Abstract
Multimorbidity (two or more coexisting conditions in an individual) is a growing global challenge with substantial effects on individuals, carers and society. Multimorbidity occurs a decade earlier in socioeconomically deprived communities and is associated with premature death, poorer function and quality of life and increased health-care utilization. Mechanisms underlying the development of multimorbidity are complex, interrelated and multilevel, but are related to ageing and underlying biological mechanisms and broader determinants of health such as socioeconomic deprivation. Little is known about prevention of multimorbidity, but focusing on psychosocial and behavioural factors, particularly population level interventions and structural changes, is likely to be beneficial. Most clinical practice guidelines and health-care training and delivery focus on single diseases, leading to care that is sometimes inadequate and potentially harmful. Multimorbidity requires person-centred care, prioritizing what matters most to the individual and the individual’s carers, ensuring care that is effectively coordinated and minimally disruptive, and aligns with the patient’s values. Interventions are likely to be complex and multifaceted. Although an increasing number of studies have examined multimorbidity interventions, there is still limited evidence to support any approach. Greater investment in multimorbidity research and training along with reconfiguration of health care supporting the management of multimorbidity is urgently needed.
Similar content being viewed by others
Introduction
Interest in multimorbidity — commonly defined as the co-occurrence of at least two chronic conditions in the same individual1 — has increased in the past few years owing to its substantial effect on the individual and the individual’s family, as well as on health systems and on society, particularly in resource-poor settings2,3,4. Multimorbidity is distinct from the related concept of comorbidity, which refers to the combined effects of additional conditions in relation to the index condition in an individual5,6,7,8. By contrast, care for multimorbidity is patient-centred and does not routinely give priority to any single condition, although in clinical care, patients and clinicians will usually focus on the most pressing problems that the patient is experiencing.
People with multimorbidity are more likely to die prematurely, be admitted to hospital and have an increased length of stay than people with a single chronic condition9,10. Multimorbidity is also associated with poorer function and health-related quality of life (HRQOL), depression and intake of multiple drugs (polypharmacy) and greater socioeconomic costs11,12,13,14,15,16,17,18. Most health care is designed to treat individual conditions rather than providing comprehensive, person-centred care2,19,20, which often leads to fragmented and sometimes contradictory care for people with multimorbidity and increases their treatment burden21 Moreover, treating one condition at a time is inefficient and unsatisfactory for both people with multimorbidity and their health-care providers22,23,24.
Multimorbidity is increasingly common owing to changes in lifestyle risk factors, notably physical inactivity and obesity, and population ageing that in part reflects improvements in survival from acute and chronic conditions2,19,25,26. Multimorbidity is associated with socioeconomic status and age3,19,25,27. However, although age is the strongest driver of multimorbidity, in absolute numbers, more people <65 years of age have multimorbidity than people ≥65 years of age, partly because more people in the general population are in that age group. Moreover, this emphasizes that multimorbidity is not just a feature of ageing19,26.
Multimorbidity is further complicated in low-income and middle-income countries (LMICs) by the overlap of compounding factors, including adverse environmental and early life stressors linked to poverty, limited social infrastructure and poorer family coping mechanisms, that translate into chronic diseases occurring at earlier ages28,29,30,31. LMICs also have a higher prevalence of multimorbidity-related financial burden32,33 and have weaknesses in health systems including a greater focus on managing acute health conditions and chronic infectious diseases3,4,33,34 and, in some countries, complete absence of services for people with multimorbidity35.
Of note, during the COVID-19 pandemic individuals with multimorbidity had greater risk of infection and adverse outcomes including hospitalization. Moreover, there has been a deficit in standardized health advice and clinical guidelines for some of the most vulnerable people with multimorbidity, notably for people in care homes, in which the effect of COVID-19 was catastrophic36,37,38,39,40. The COVID-19 pandemic also demonstrated the fragility of public health systems worldwide, and the prioritization of acute care has further compromised long-term chronic care, including mental health care40,41,42.
Overall, the pandemic highlights the urgent need for action to deal with the increasing burden of chronic conditions and multimorbidity worldwide through better prevention and management and a reconfiguration of health care to achieve an appropriate balance of disease-oriented specialist care and person-centred generalist and primary care43,44. Moreover, health systems should take into account what matters most to people, such as continuity of care35, competent care, user experience, health outcomes and confidence in the system, to advance towards high-quality health care45. Changing health-care delivery requires updating the training of the next generation of health-care providers and increasing emphasis on primary prevention strategies, including lifestyle-focused and population-wide prevention efforts, many of which will be deployed outside the health-care delivery system.
This Primer provides a global overview of the epidemiology, potential underlying mechanisms and pathophysiology, diagnosis, prevention, management and outcomes of multimorbidity. Moreover, this Primer highlights the need for improved management and enhanced support to primary care and public health and ends with a call to action for future research. For consistency, the term ‘multimorbidity’ is used throughout, acknowledging that ‘multiple chronic conditions’ is also often used in the literature and considered more lay person friendly46. In this Primer, multimorbidity is defined as the co-occurrence of at least two chronic conditions in the same individual, as this definition is the most commonly used and is the accepted definition used by WHO1,47. Given that multimorbidity should have a person-centred approach and does not intrinsically prioritize one individual condition over others5,6, this Primer does not follow a structure focusing on certain individual diseases or conditions separately, but refers to individual conditions, comorbidities and clusters of conditions when relevant throughout.
Epidemiology
Although the presence of two or more chronic conditions is the most widely cited and accepted definition of multimorbidity (Box 1), the way multimorbidity is defined (for example, the number of coexisting conditions needed to qualify as having multimorbidity) and measured is highly variable depending on the number of conditions considered and how they are measured48,49,50. The simple two or more chronic condition definition has been criticized for including people with combinations of conditions that do not meaningfully affect the individual (such as well-controlled hypertension, mild eczema and high cholesterol), which has led to suggestions of alternative definitions of ‘complex multimorbidity’, such as the “co-occurrence of three or more chronic conditions affecting three or more different body systems within one person”51. Regardless, at the patient (and household) level, having more than one condition, including a mental health disorder, translates into a higher health-care load and treatment burden, which is equally important to or more important than the precision in the ‘technical’ definition of multimorbidity35,52,53.
Although plausible, the clinical or research use of the concept of complex multimorbidity is not well established54,55. One systematic review of 566 studies of multimorbidity found that simple counts (counting the number of conditions an individual has) or weighted condition counts (introducing a weighting for included conditions based on severity and/or impact) are commonly used in research, but the number of conditions included in measures varies from 2 to 285 (median 18)56. Only eight physical conditions were included (diabetes mellitus, stroke, cancer, chronic obstructive pulmonary disease, hypertension, coronary heart disease, chronic kidney disease and heart failure) in >50% of studies, and 21.5% of studies did not include any mental health condition56. The relative value of simple condition counts versus weighted indexes is debated5,43,57,58,59. Some systematic reviews have concluded that counts and weighted measures are equally effective at predicting the majority of outcomes and an overview of systematic reviews could not identify consensus on this issue, and suggested that choice of measure should be determined based on study aims57,58,59. How indices should be weighted (for example, by HRQOL or other outcomes) is also debated, and the most appropriate weighting is likely to vary depending on the purpose of the study49,60, the source and type of data available, the population source and the effect being considered49,57,61,62. Further adding to the variability is whether risk factors and symptoms such as urinary incontinence, pain or obesity are included. A large cohort study found that including risk factors increased only the prevalence of multimorbidity, whereas including symptoms increased both prevalence and association with patient outcomes48.
