. 20, 763–776 (2004).
Mikolasevic, I. et al. Liver disease during pregnancy: a challenging clinical issue. Med. Sci. Monit. 24, 4080–4090 (2018).
Haram, K., Svendsen, E. & Abildgaard, U. The HELLP syndrome: clinical issues and management. A review. BMC Pregnancy Childbirth 9, 8 (2009).
Sibai, B. M. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing. Am. J. Obstet. Gynecol. 162, 311–316 (1990).
Miller, E. C. & Vollbracht, S. Neurology of preeclampsia and related disorders: an update in neuro-obstetrics. Curr. Pain Headache Rep. 25, 40 (2021).
Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33, 130–137 (2009).
Miller, E. C. Preeclampsia and cerebrovascular disease. Hypertension 74, 5–13 (2019).
Yousif, D. et al. Autonomic dysfunction in preeclampsia: a systematic review. Front. Neurol. 10, 816 (2019).
Mahendra, V., Clark, S. L. & Suresh, M. S. Neuropathophysiology of preeclampsia and eclampsia: a review of cerebral hemodynamic principles in hypertensive disorders of pregnancy. Pregnancy Hypertens. 23, 104–111 (2021).
Andersson, M. et al. Signs of neuroaxonal injury in preeclampsia — a case control study. PLoS ONE 16, e0246786 (2021).
Nardozza, L. M. M. et al. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet. 295, 1061–1077 (2017).
Takahashi, M. et al. Fetal growth restriction as the initial finding of preeclampsia is a clinical predictor of maternal and neonatal prognoses: a single-center retrospective study. BMC Pregnancy Childbirth 21, 1–8 (2021).
Wojtowicz, A. et al. Early-and late-onset preeclampsia: a comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int. J. Hypertens. 2019, 4108271 (2019).
Madazli, R. et al. Comparison of clinical and perinatal outcomes in early-and late-onset preeclampsia. Arch. Gynecol. Obstet. 290, 53–57 (2014).
Egbor, M., Ansari, T., Morris, N., Green, C. & Sibbons, P. Maternal medicine: morphometric placental villous and vascular abnormalities in early‐and late‐onset pre‐eclampsia with and without fetal growth restriction. BJOG 113, 580–589 (2006).
Brown, M. A., Lindheimer, M. D., de Swiet, M., Van Assche, A. & Moutquin, J. M. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens. Pregnancy 20, ix–xiv (2001).
Lai, J., Syngelaki, A., Nicolaides, K. H., von Dadelszen, P. & Magee, L. A. Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes. Am. J. Obstet. Gynecol. 224, 518.e1–518.e11 (2021).
Reddy, M. et al. The impact of the definition of preeclampsia on disease diagnosis and outcomes: a retrospective cohort study. Am. J. Obstet. Gynecol. 224, 217.e1–217.e11 (2021).
Sinkey, R. G. et al. Prevention, diagnosis, and management of hypertensive disorders of pregnancy: a comparison of international guidelines. Curr. Hypertens. Rep. 22, 66–66 (2020).
Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet 398, 341–354 (2021).
Agrawal, S., Cerdeira, A. S., Redman, C. & Vatish, M. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension 71, 306–316 (2018).
Veisani, Y., Jenabi, E., Delpisheh, A. & Khazaei, S. Angiogenic factors and the risk of preeclampsia: a systematic review and meta-analysis. Int. J. Reprod. Biomed. 17, 1–10 (2019).
Duhig, K. E. et al. Prognostic indicators of severe disease in late preterm pre-eclampsia to guide decision making on timing of delivery: the PEACOCK study. Pregnancy Hypertens. 24, 90–95 (2021).
Lim, S. et al. Biomarkers and the prediction of adverse outcomes in preeclampsia: a systematic review and meta-analysis. Obstet. Gynecol. 137, 72–81 (2021).