This variability makes comparison of prevalence and effect of multimorbidity across populations difficult. Moreover, it highlights the importance of considering and clarifying which multimorbidity framework is used in individual studies as well as calls for a consensus process to identify the most relevant definitions to use in future studies. A modified Delphi study aimed to develop consensus on the definition and measurement of multimorbidity in research. In this study, consensus was reached among professionals and people with chronic conditions that multimorbidity should be defined as two or more chronic conditions. Furthermore, consensus was also reached on a list of conditions to always include and usually include in multimorbidity measures63.
Prevalence
The estimated prevalence of multimorbidity depends on the definition used27 but, overall, many findings are consistent across studies27 (Fig. 1). Globally, about one-third of adults64, including a substantial proportion in LMICs65,66,67, and more than half of all adults with any chronic condition19 have multimorbidity25. Systematic reviews focusing on community-based studies in both high-income countries (HICs) and LMICs have found a prevalence of 15–43%28,64,68,69. A scoping review in LMICs found a prevalence of 3% to 68% in adults, with most of the evidence from Brazil, China, South Africa, India, Mexico and Iran70, and 43% in adults in Latin America and the Caribbean68. Prevalence estimates are generally lower in LMICs than in HICs (Fig. 1a,b). The reasons for this difference are not known but methodological factors and differential survival are plausible hypotheses. Of note, depression is two to three times more common in people with multimorbidity than in people without multimorbidity or those with no chronic physical condition18.
a | Prevalence estimates of multimorbidity according to age in high-income countries; data from refs.27,313,314,315,316,317,318,319. b | Prevalence estimates of multimorbidity according to age in low-income and middle-income countries; data from refs.66,67,320,321,322,323. The prevalence of multimorbidity increases with age, although estimates vary among studies. Apart from differences across regions, differences among studies may arise from the recruitment method and sample size, data collection and the operational definition of multimorbidity used, which includes the number of diagnoses considered (such as two or more, or three or more), and the conditions considered. The most appropriate estimates for a given population are probably those obtained from a large sample and using the most prevalent long-term conditions with a high effect or burden in that population. When comparing prevalence estimates of multimorbidity between high-income countries and low-income and middle-income countries, lower age-specific rates are observed in low-income and middle-income countries. To our knowledge, the reason for this difference has not been addressed in prevalence studies, and whether the difference is due to factors such as ascertainment of conditions (such as fewer conditions diagnosed), effects linked to survival (such as shorter survival after acute events), or to a true difference, remains to be determined. c | Prevalence of multimorbidity (defined as 2 or more of 40 conditions)19 by age between the most and least affluent tenths of the population. Multimorbidity prevalence increases steeply with age in all groups, and, apart from in the very oldest, is consistently higher in the less affluent with the largest difference between groups in middle age.
Although less commonly reported, some children and adolescents have multimorbidity and risk of associated disability19,27,71,72. Multimorbidity is strongly associated with age, with a prevalence of 30% among people aged 45–64 years, 65% among those aged 65–84 years and 82% among those aged ≥85 years19,27. In addition, multimorbidity is more common in women than in men, with a weighted difference in prevalence of 6.5%71. Moreover, multimorbidity has a higher odds in groups with lower education levels than in those with higher education levels73. Individuals living in the most deprived areas consistently experience higher prevalence of multimorbidity than their more affluent counterparts across the lifespan (Fig. 1c), and also experience more complex combinations of physical and mental health multimorbidity19.
Although the available literature on multimorbidity is largely dominated by studies in HICs70, studies in LMICs have also found that multimorbidity is common and associated with age, sex and social status, although the prevalence of multimorbidity is higher among adults with higher socioeconomic status in some countries, but not in others28,66,74. Reasons for these differences are largely unknown, but might relate to differences in access to health care, obtaining a diagnosis, health-seeking behaviour and longevity75.
Condition clusters
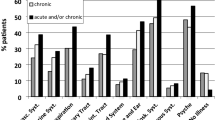
The identification of clusters of conditions is an alternative to both simple counts and weighted indices28,43,76. The most appropriate methods to identify and analyse clusters is debated. Factor analysis or hierarchical clustering methods were predominantly used in studies included in recent systematic reviews77,78,79, with smaller numbers of studies using latent class, network and multiple correspondence analysis. The two most consistent and replicable clusters across available studies included cardiometabolic conditions and mental health conditions, respectively, although clusters including musculoskeletal conditions and allergic conditions have also been identified77,78,79. Although the evidence is still limited, some data suggest that certain clusters, in particular those including mental health conditions (such as depression), are associated with poorer health80,81, functional limitations82 and higher health-care costs compared with other clusters83. However, few replication studies have been carried out, and available studies suggest that observed condition clusters are not usually replicable using different methods and/or in different datasets77,79,84,85,86. Further research is needed to obtain a better understanding of multimorbidity clusters, their importance for care and their trajectories over time across different age ranges, sex, genders and racial groups87,88,89. This research will identify opportunities for early intervention to address sex and gender, ethnic and socioeconomic inequality in multimorbidity90,91.
Multimorbidity trajectories
Relatively few studies have thoroughly examined multimorbidity trajectories over time. One scoping review on multimorbidity trajectories compiled evidence from 34 studies, and found significant associations between multimorbidity and adverse outcomes, such as reduced reported health, and increased risk of disability and mortality92. However, no studies were from LMICs and heterogeneous methods were used. Additional longitudinal data and analysis are important to obtain a better understanding of the development and acceleration of multimorbidity and its inequalities based on social status73,93,94,95.
Health-care utilization and economic effect
People with multimorbidity are more likely to die prematurely54,96. Furthermore, multimorbidity is linked with increased health-care utilization10,17,97. Multimorbidity accounts for 78% of all consultations in primary care in HICs97, people with multimorbidity have more frequent hospital admissions with longer lengths of hospital stay than people with one or no conditions10,97,98, and there is an almost exponential relationship between the number of chronic conditions and their associated costs due to increased health-care utilization17. This higher health-care utilization, coupled with multiple pharmacological treatments that is common among people with multimorbidity15,17, leads to higher treatment burden21,99,100, and also places financial strain on patients and health-care systems. Households can experience very high health expenditures associated with the management of chronic conditions and multimorbidity32,101,102. Informal caregiving, provided by family relatives mostly without financial compensation, many of whom have to stop working to devote to caregiving103,104, adds to the societal and household economic burden of multimorbidity.
Mechanisms/pathophysiology
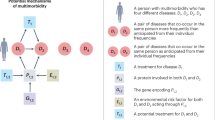
Studying the mechanisms and pathophysiology of multimorbidity is complicated by the heterogeneity of patients. Patients may have concordant multimorbidity (for example, cardiovascular multimorbidity (such as a combination of atrial fibrillation, coronary heart disease and heart failure)) where conditions have a shared pathophysiology or shared approaches to management, or discordant multimorbidity (such as a combination of chronic obstructive pulmonary disease, depression, dyspepsia and osteoarthritis) whereby the conditions have unrelated pathophysiology and differing treatments that may even be contradictory105. Nonetheless, the emerging literature on pathophysiology and mechanisms of multimorbidity provides evidence of some common multifactorial pathways106 (Fig. 2). Mechanisms can be considered in three broad areas: ageing and inflammation; socioeconomic, psychosocial and behavioural determinants of health; and medication-related. Each of these issues is discussed in turn in the sections below.