Liu, Y. et al. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Arch. Gynecol. Obstet. 292, 507–518 (2015).
Huhn, E. A. et al. Diagnostic accuracy of different soluble fms-like tyrosine kinase 1 and placental growth factor cut-off values in the assessment of preterm and term preeclampsia: a gestational age matched case-control study. Front. Med. 5, 325–325 (2018).
Duhig, K. E. et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 393, 1807–1818 (2019).
Ovayolu, A. et al. Measuring the status of maternal serum thiol/disulfide couples in the diagnosis and/or the determination of the severity of late-onset preeclampsia. J. Matern. Fetal Neonatal Med. https://doi.org/10.1080/14767058.2021.1904393 (2021).
Cheng, S. et al. Novel blood test for early biomarkers of preeclampsia and Alzheimer’s disease. Sci. Rep. 11, 15934–15934 (2021).
Uzun, N., Sarııbrahım Astepe, B., Uzun, F. & Kale, E. Predictive value of maternal serum podocalyxin in the diagnosis of preeclampsia: a prospective case-control study. Ginekol. Pol. https://doi.org/10.5603/GP.a2021.0108 (2021).
Sibai, B. M. et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am. J. Obstet. Gynecol. 169, 1000–1006 (1993).
Douglas, K. A. & Redman, C. W. Eclampsia in the United Kingdom. BMJ 309, 1395–1400 (1994).
Zwertbroek, E. F. et al. Prediction of progression to severe disease in women with late preterm hypertensive disorders of pregnancy. Acta Obstet. Gynecol. Scand. 96, 96–105 (2017).
von Dadelszen, P. et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet 377, 219–227 (2011).
Koopmans, C. M. et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet 374, 979–988 (2009).
Broekhuijsen, K. et al. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet 385, 2492–2501 (2015).
Bian, X. et al. Short-term prediction of adverse outcomes using the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor) ratio in Asian women with suspected preeclampsia. Hypertension 74, 164–172 (2019).
Reddy, M. et al. Role of placental, fetal and maternal cardiovascular markers in predicting adverse outcome in women with suspected or confirmed pre-eclampsia. Ultrasound Obstet. Gynecol. 59, 596–605 (2022).
Ukah, U. V. et al. Assessment of the fullPIERS risk prediction model in women with early-onset preeclampsia. Hypertension 71, 659–665 (2018).
O’Gorman, N. et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 214, 103.e1–103.e12 (2016).
Tan, M. Y. et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet. Gynecol. 52, 186–195 (2018).
Wright, D., Wright, A. & Nicolaides, K. H. The competing risk approach for prediction of preeclampsia. Am. J. Obstet. Gynecol. 223, 12–23.e17 (2020).
Poon, L. C. et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 145 (Suppl. 1), 1–33 (2019).
Rolnik, D. L. et al. Routine first trimester combined screening for preterm preeclampsia in Australia: a multicenter clinical implementation cohort study. Int. J. Gynaecol. Obstet. https://doi.org/10.1002/ijgo.14049 (2021).
Park, F., Deeming, S., Bennett, N. & Hyett, J. Cost-effectiveness analysis of a model of first-trimester prediction and prevention of preterm pre-eclampsia compared with usual care. Ultrasound Obstet. Gynecol. 58, 688–697 (2021).
Sotiriadis, A. et al. ISUOG Practice Guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet. Gynecol. 53, 7–22 (2019).
Litwinska, M., Syngelaki, A., Wright, A., Wright, D. & Nicolaides, K. H. Management of pregnancies after combined screening for pre-eclampsia at 19–24 weeks’ gestation. Ultrasound Obstet. Gynecol. 52, 365–372 (2018).
Black, C. et al. Prediction of preterm pre-eclampsia at midpregnancy using a multivariable screening algorithm. Aust. NZ J. Obstet. Gynaecol. 60, 675–682 (2020).
Wright, D., Dragan, I., Syngelaki, A., Akolekar, R. & Nicolaides, K. H. Proposed clinical management of pregnancies after combined screening for pre-eclampsia at 30–34 weeks’ gestation. Ultrasound Obstet. Gynecol. 49, 194–200 (2017).
Panaitescu, A. et al. Screening for pre-eclampsia at 35–37 weeks’ gestation. Ultrasound Obstet. Gynecol. 52, 501–506 (2018).
Teoh, S. S. Y. et al. Low serum levels of HtrA3 at 15 weeks of gestation are associated with late-onset preeclampsia development and small for gestational age birth. Fetal Diagn. Ther. 46, 392–401 (2019).
Rasmussen, M. et al. RNA profiles reveal signatures of future health and disease in pregnancy. Nature 601, 422–427 (2022). This paper demonstrates that circulating placental RNA can predict pre-eclampsia risk, which has exciting potential as a predictive biomarker.
Moufarrej, M. N. et al. Early prediction of preeclampsia in pregnancy with cell-free RNA. Nature 602, 689–694 (2022).
Panaitescu, B. et al. ELABELA plasma concentrations are increased in women with late-onset preeclampsia. J. Matern. Fetal Neonatal Med. 33, 5–15 (2020).
Alici Davutoğlu, E. et al. Evaluation of maternal serum hypoxia inducible factor-1α, progranulin and syndecan-1 levels in pregnancies with early- and late-onset preeclampsia. J. Matern. Fetal Neonatal Med. 31, 1976–1982 (2018).
Beaufils, M., Uzan, S., Donsimoni, R. & Colau, J. C. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet 1, 840–842 (1985).
Hauth, J. C. et al. Low-dose aspirin therapy to prevent preeclampsia. Am. J. Obstet. Gynecol. 168, 1083–1091 (1993).
Imperiale, T. F. & Petrulis, A. S. A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. JAMA 266, 260–264 (1991).
Wallenburg, H. C. S., Makovitz, J. W., Dekker, G. A. & Rotmans, P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet 327, 1–3 (1986).
Askie, L. M., Duley, L., Henderson-Smart, D. J. & Stewart, L. A. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 369, 1791–1798 (2007).
Kinshella, M.-L. W. et al. Calcium for pre-eclampsia prevention: a systematic review and network meta-analysis to guide personalised antenatal care. BJOG https://doi.org/10.1111/1471-0528.17222 (2022).
Grobman, W. A. et al. Labor induction versus expectant management in low-risk nulliparous women. N. Engl. J. Med. 379, 513–523 (2018).
Aldika Akbar, M. I. et al. INOVASIA study: a multicenter randomized clinical trial of pravastatin to prevent preeclampsia in high risk patients. Am. J. Perinatol. https://doi.org/10.1055/a-1798-1925 (2022).
Döbert, M. et al. Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation 144, 670–679 (2021).
Tarry-Adkins, J. L., Ozanne, S. E. & Aiken, C. E. Impact of metformin treatment during pregnancy on maternal outcomes: a systematic review/meta-analysis. Sci. Rep. 11, 9240 (2021).
Syngelaki, A. et al. Metformin versus placebo in obese pregnant women without diabetes mellitus. N. Engl. J. Med. 374, 434–443 (2016).
Irwinda, R., Hiksas, R., Lokeswara, A. W. & Wibowo, N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal-fetal outcome: systematic review and meta-analysis. Womens Health 18, 17455057221111066 (2022).
Cruz-Lemini, M., Vázquez, J. C., Ullmo, J. & Llurba, E. Low-molecular-weight heparin for prevention of preeclampsia and other placenta-mediated complications: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 226, S1126–S1144.e17 (2022).
Sibai, B. M. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am. J. Obstet. Gynecol. 205, 191–198 (2011).
Easterling, T. et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet 394, 1011–1021 (2019).