Development of multimorbidity is affected by several factors. Mechanisms underlying the development of multimorbidity are frequently interrelated and may be synergistic. Mechanisms can be considered in three areas: underlying biological mechanisms relating to ageing and inflammation; broader determinants of health such as socioeconomic, psychosocial and behavioural social determinants; and medication-related.
Ageing, inflammation and multimorbidity
The body of literature on the mechanisms connecting ageing and the development of multimorbidity is increasing107,108,109,110. The ‘hallmarks of ageing’111 include genomic instability, epigenetic effects, telomere attrition, loss of proteostasis, altered intercellular communication, mitochondrial dysfunction, deregulated nutrient sensing, cellular senescence and stem cell exhaustion. These hallmarks have been postulated to be possible targets for future pharmacological developments to prevent or slow development of multimorbidity112.
Genomic instability is important because it is key to maintaining the health of cells and tissues, but it can be adversely affected by a range of internal and external factors113. Internal factors that can have negative effects include generation of reactive oxygen species (ROS) and spontaneous hydrolytic reactions, whereas external factors include chemicals in the environment or ultraviolet radiation112. Long-term epigenetic changes, which is how behaviours and environment influence gene function, have been postulated to have an important role in understanding development of multimorbidity and affect gene function through effects on histones, DNA methylation and microRNA dysregulation114. Both genomic instability and epigenetic changes are associated with development of certain cancers115 and chronic inflammatory disease116.
Telomere attrition can be increased by oxidative stress117 and telomeres shorten with age118, but the mechanisms underpinning these changes and the effects on human health remain uncertain119,120. A study examining the relationship between telomere length and development of multimorbidity did not find an association; however, in men, longer telomeres were associated with a lower risk of multimorbidity, including mental health conditions121. However, another study did show a relationship between telomere shortening in people with multimorbidity who also experienced sarcopenia or frailty122. Although the literature on direct mechanisms connecting telomere shortening to chronic disease remains sparse, there is growing evidence of links between telomere shortening and carcinogenesis123, inflammatory conditions (such as inflammatory bowel disease124 and kidney fibrosis125) and certain neurodegenerative disorders (such as Alzheimer disease)126. Interest is growing in the potential of telomere shortening to serve as a prognostic marker, and this may be an area worthy of further investigation in relation to multimorbidity.
Loss of proteostasis, including impaired autophagy, protein misfolding and reduced translation fidelity, is associated with ageing and age-related diseases112,127,128. Moreover, alterations in proteostasis have been suggested to have a role in the development of neurodegenerative diseases such as Parkinson disease and Alzheimer disease127. Altered intercellular communication (which can be neuronal, endocrine or neuroendocrine) that occurs with ageing can also lead to decreases in tissue health and are often associated with an increase in inflammatory signalling known as ‘inflammageing’129. Deregulated nutrient sensing can affect multiple signalling pathways that seem to affect longevity (for example, the insulin-like growth factor signalling pathway). Anabolic signalling has been suggested to promote ageing, whereas decreased nutrient signalling secondary to calorie-restricted diets or stimulation of sirtuins (signalling proteins involved in maintaining cellular haemostasis) promotes longevity. The role of insulin-like growth factors on the cells of bone development have been the subject of clinical studies aimed at treating osteoporosis, but benefits remain uncertain128.
Mitochondrial dysfunction can be exacerbated by oxidative stress130 and has a role in stem cell function131 and cellular senescence132. The mechanisms underlying the adverse effects of mitochondrial dysfunction have been studied in mice133. This research demonstrated that mice with T cells deficient in a mitochondrial DNA-stabilizing protein have features associated with ageing including abnormalities of neurological, metabolic, muscular and cardiovascular function and that these changes produce effects similar to inflammageing133. This research suggested that mitochondrial dysfunction was controlled by mitochondrial transcription factor A, which is associated with inflammageing, and is a predictor of multimorbidity, contributing to the evidence that mitochondria have a causal role in senescence134.
Cellular senescence is associated with chronic inflammation110,135. Cellular senescence results in senescent cells that can remain metabolically active and affect other cells through a senescence-associated secretory phenotype136 that can secrete inflammatory mediators. This can lead to the promotion of a pro-inflammatory state that may be associated with age-related chronic diseases and, in turn, multiple chronic diseases (multimorbidity)110,136. Multiple factors can stimulate cellular senescence. External factors include metabolic signals (such as high levels of glucose), hypoxia and ROS whereas internal factors include telomeric dysfunction, DNA damage and mitochondrial dysfunction137. Senescent cells accumulate in multiple chronic diseases such as diabetes mellitus and cardiovascular disease138,139.
Stem cell exhaustion111 is a typical attribute of ageing that is associated with cellular senescence. Stem cells are required to generate new cells as old cells are lost or damaged, and without sufficient proliferating stem cells responses to damage or injury will be inadequate, resulting in impaired cell replacement and recovery139. Genomic stability and proteostasis are important for stem cell function, illustrating the connection between the various hallmarks of ageing. Stem cell exhaustion has been linked with development of chronic lung diseases such as chronic obstructive pulmonary disease140.
One study explored the relationship between the hallmarks of ageing and multiple age-related diseases through text mining the literature, genome-wide association studies and examination of electronic health records (EHRs)141. This study found that five hallmarks of ageing (altered intercellular communication, mitochondrial dysfunction, deregulated nutrient sensing, cellular senescence and stem cell exhaustion) occurred more often in multimorbidity than expected by chance across different age groups141. Interest is increasing in the geroscience hypothesis, which suggests that health can be enhanced by focusing on the mechanisms of ageing rather than single diseases. A growing number of studies are investigating geroscience-informed therapeutic approaches112 aiming to reduce or slow the effects of or development of multimorbidity. Research in these domains is likely to further intensify in the future.
Whether the hallmarks of ageing work individually, together or interactively is unclear, and only some have been validated in clinical studies142. Biomarker studies have suggested that the build-up of senescent cells affects allostasis143 resulting in increased allostatic load, which has been proposed as a gauge of the aggregate physiological burden on the body required to maintain internal stability144. Allostatic load can be assessed by measuring multisystem biomarkers that are an indicator of multisystem physiological dysregulation. Allostatic load is a measure of the cumulative effect of chronic stress and probably also life events (as described in the section Social, psychosocial and behavioural factors). It has been associated with a range of health conditions including diabetes mellitus, musculoskeletal disorders, cancer, and mood and anxiety disorders, with evidence that those experiencing high levels of stress and psychological distress have higher allostatic loads145.
Although our understanding of the hallmarks of ageing and their relationship with multimorbidity is limited, some biomarkers, particularly those related to oxidative stress, may be markers of some of these mechanisms of ageing and inflammation (see the section Diagnosis, screening and prevention, below).