Nij Bijvank, S. W. et al. Nicardipine for treating severe antepartum hypertension during pregnancy: Nine years of experience in more than 800 women. Acta Obstet. Gynecol. Scand. https://doi.org/10.1111/aogs.14406 (2022).
Impey, L. Severe hypotension and fetal distress following sublingual administration of nifedipine to a patient with severe pregnancy induced hypertension at 33 weeks. Br. J. Obstet. Gynaecol. 100, 959–961 (1993).
Magee, L. A., Namouz-Haddad, S., Cao, V., Koren, G. & von Dadelszen, P. Labetalol for hypertension in pregnancy. Expert Opin. Drug Saf. 14, 453–461 (2015).
Wiciński, M., Malinowski, B., Puk, O., Socha, M. & Słupski, M. Methyldopa as an inductor of postpartum depression and maternal blues: a review. Biomed. Pharmacother. 127, 110196 (2020).
Magee, L. A. et al. Less-tight versus tight control of hypertension in pregnancy. N. Engl. J. Med. 372, 407–417 (2015).
Abalos, E., Duley, L., Steyn, D. W. & Gialdini, C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst. Rev. 10, CD002252 (2018).
Chappell, L. C. et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet 394, 1181–1190 (2019).
Beardmore-Gray, A. et al. Planned delivery or expectant management in preeclampsia: an individual participant data meta-analysis. Am. J. Obstet. Gynecol. 227, 218–230.e8 (2022).
Poon, L. C. et al. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am. J. Obstet. Gynecol. 217, 585.e1–585.e5 (2017).
Bullo, M., Tschumi, S., Bucher, B. S., Bianchetti, M. G. & Simonetti, G. D. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension 60, 444–450 (2012).
Duley, L. & Gulmezoglu, A. M. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.Cd002960 (2001).
Altman, D. et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie trial: a randomised placebo-controlled trial. Lancet 359, 1877–1890 (2002).
Lam, M. T. C. & Dierking, E. Intensive care unit issues in eclampsia and HELLP syndrome. Int. J. Crit. Illn. Inj. Sci. 7, 136–141 (2017).
Mosca, L. et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation 123, 1243–1262 (2011).
Seely, E. W. et al. Cardiovascular health after preeclampsia: patient and provider perspective. J. Womens Health 30, 305–313 (2021).
Cairns, A. E. et al. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertension 72, 425–432 (2018).
Kitt, J. A. et al. Short-term postpartum blood pressure self-management and long-term blood pressure control: a randomized controlled trial. Hypertension 78, 469–479 (2021).
Mongraw-Chaffin, M. L., Cirillo, P. M. & Cohn, B. A. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 56, 166–171 (2010).
Dall’Asta, A. et al. Cardiovascular events following pregnancy complicated by pre‐eclampsia with emphasis on comparison between early‐and late‐onset forms: systematic review and meta‐analysis. Ultrasound Obstet. Gynecol. 57, 698–709 (2021).
Rich-Edwards, J. W. et al. Randomized trial to reduce cardiovascular risk in women with recent preeclampsia. J. Womens Health 28, 1493–1504 (2019).
Paauw, N. D., Luijken, K., Franx, A., Verhaar, M. C. & Lely, A. T. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin. Sci. 130, 239–246 (2016).
Vikse, B. E., Irgens, L. M., Leivestad, T., Skjærven, R. & Iversen, B. M. Preeclampsia and the risk of end-stage renal disease. N. Engl. J. Med. 359, 800–809 (2008).
Postma, I. R. et al. Cerebral white matter lesions, subjective cognitive failures, and objective neurocognitive functioning: a follow-up study in women after hypertensive disorders of pregnancy. J. Clin. Exp. Neuropsychol. 38, 585–598 (2016).
Nuckols, V. R. et al. Twenty-four-hour blood pressure variability is associated with lower cognitive performance in young women with a recent history of preeclampsia. Am. J. Hypertens. 34, 1291–1299 (2021).
Bellamy, L., Casas, J.-P., Hingorani, A. D. & Williams, D. J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335, 974 (2007).
Aukes, A. et al. Long‐term cerebral imaging after pre‐eclampsia. BJOG 119, 1117–1122 (2012).
Hammer, E. S. & Cipolla, M. J. Cerebrovascular dysfunction in preeclamptic pregnancies. Curr. Hypertens. Rep. 17, 1–8 (2015).
Postma, I. R., Slager, S., Kremer, H. P., de Groot, J. C. & Zeeman, G. G. Long-term consequences of the posterior reversible encephalopathy syndrome in eclampsia and preeclampsia: a review of the obstetric and nonobstetric literature. Obstet. Gynecol. Surv. 69, 287–300 (2014).
Wang, F., Zhang, W., Cheng, W., Huo, N. & Zhang, S. Preeclampsia and cancer risk in women in later life: a systematic review and meta-analysis of cohort studies. Menopause 28, 1070–1078 (2021).
Benagiano, M., Mancuso, S., Brosens, J. J. & Benagiano, G. Long-term consequences of placental vascular pathology on the maternal and offspring cardiovascular systems. Biomolecules 11, 1625 (2021).
Williams, D. Long-term complications of preeclampsia. Semin. Nephrol. 31, 111–122 (2011).
Hoodbhoy, Z. et al. Cardiovascular dysfunction in children exposed to preeclampsia during fetal life. J. Am. Soc. Echocardiogr. 34, 653–661 (2021).
Lazdam, M. et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension 60, 1338–1345 (2012).
van Wassenaer, A. G. et al. Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 204, e1–e9 (2011).
Stern, C. et al. The impact of severe preeclampsia on maternal quality of life. Qual. Life Res. 23, 1019–1026 (2014).
Cetin, O., Guzel Ozdemir, P., Kurdoglu, Z. & Sahin, H. G. Investigation of maternal psychopathological symptoms, dream anxiety and insomnia in preeclampsia. J. Matern. Fetal Neonatal Med. 30, 2510–2515 (2017).
Rep, A., Ganzevoort, W., Bonsel, G. J., Wolf, H. & de Vries, J. I. Psychosocial impact of early-onset hypertensive disorders and related complications in pregnancy. Am. J. Obstet. Gynecol. 197, 158.e1–158.e6 (2007).
Hoedjes, M. et al. Postpartum depression after mild and severe preeclampsia. J. Womens Health 20, 1535–1542 (2011).
Hoedjes, M. et al. Poor health‐related quality of life after severe preeclampsia. Birth 38, 246–255 (2011).
Hoedjes, M. et al. Symptoms of post-traumatic stress after preeclampsia. J. Psychosom. Obstet. Gynaecol. 32, 126–134 (2011).
Gaugler-Senden, I. P. et al. Maternal psychosocial outcome after early onset preeclampsia and preterm birth. J. Matern. Fetal Neonatal Med. 25, 272–276 (2012).
Taylor, E. B. & George, E. M. Animal models of preeclampsia: mechanistic insights and promising therapeutics. Endocrinology https://doi.org/10.1210/endocr/bqac096 (2022).
Waker, C. A., Kaufman, M. R. & Brown, T. L. Current state of preeclampsia mouse models: approaches, relevance, and standardization. Front. Physiol. 12, 681632 (2021).
Davisson, R. L. et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 39, 337–342 (2002).
Kumasawa, K. et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl Acad. Sci. USA 108, 1451–1455 (2011).
Takimoto, E. et al. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science 274, 995–998 (1996).
Bohlender, J., Ganten, D. & Luft, F. C. Rats transgenic for human renin and human angiotensinogen as a model for gestational hypertension. J. Am. Soc. Nephrol. 11, 2056 (2000).
Doridot, L. et al. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension 61, 662–668 (2013).