Social, psychosocial and behavioural factors
Socioeconomic, psychosocial and behavioural determinants of health are associated with development of multimorbidity108. Socioeconomic deprivation (measured by household income, total household wealth or household area146) and lower education level are associated with higher multimorbidity prevalence73,146,147,148,149 and development of multimorbidity at a younger age19. The opposite may apply in LMICs, whereby some studies have suggested an associated between multimorbidity and higher incomes73. A systematic review of 24 studies examining the relationship between socioeconomic deprivation, education level or income found that a lower education level was associated with a 64% increased odds of multimorbidity compared with a higher education level73, whereas another review including 42 studies found that multimorbidity was over four times more likely in people with the lowest incomes than in those with the highest incomes146. Other studies have shown that multimorbidity occurs a decade earlier in those from more socioeconomically deprived backgrounds19.
A range of lifestyle factors including positive smoking status, high alcohol intake, decreased physical activity and poor diet quality are associated with development of multimorbidity150,151. However, findings are mixed, and it remains unclear which factors are the most important, making it difficult to draw firm conclusions. A Canadian study involving 1,196 participants examined the association between common lifestyle factors (such as current or past smoking, high-risk alcohol consumption, physical inactivity and low fruit and vegetable consumption) and found that smoking was the most important factor152. This study also found that the presence of combinations of unhealthy lifestyles (such as current or past smoking and physical inactivity) increased the risk of multimorbidity152. Of note, this study did not show an increased risk of multimorbidity with physical inactivity, which is in contrast to the findings of other studies, such as one study using data from the China Health and Retirement Longitudinal Study that demonstrated a 45% increased risk of multimorbidity with low levels of physical activity153. By contrast, an Australian study involving 53,867 participants aged 45–64 years who were free of 11 predefined chronic conditions at baseline showed that among the top multimorbidity predictors were current or past smoking, and increased age, high BMI and high intake of chicken or red meat in both sexes, but that other behavioural factors including physical inactivity, alcohol consumption and excessive or insufficient sleep duration were also important154. A study from India in 699,686 women showed that the risk of multimorbidity was 87% higher in women who smoked or chewed tobacco and was 18% higher in those who consumed alcohol than in women who did not smoke or chew tobacco or consume alcohol, respectively155. Factors such as smoking promote cellular senescence through inflammatory effects, oxidative stress and DNA damage156, whereas exercise prevents cellular senescence156,157, highlighting the probable interplay between socioeconomic, psychosocial and behavioural determinants and the hallmarks of ageing.
Interest is increasing in emerging lifestyle factors as potentially preventable factors in the development of chronic illness, such as cardiovascular and metabolic disease158,159 and their role in development of multimorbidity. Emerging lifestyle factors include high television viewing time160 or sedentary behaviour, sleep duration161 (both too much and too little), and levels of social participation (such as loneliness)108,153,158,162,163,164. Short sleep duration was associated with the number of multimorbid conditions in 1,508 respondents of the European Health Examination Survey164. Moreover, data from the US 2005–2006 National Health and Nutrition Examination Survey (NHANES) suggests that sedentary behaviour is associated with multimorbidity, after adjusting for light-intensity physical activity and adherence to moderate-to-vigorous physical activity guidelines165. Loneliness and social isolation have also been suggested to be associated with multimorbidity166; however, 1 systematic review found that only eight studies have examined these issues and found that, although cross-sectional and longitudinal studies suggested an association between loneliness and multimorbidity, the evidence for social isolation is under-researched166. The mechanisms underlying many of these associations remain uncertain with some suggesting, for example, that the relationship between multimorbidity and sleep disturbance could be bidirectional167, and others suggesting that sleep disturbance could be a surrogate measure of loss of resilience or multisystem homeostatic dysregulation163. Moreover, social relationships have been suggested to moderate the effects of stress on health and well-being in the stress buffering hypothesis168.
Adverse childhood experiences (ACEs)169,170 are also associated with increased severity and complexity of multimorbidity170. There are a range of hypotheses for potential underlying mechanisms171, including chronic activation of the hypothalamic–pituitary–adrenal axis by persistent stress secondary to ACEs, leading to increased allostatic load. Other work has suggested that ACEs are associated with increased cortisol levels and chronic inflammation172 or with DNA methylation of certain genes and telomere length shortening, possibly increasing the risk of conditions of ageing173,174.
Lacking control over one’s life (the extent to which people believe what happens in their life is determined by factors outside their control)148 has also been implicated in development of multimorbidity. Lack of control may exacerbate anxiety, thereby promoting a chronic stress response and increasing the risk of unhealthy behaviours such as smoking108. Research into the interplay between stress and multimorbidity is in its infancy, but has indicated an association with increased hospitalizations and mortality175,176. Stress could be a modifiable risk factor for multimorbidity, particularly as it might be associated with decisions about unhealthy behaviours, but its effects may also be explained through an effect on allostatic load177. Some have posited that the social hallmarks of ageing should be integrated with the work on the biology of ageing to enhance understanding of the factors associated with human ageing and the development of multimorbidity178.
Although evidence for the social determinants of multimorbidity is increasing, more research is required to help understand which factors or combination of factors are the most important to target. A key gap in knowledge is of determinants of different multimorbidity patterns, particularly in LMICs92.
Medication-related mechanisms
Medications and polypharmacy may also contribute to development of multimorbidity. Several medications are associated with increased risk of diabetes mellitus and dyslipidaemia (for example, antipsychotics179). Similarly, medications with anticholinergic effects are associated with increased risk of cardiovascular events and cognitive impairment or dementia170.
In practical terms, this could lead to patients receiving treatment for specific single conditions, and developing additional chronic conditions as a direct consequence of the treatment of the initial condition (for example, oral steroids for polymyalgia rheumatica contributing to the development of diabetes mellitus, cataracts and osteoporosis). In this way, medications can contribute to the development of multimorbidity. Equally, polypharmacy can increase the risk of drug–drug interactions or drug–condition interactions, also causing or adding to multimorbidity. For example, co-prescription of NSAIDs for arthritis and selective serotonin reuptake inhibitors for depression can result in gastrointestinal bleeding180.
In summary, many interrelationships are involved in the development of multimorbidity, including ageing and inflammation, socioeconomic, psychosocial and behavioural social determinants and medications. Figure 2 summarizes key influences on development of multimorbidity, and illustrates the shared pathways to the development of multimorbidity. Mechanisms underpinning development of multimorbidity are frequently interrelated and may be synergistic.
Diagnosis, screening and prevention
Multimorbidity is not a condition or disease in the usual sense, so conventional ideas of diagnosis and screening are not strictly relevant. The focus of this section is, therefore, on the detection and diagnosis of multimorbidity that is significant or severe from the perspective of the patient or the clinician, and that, therefore, requires an approach to care which is more than simply optimizing care for every individual condition present.
Diagnosis in clinical practice
As multimorbidity is the coexistence of two or more chronic conditions, ‘diagnosing’ multimorbidity in clinical practice is rarely a problem because the clinician and patient usually agree which conditions are currently active or relevant. However, deciding (or diagnosing) when multimorbidity is sufficiently severe or impactful that it requires specific attention or when the management of a single disease needs to be adapted (including not following single-disease guidelines or shifting to more palliative approaches to care) is more difficult20.