Singh, J., Ahmed, A. & Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 58, 716–724 (2011).
Alexander, B. T. et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37, 1191–1195 (2001).
Cavanagh, D., Rao, P. S., Tung, K. S. K. & Gaston, L. Eclamptogenic toxemia: the development of an experimental model in the subhuman primate. Am. J. Obstet. Gynecol. 120, 183–196 (1974).
Yallampalli, C. & Garfield, R. E. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am. J. Obstet. Gynecol. 169, 1316–1320 (1993).
Fan, M. et al. LPS induces preeclampsia-like phenotype in rats and HTR8/SVneo cells dysfunction through TLR4/p38 MAPK pathway. Front. Physiol. 10, 1030 (2019).
Kohli, S. et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 128, 2153–2164 (2016).
Kalkunte, S. et al. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am. J. Pathol. 177, 2387–2398 (2010).
Baijnath, S., Soobryan, N., Mackraj, I., Gathiram, P. & Moodley, J. The optimization of a chronic nitric oxide synthase (NOS) inhibition model of pre-eclampsia by evaluating physiological changes. Eur. J. Obstet. Gynecol. Reprod. Biol. 182, 71–75 (2014).
Soobryan, N. et al. The effects of sildenafil citrate on uterine angiogenic status and serum inflammatory markers in an L-NAME rat model of pre-eclampsia. Eur. J. Pharmacol. 795, 101–107 (2017).
Baijnath, S., Murugesan, S., Mackraj, I., Gathiram, P. & Moodley, J. The effects of sildenafil citrate on urinary podocin and nephrin mRNA expression in an L-NAME model of pre-eclampsia. Mol. Cell Biochem. 427, 59–67 (2017).
Possomato-Vieira, J. S., Gonçalves-Rizzi, V. H., Graça, T. U., Nascimento, R. A. & Dias-Junior, C. A. Sodium hydrosulfide prevents hypertension and increases in vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 in hypertensive pregnant rats. Naunyn Schmiedebergs Arch. Pharmacol. 389, 1325–1332 (2016).
Oludare, G. O., Jinadu, H. D. & Aro, O. O. L-arginine attenuates blood pressure and reverses the suppression of angiogenic risk factors in a rat model of preeclampsia. Pathophysiology 25, 389–395 (2018).
Kasture, V. et al. Maternal omega-3 fatty acids and vitamin E improve placental angiogenesis in late-onset but not early-onset preeclampsia. Mol. Cell Biochem. 461, 159–170 (2019).
Goulopoulou, S. Maternal vascular physiology in preeclampsia. Hypertension 70, 1066–1073 (2017).
Feng, X. et al. Comparison of vascular responses to vasoconstrictors in human placenta in preeclampsia between preterm and later term. Curr. Pharm. Biotechnol. 21, 727–733 (2020).
Langston-Cox, A., Leo, C. H., Tare, M., Wallace, E. M. & Marshall, S. A. Sulforaphane improves vascular reactivity in mouse and human arteries after “preeclamptic-like” injury. Placenta 101, 242–250 (2020).
Schneider, H. & Huch, A. Dual in vitro perfusion of an isolated lobe of human placenta: method and instrumentation. Contrib. Gynecol. Obstet. 13, 40–47 (1985).
Jain, A. et al. Hypoxic treatment of human dual placental perfusion induces a preeclampsia-like inflammatory response. Lab. Invest. 94, 873–880 (2014).
May, K. et al. Perfusion of human placenta with hemoglobin introduces preeclampsia-like injuries that are prevented by α1-microglobulin. Placenta 32, 323–332 (2011).
Guller, S. et al. Protein composition of microparticles shed from human placenta during placental perfusion: potential role in angiogenesis and fibrinolysis in preeclampsia. Placenta 32, 63–69 (2011).
Guller, S., Ma, Y., Malek, A., Di Santo, S. & Schneider, H. Differential release of plasminogen activator inhibitors (PAIs) during dual perfusion of human placenta: implications in preeclampsia. Placenta 28, 278–285 (2007).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564, 263–267 (2018).
Dimitriadis, E., Menkhorst, E., Saito, S., Kutteh, W. H. & Brosens, J. J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers 6, 98 (2020).
Adorno, M., Maher-Griffiths, C. & Grush Abadie, H. R. HELLP syndrome. Crit. Care Nurs. Clin. North Am. 34, 277–288 (2022).
Author information
Authors and Affiliations
Contributions
Introduction (E.M.); Epidemiology (E.M., D.L.R. and C.W); Mechanisms/pathophysiology (E.D., E.M., W.Z. and G.E.-G.); Diagnosis, screening and prevention (D.L.R., F.d.S.C., J.H. and K.N.); Management (D.L.R., K.K., R.P.V.F. and J.H.); Quality of life (W.Z. and F.d.S.C.); Outlook (E.D.); Overview of Primer (E.M. and E.D.)
Corresponding author
Ethics declarations
Competing interests
F.d.S.C. has Research Funding from ThermoFisher for an angiogenic marker study (sFLT1/PGF). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks M. K. Santillan; S. R. Hansson; R. Dechend; P. Nunes, who co-reviewed with V. C. Sandrim; E. Toivonen, who co-reviewed with H. M. Laivuori; and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Anticardiolipin antibodies
-
Auto-antibodies found in antiphospholipid syndrome.
- Antiphospholipid syndrome
-
Autoimmune disease characterized by recurring thromboses, recurrent pregnancy loss and thrombocytopenia.
- Chorionicity
-
The number of placentas in a pregnancy.
- Cytotrophoblasts
-
Trophoblast progenitor stem cells located interior to the syncytiotrophoblast in placental villus and in cell columns that anchor placental villus to the decidua.
- Decidua
-
The modified endometrial layer during pregnancy into which the placenta is anchored.
- Extravillous trophoblasts
-
Differentiated cytotrophoblasts that invade from cell columns into the decidua and engraft maternal spiral arteries.
- Lupus anticoagulant
-
Immunoglobulin that binds to phospholipids and increases the risk of developing thromboses.
- Placental malperfusion
-
Reduced maternal blood flow to the intervillous space of the placenta.
- Primiparity
-
Term used to describe a first pregnancy and/or to have given birth only once.
- Syncytiotrophoblast
-
Multinucleated, continuous trophoblast that covers the entire surface of the placental villus and is in direct contact with maternal blood.
- Trophoblast
-
Non-embryonic fetal cells derived from the trophectoderm of the blastocyst.
- Uterine spiral arteries
-
Small arteries that supply blood to the endometrium of the uterus during the menstrual cycle and the intervillus space of the placenta during pregnancy.
- Zygosity
-
Genetic similarity of fetuses in multifetal pregnancies.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dimitriadis, E., Rolnik, D.L., Zhou, W. et al. Pre-eclampsia. Nat Rev Dis Primers 9, 8 (2023). https://doi.org/10.1038/s41572-023-00417-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-023-00417-6
This article is cited by
-
Pregnancy outcomes of Fabry disease in Austria (PROFABIA)-a retrospective cohort-study
Orphanet Journal of Rare Diseases (2024)
-
Autophagy-related biomarkers in preeclampsia: the underlying mechanism, correlation to the immune microenvironment and drug screening
BMC Pregnancy and Childbirth (2024)
-
Postpartum and interpregnancy care of women with a history of hypertensive disorders of pregnancy
Hypertension Research (2024)
-
Cell-Free Nucleic Acids for Early Prediction of Preeclampsia
Current Hypertension Reports (2024)
-
PBPK/PD Modeling of Nifedipine for Precision Medicine in Pregnant Women: Enhancing Clinical Decision-Making for Optimal Drug Therapy
Pharmaceutical Research (2024)