From this perspective, the UK National Institute for Health and Care Excellence (NICE) guideline on multimorbidity recommends that clinicians actively consider whether an individual patient requires an approach to care that specifically accounts for multimorbidity181, if the patient requests such care or if the patient has any of the following features: finding it difficult to manage treatment or usual activities; receiving care from multiple services; having both physical and mental health chronic conditions; frequently seeking unplanned or emergency care; taking multiple medicines; or having frailty182. Of note, although frailty and multimorbidity are highly associated, they are not the same43,183. Although 72% of individuals with frailty have multimorbidity, only 16% of individuals with multimorbidity have frailty184. Both conditions are associated with lower socioeconomic status and neither is restricted to older adults19,183,185. However, the coexistence of frailty and multimorbidity is associated with increased risk of mortality183, even after adjusting for the number of conditions, sociodemographic factors and lifestyle. Thus, identifying pre-frailty and frailty in patients with multimorbidity is important to prevent frailty progression, reduce the risk of adverse outcomes and optimize treatment.
The NICE guideline on multimorbidity also recommends that clinicians screen for patients who might require an approach to care that accounts for multimorbidity using EHRs or opportunistically identify patients during routine care. The recommended screening tools for use with EHRs have been validated in the UK to predict emergency hospital admission and to identify polypharmacy, but the same principles apply internationally. Key markers to opportunistically identify patients who require such a different approach (Fig. 3) are consideration of condition burden, treatment burden and frailty. Condition burden and treatment burden can only be assessed by asking the patient and/or carer about their experience of health and care181. For assessing the presence of frailty, then simple measures such as informal or formal assessment of gait speed, self-reported health, timed up-and-go tests or the PRISMA-7 questionnaire are recommended in the NICE guideline and are well correlated with gold standard frailty assessment, and are useful screening tools181. Of note, very intensive evaluation (such as that carried out in Comprehensive Geriatric Assessment (CGA)) is not recommended in the NICE guideline for diagnosis of problematic multimorbidity in all patients, as CGA is too resource-intensive for routine use. Instead, NICE considers CGA to be a combined assessment and intervention to be used in selected patients where there is agreement that a different approach to care is needed (see Management, below).
Adaptation of care to account for multimorbidity may be needed because the patient experiences a high condition burden and/or because the patient experiences a high treatment burden. Condition burden is related to the severity, complexity and interaction of the effects of individual conditions. For example, diabetes mellitus and hypertension is a combination that is relatively unproblematic because cardiovascular disease prevention is a common goal, whereas combinations such as diabetes mellitus, schizophrenia and chronic obstructive pulmonary disease (COPD) have more complex interactions between conditions (for example, schizophrenia has effects on motivation that may make lifestyle change difficult) and between medications (for example, antipsychotics adversely affect glucose metabolism, and share anticholinergic adverse effects with some COPD medications). Treatment burden is related to the effect of treatments, including the complexity of follow-up in relation to the number of different professionals, services, appointments and admissions, and complexity of treatment, particularly in relation to polypharmacy.
Less-specific guidance on diagnosis or screening of multimorbidity is present in other guidance documents internationally, which tend to start from the recognition that the patient has multiple conditions. However, other guidelines recommend agreement with the patient about their most important outcomes or priorities, which may be associated with specific conditions or may not be condition specific186,187,188. Such a patient-centred approach is critical to ensuring that care is tailored to the individual. The range of personal circumstances that are important to the individual and relevant to care will often include diagnosed conditions but also potentially broader issues that affect health and care, for example living circumstances, social disadvantage and health literacy, all of which can influence an individual’s capacity to cope with a given level of treatment burden99,100.
However, some combinations of conditions may not be immediately problematic for the individual patient but carry considerable future risk that may need to be managed (such as hypertension, hyperlipidaemia, obesity, impaired glucose tolerance and previous myocardial infarction without current symptoms). Patient-centred care that focuses on high condition and/or treatment burden as experienced by the patient, therefore, has to be balanced against managing disease and future risk. Accordingly, predicting poor health outcomes and limited life expectancy is an important parallel strategy in identifying patients with multimorbidity who need an approach to care different from the more common disease or specialty-oriented models of care as reflected in condition-specific clinical guidelines.
A practical example of the diagnostic and management challenges facing clinicians is a patient with heart disease and chronic respiratory disease who has breathlessness and fatigue. A generalist approach is needed to identify the likely cause of these symptoms, which could relate to either condition and/or be compounded by coexisting depression and anxiety. Similarly, people with a combination of diabetes mellitus, heart disease and arthritis often find that pain caused by active arthritis limits their capacity to exercise and in some this contributes to difficulties maintaining a healthy body weight, thereby adversely affecting their diabetes and heart disease. Accordingly, even in someone with poor diabetes control, pain management might be the immediate priority. This approach, which focuses on improving outcomes prioritized by the patient and improving experience of care, rather than focusing on the condition count, parallels a change in thinking about polypharmacy from considering the total number of medications (often used in research studies) to focusing on appropriate polypharmacy from a patient perspective189,190.
From a patient and clinical perspective, multimorbidity may, therefore, be present but not problematic, and the diagnostic problem is identification of multimorbidity, which requires a specific approach to care that goes beyond single-condition treatments. A combination of systematic screening of EHR data to identify patients for review, and opportunistic case finding during routine care is required. However, the core of diagnosis comprises the clinician actively working with the patient (and/or carer) to understand their experience while also using clinical judgement and agreeing a management plan with the patient.
Physiological and serum biomarkers
Several physiological and serum biomarkers may in the future be useful to help us understand determinants of multimorbidity and could also potentially be used to identify individuals at risk of adverse outcomes. Several physiological biomarkers are associated with development of multimorbidity191, including higher baseline blood pressure, reduced hand grip strength192,193,194, high waist–hip ratio and high BMI150,191,195,196, and lung function indices, such as reduced forced expiratory volume in 1 s (ref.197). Some evidence suggests a link between the levels of a range of serum biomarkers and multimorbidity including higher cystatin C, C-reactive protein and lipoprotein levels, lower dehydroepiandrosterone sulfate levels, higher IL-6 levels198, lower serum glutathione levels199, and higher diacron reactive oxygen metabolite and HbA1c levels200. This area is rapidly evolving with the potential for new biomarkers to be identified. For example, one study found an association between high total serum homocysteine and low methionine levels with more rapid development of cardiovascular multimorbidity201. Data on the use of some biomarkers for multimorbidity, such as vitamin D, are conflicting202,203.
Of note, as yet there is no clear evidence to support the use of physiological and serum biomarkers to target treatments or interventions in patients with multimorbidity. Two systematic reviews have highlighted the insufficient literature on this topic191,198 and suggest that there is an urgent need for additional good quality studies to aid understanding and inform targeting of potential future interventions (for example, to help individualize care) aimed at reducing or delaying development of multimorbidity. Future research on biomarkers for multimorbidity may identify biomarkers of sufficient predictive value to be used as screening tools in clinical practice or research.
Prevention
Primary prevention of multimorbidity has not been studied robustly, in part because such studies would potentially need long-term interventions and decades of follow-up to evaluate possible long-term benefits. Preventive measures for multimorbidity are related to psychosocial and behavioural factors, including the broad social determinants of health perspective, as described in Mechanisms/pathophysiology. The a healthy lifestyle (engaging in physical activity, not smoking, eating five portions of fruit and vegetables per day and not consuming alcohol in excess) seems to be associated with an increased life expectancy regardless of multimorbidity204. Moreover, as physical inactivity is a risk factor for several chronic conditions, it is of particular relevance for the prevention of multimorbidity in all age groups205, especially in individuals from socioeconomically deprived backgrounds who are more vulnerable to unhealthy lifestyle factors204, given the negative health effects associated with social deprivation.
Population level and structural changes are necessary to effectively prevent multimorbidity and limit its progression. These changes could focus on influencing the determinants of health by reducing the effects of a particular risk factor across the whole population206,207,208 (focusing, for example, on hypertension209, smoking210 and obesity211,212 at the population level). Moreover, population-level approaches may be needed to overcome structural racism and economic barriers213 and to address early determinants of multimorbidity, including socioeconomic deprivation and lower education level, to aid with the prevention of multimorbidity70,214,215.
Management
Most clinical practice guidelines and organization of health care focus on managing single diseases216. Cumulatively implementing a single-disease approach for patients with multimorbidity can lead to care that is impractical or even harmful20,186,217,218,219, particularly as the number and complexity of conditions increase. Management of multimorbidity requires an appropriate balance between a single-disease focus and multimorbidity care. Moreover, multimorbidity requires care that is both patient-centred and family-centred, prioritizing what matters most to the individual and the individual’s carers, ensuring care that is effectively coordinated and minimally disruptive, and aligns with patient values and priorities220. Recognizing the social, family and care context in which health-care activities are managed, decisions are made and care is experienced is essential, particularly in those with more complex health needs. The need for an individualized, patient-centred approach to care means that there is no single multimorbidity management pathway. The patients and care settings are heterogeneous and care approaches will vary from potentially curative to palliative approaches. This paradigm shift from a management approach focusing on a single condition to a multimorbidity approach to care challenges conventional approaches to care delivery and needs to be supported by research that can inform evidence-based treatments for multimorbidity with a broad focus on identifying and addressing the needs of the patient and the patient’s carers.
Evidence-based multimorbidity care
Given the challenges of managing multimorbidity, potential interventions are likely to be complex and multifaceted if they are to address the varied needs of the individual. Although an increasing numbers of studies have examined interventions for multimorbidity, evidence is still too limited to support any specific approach. One 2016 Cochrane review (which was corrected and re-published in 2021) included studies targeting multimorbidity (eight studies) and comorbidity (eight studies)221, and suggested that interventions targeting comorbidity or common clusters of conditions that include depression may improve mental health outcomes, but found no clear evidence of effectiveness for interventions targeting multimorbidity more broadly. Comorbidity interventions can be designed to address the challenges of patients with those specific conditions; for example, an intervention for people with diabetes mellitus and comorbid depression will combine elements of diabetes-focused care with psychotherapy or escalation of antidepressant medication. The most consistent evidence for comorbidity studies relates to collaborative care approaches for comorbid depression, which improve depression outcomes95. Interventions for comorbidity that have targeted depression222 or dementia care223, have tended to target the index condition (depression or dementia) with less focus on the other comorbid conditions, meaning they do not really address the multimorbidity experienced by the patient.
A 2021 systematic review included studies published up to 2019 and focused on trials of interventions targeting multimorbidity only (excluding comorbidity studies) and identified 8 further studies totalling 16 randomized controlled trials (RCTs)224. Most of these trials included older patients (mean age >70 years in 11 of the 16 studies) and individuals with at least three conditions. Interventions targeting multimorbidity need to be focused, yet generic, and, in this systematic review, interventions were provided by a range of disciplines based in established primary care systems in HICs. Interventions were divided broadly into three groups: care coordination225 combined with self-management support226, self-management support alone and medicines management227. Although there was no clear evidence of effectiveness for any specific intervention type, a combination of care coordination and self-management support was suggested to improve the patient experience of care. Another focus of multimorbidity trials has been enhancing self-management support; however, despite 12 of the 16 RCTs having this aim, no clear evidence of effect on self-management or health behaviours was found221. CGA involves specialist multidisciplinary assessment of older patients and care to address biopsychosocial needs and can be considered in older patients with multimorbidity. There is evidence that CGA improves outcomes in hospitalized patients228, although the effect on outcomes in primary care and community settings is less clear, and it is a very resource-intensive intervention229.
Four of the 16 RCTs in the 2021 systematic review evaluated medicines management-type interventions with mixed effects, which may have related to inappropriate patients being targeted, for example, in those with little room for improvement. A more recent RCT from Ireland also evaluated a medicines management intervention in individuals with multimorbidity, targeting older adults taking at least 15 medicines, and found a significant small reduction in the number of medicines, although no significant effect on the appropriateness of medicines was reported230.
Most existing trials have focused on older people but it is also important to address the needs of younger individuals who have different challenges, such as working and child-minding as well as managing their multimorbidity, particularly those in the poorest socioeconomic groups who develop multimorbidity earlier19. The CarePlus study in Scotland, UK, specifically targeted socioeconomically disadvantaged adults with multimorbidity with a multilevel intervention supporting practitioners and patients that reported a significant effect on negative well-being, although no effect on a range of other outcomes231. The CarePlus intervention was cost-effective within recommended UK funding thresholds, although this finding needs to be replicated in larger trials in other settings. Several challenges have arisen in existing trials of multimorbidity interventions (Table 1). Of note, interventions for multimorbidity have mostly been developed within well-established health-care delivery structures with strong primary care networks in HICs, with only limited development in LMICs43.
Evidence-based clinical guidelines
The limited available evidence on the treatment of multimorbidity makes it challenging for clinical guideline development, although a small number of guidelines have been developed internationally181,188,232. The consensus across these guidelines is presented in Box 2 (ref.233).
The general lack of evidence on which to base guideline recommendations has led to a reliance on consensus233. Although the evidence that multimorbidity care has major advantages over parallel care for single chronic conditions remains weak and inconsistent, qualitative research with patients and practitioners highlights the need for change. This research emphasizes the challenges patients face managing multiple conditions in fragmented medical systems that have largely been designed for the care of single chronic conditions and have not prioritized care coordination234,235. The NICE guideline on multimorbidity calls for a reorientation of care to address multimorbidity and highlights the importance of recognizing and addressing treatment burden for patients100,181.
Managing medicines is a key part of existing clinical guidelines for multimorbidity with an emphasis on patients with complex polypharmacy (those taking ten or more medicines regularly). Medicines management for multimorbidity typically includes an emphasis on deprescribing and/or addressing indicators of prescribing appropriateness. In the extensive literature on polypharmacy, potentially inappropriate prescribing and deprescribing, some systematic reviews have found an impact on validated measures of appropriate prescribing, but there is less clear evidence of effect on clinical outcomes and well-being189,190,236,237. Given the overlap between multimorbidity and polypharmacy, clinical guidelines for each condition often overlap233. One systematic review identified four guidelines for polypharmacy and four guidelines for multimorbidity with overlapping principles and recommendations including targeting those in need of intervention, undertaking holistic assessments of conditions and patient priorities, evaluating physiological status (frailty), reviewing medicines, individualizing management and ensuring appropriate monitoring233.
Multimorbidity and polypharmacy guidelines differ from single disease-oriented guidelines primarily in their generic focus and wider applicability. However, clinicians will still probably use elements of single-condition guidelines based on patient priorities, risk factors and symptoms. However, accounting for multimorbidity in single-disease guidelines is a key challenge. RCTs routinely exclude many patients with the target condition, notably older individuals and those with multimorbidity, co-prescribing or frailty238,239,240. Indeed, one systematic review of 50 studies of trial inclusion and exclusion criteria encompassing 305 trials and 31 physical conditions found that more than half of the trials excluded more than half of patients with the conditions studied241. Even patient cohorts in trials that are specifically conducted in older people are likely to significantly differ from the clinical population242 owing to explicit and implicit exclusion criteria or biases in trial recruitment (such as exclusion of house-bound individuals and those in care homes)243. These issues suggest that while treatment benefits observed in trials may be generally applicable to those with multimorbidity, the precise benefit in populations excluded from clinical trials may differ owing to varying baseline risk244 or increased treatment harms245.
In guidelines, the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system is used to determine the certainty of the evidence underpinning the clinical recommendations. GRADE accounts for indirectness of evidence, relating to the applicability of the evidence in terms of populations included and differences in trial design, interventions and outcomes246. A finding of serious limitations due to indirectness weakens the guideline recommendation for all patients, which may unfairly downgrade strong evidence for the population studied in the trial. The implication is that, rather than downgrading a global recommendation, developers of single-disease guidelines should make nuanced recommendations that explicitly account for variation in the strength of evidence for different groups of patients. Considering coexisting conditions at all major steps in the development of single-disease guidelines is necessary to frame questions so that indirectness to populations with multimorbidity can be identified247.
Both intervention studies and clinical guidelines need to identify and target patients who have significant treatment burden and who are in danger of being overwhelmed by self-management, which can result in poor adherence to treatment and adverse outcomes99,100,248. Patient-reported measures of treatment burden exist249,250,251, but their ability to predict adverse outcomes remains uncertain. There is increasing emphasis on understanding factors that influence an individual’s capacity to self-manage, which can vary over time as illnesses accumulate and personal circumstances change99,100,248,252,253 (Fig. 4). These factors include the work involved in taking medicines, self-monitoring, attending appointments and following health professional recommendations. Implications for clinical practice based on available evidence and clinical guidelines are summarized in Box 3 and global barriers and opportunities for multimorbidity management are summarized in Box 4.
Multimorbidity is often associated with high treatment burden, and patients might have lower capacity to self-manage and cope. Treatment burden is strongly associated with the number of chronic conditions217,324. Patient-reported measures of treatment burden exist249,250,251 but their ability to predict adverse outcomes is uncertain. The individual’s capacity to self-manage can vary over time as illnesses accumulate and personal circumstances change99,100,248,252,253.
Outcomes of care
Outcomes of care can be considered from both a care delivery and a research perspective. In clinical practice, the outcomes to prioritize can be decided between the patient, the patient’s carers and clinicians, by identifying the outcomes that matter most to the patient. In research, there is a need to systematize and harmonize outcomes to compare results across studies. A core outcome set for multimorbidity was developed by an expert panel, including multidisciplinary expert clinicians, researchers and patients from 13 countries254. HRQOL (see Quality of life, below), mental health and mortality are essential core outcomes in multimorbidity research. The other 17 core outcomes were grouped across the following domains: patient-reported effects and behaviours; physical activity and function; consultation-related; and health systems (Box 5). Another outcome set has been developed for measuring quality of care in multimorbidity using data from EHRs255, and recent256 and ongoing work257 aims to identify core outcomes in trials of prevention and treatment of multimorbidity in LMICs. Although cost outcomes of care are important to patients and health systems, there has been limited consideration of cost-effectiveness in trials of multimorbidity interventions, and existing studies have focused on health systems rather than patient costs or financial burden221.
From the patient perspective, managing multimorbidity is challenging owing to the burden of illness and the burden of treatment258,259. Treatment burden can be measured as an outcome of care251 in both clinical practice and research as new interventions should reduce rather than add to treatment burden. Moreover, some evidence shows that treatment burden also affects caregivers, and poses a pervasive challenge for both health-care providers and systems17,260,261,262,263,264,265,266,267,268,269. Furthermore, psychological distress experienced by patients with problematic multimorbidity and their caregivers may lead to fragmented and ruptured continuity of care and, therefore, complicate management11,270.
Multimorbidity outcomes include some promising indices of multimorbidity developed to predict mortality, health expenditures and physical functioning271,272,273,274,275,276, but there are few formal prediction tools181, and they require validation using high-quality data before their use can be recommended. These tools are primarily research outcomes and have not been developed or used to support clinical practice. Evidence supporting the use of prediction tools in primary care are particularly important given the opportunity to provide holistic patient-centred care in this setting.
Most of the available evidence on outcomes in multimorbidity pertains to HICs with minimal reports from LMICs277. Research in sub-Saharan Africa has examined the use of theoretical frameworks to aid understanding of chronic disease management and multimorbidity issues, such as the cumulative complexity model and burden of treatment theory35, in LMICs278. This preliminary work suggests that frameworks developed in HICs are generally applicable to LMICs but that there are some key differences and the absence of or limited access to required treatments is a key additional identified burden. A contextualized patient-reported measure to assess the effect of multimorbidity treatment and self-management burden on HRQOL and patient well-being could optimize patient-centred care delivery in these resource-constrained settings75.
Quality of life
Management of multimorbidity aims to improve patient outcomes. HRQOL is an essential outcome in multimorbidity research. Many observational studies have consistently shown that multimorbidity is associated with poor HRQOL and psychological well-being across the lifespan14,80,279,280,281. Some studies have suggested that this effect on HRQOL is stronger in younger individuals282, which some have suggested may be due to the accompanying life changes or biographical disruptions in younger people253. Others have indicated a more severe deterioration in well-being in older individuals80, with a less steep reduction in HRQOL as the number of conditions increases281. A higher number of conditions is associated with greater reductions in HRQOL282, and clusters of multimorbidity including both mental and physical conditions are also associated with poorer well-being81. Moreover, higher deprivation is associated with a more marked decrease in HRQOL with multimorbidity282. The association between HRQOL and multimorbidity is stronger when disease severity is taken into account280,283.
Outlook
Multimorbidity is a major global health challenge that is increasing in prevalence. Further evidence to support effective management and improve patient outcomes is needed, particularly in LMICs (Table 2).
Epidemiology and mechanisms
More longitudinal studies are needed that examine multifactorial pathways and disease trajectories across age, sex, gender, racial and socioeconomic groups. Moreover, other studies are needed to evaluate the use and clinical importance of multimorbidity clusters. Especially given the experience of the COVID-19 pandemic, a syndemic approach (considering interactions between conditions and factors affecting the interactions) is needed to address the shared social determinants of multimorbidity31,284.
Management
Most care for multimorbidity takes place in and is coordinated from primary care, and home-based and ambulatory settings, and these need to be reconfigured to address both acute episodic illnesses and chronic care, ensuring patient-centred and family-centred approaches that reduce treatment burden. Specialty care may be needed for those with more complex health needs and health systems need better integration of primary and specialty care and improved communication between care systems. Moving away from siloed care for individual conditions is urgently needed to improve quality of care and safety.
Interventions for multimorbidity
Clinicians, health managers and policy makers need guidance on how to develop interventions for multimorbidity owing to a paucity of evidence for management of this condition. These interventions should be based on known problems, which include lack of coordination, duplication, treatment burden, single-disease focus and problematic polypharmacy. Three key areas need to be considered, including targeting the appropriate patients and addressing their priorities, including their caregivers; supporting self-management and healthy behaviours; and delivering health and social care with a focus on interdisciplinary care and professional expertise (for example, in medicines management). Self-management support is part of many patient-oriented interventions, and is used widely in many single-disease programmes. Motivational interviewing is a critical component of self-management given the relationship between the accumulation of unhealthy behaviours and multimorbidity152. Of note, the concept of self-management may not entirely match the lived experience of people with multimorbidity: older adults frequently receive care from family or friends and are more likely to do so as their health worsens. Although self-management support has the potential to improve outcomes and reduce health-care utilization, evidence underpinning its effect in multimorbidity is limited221. However, self-management remains a key area for consideration in the evaluation of interventions in chronic diseases285.
Healthy behaviours are often a focus of self-management support interventions (for example, improving physical activity and participating in exercise therapy), and behavioural interventions targeting lifestyle behaviours may improve HRQOL and physical function and reduce depression286. Exercise has important health effects, including reducing blood pressure, inflammation, constipation, risk of thrombosis and, in those with diabetes mellitus, blood glucose levels, in addition to improving mood and mental health, pulmonary capacity, oxygen flow, muscle strength and stimulating metabolism287. A meta-analysis has suggested that exercise therapy is safe and effective in improving physical and psychosocial health in people with multimorbidity288. Given the demonstrated clinical effects of exercise on at least 26 chronic conditions287, it is promising both for treatment and prevention, especially when combined with other self-management supports. Importantly, barriers to participation need to be overcome to ensure adherence and effects in the long term289. Various international studies aim to investigate exercise therapy and self-management support for people with multimorbidity (such as MOBILIZE and PERFORM). Other health behaviours (healthy food, avoidance of smoking and responsible alcohol consumption) need to be considered when optimizing care of patients with multimorbidity, although an overemphasis on personal behaviours may not be appropriate or as effective as addressing broader socioeconomic determinants of health. Interventions that target both upstream and downstream determinants of health is essential290, and even those targeting individual behaviour need to take account of potential prevention burden (shifting responsibility for prevention to individuals) if they are to address health inequalities291.
Digital health and multimorbidity
More innovative approaches to the management of multimorbidity include interventions that incorporate digital health solutions. Going forward, following experiences of remote care delivery during the COVID-19 pandemic, interventions and care delivery need to consider the potential of digital health and artificial intelligence to reduce treatment burden and/or enhance patient capacity to self-manage and negotiate health-care systems. However, such interventions will need to consider how to prevent the increasing use of digital health from contributing to widening health inequality. Moreover, there are concerns about an increasing digital health literacy gap, which is more common in older individuals292, people of lower socioeconomic status, those with learning and other disabilities and those with language barriers293,294.
Personalized approaches
Although only in its infancy, personalized treatment, or precision medicine, targeted to the needs of the individual is promising for people with multimorbidity and might lead to health advantages by improving the effectiveness of, and reducing the number of adverse events from, various interventions295. We also need to consider how to help the increasing number of patients with multimorbidity and cognitive impairment and those with invisible disabilities such as chronic pain and fatigue (which is associated with many chronic conditions). More trials are needed to build an evidence base on how to manage multimorbidity and to help produce clinical guidelines. Co-design of interventions with patients, carers and clinicians has been lacking to date, although it may improve the effectiveness of interventions296. Trials of interventions for conditions sharing common characteristics and risk factors are also needed, particularly in terms of prevention of further disability, frailty and worsening health outcomes.
Models of care
Owing to the complexity of multimorbidity management, ensuring that clinical practice incorporates interdisciplinary care makes sense. Interdisciplinary teams have been central to the interventions published to date221. New models of integrated care that are being developed in many countries include teams of allied professionals joining doctor-led practices297,298,299. Enhancing teamwork could increase the likelihood of effective interventions for multimorbidity. Mechanisms to enhance teamwork are summarized in the Patient-Centred Innovation for Persons with Multimorbidity (PACEinMM) evidence-informed framework300, which highlights the following aspects: need for a shared team philosophy or vision; strong team relationships with a dedicated person acting as a bridge between the patient and the rest of the team; connectedness with all the components of the health-care system and the community to avoid duplication and work in silos; professional training specific to integrated care and enhanced patient relationships. This framework complements Wagner’s Chronic Care Model301 by identifying conditions under which productive interactions between the patient and the interdisciplinary teams may occur. Moreover, there is an increasing focus on ‘minimally disruptive medicine’218 which calls for clinicians to identify the patient’s burden of treatment, taking account of factors that influence capacity to self-manage; encourages a focus on care coordination; and prioritization from the patient perspective. Social prescribing is increasingly being adopted and aligns well with a patient-centred approach to multimorbidity302. However, despite its increasing popularity it does not have a strong evidence base and there are a wide range of definitions and types of approaches being adopted303. One potential model is the use of practice-based link workers who implement social prescribing, and there are two small trials exploring its effects in multimorbidity304,305.
Addressing the challenge of multimorbidity facing health systems requires a resilient health workforce and processes to tackle the interplay of health-system emergencies (such as pandemics and the health effects of climate change) with effective management of ongoing multimorbidity. Multimorbidity management will also require augmented skills in multidisciplinary team-based care through inter-professional learning and communication. Globally, health care is still predominantly organized around single conditions, and reimbursement models often reinforce this focus. This approach and structure needs to urgently change to enable a rebalancing between generalism and specialism in health-care systems. All aspects of health-care delivery need this reorientation by clinician training, producing policies and guidelines around clinical care delivery, adapting the places where health care is delivered to incorporate home-based and community-based care, and developing reimbursement models that recognize complexity. Although elements of specialty care delivery should be retained, more generalism is required both in primary care and in generalist specialist care across all ages. Beyond the dichotomies within clinical specialties, it remains critical and essential that patients, caregivers and families are at the core of services and receive high-quality care throughout the multiple ongoing interactions between patients and the health system. Closing the physical–mental health divide in health-care systems is also critical for managing complex physical–mental health multimorbidity. This requires both physical and mental health specialists taking at least some responsibility for the other condition (for example, cardiologists thinking about depression and psychiatrists thinking about smoking and cardiovascular risk), particularly in those with enduring serious mental illness. We need clinicians who are able to work effectively across the health-care divide. Relationships have been suggested to be the ‘silver bullet’ of general practice, enhancing trust, and there is growing evidence that continuity matters and is associated with improved outcomes306,307. For multimorbidity, we need to focus on promoting relationships both between practitioners, patient and caregivers and between health professionals to enhance care coordination and reduce fragmentation of care.
References
Valderas, J. M., Starfield, B., Sibbald, B., Salisbury, C. & Roland, M. Defining comorbidity: implications for understanding health and health services. Ann. Fam. Med. 7, 357–363 (2009).
Salisbury, C. Multimorbidity: redesigning health care for people who use it. Lancet 380, 7–9 (2012).
Atun, R. Transitioning health systems for multimorbidity. Lancet 386, 721–722 (2015).
Beran, D., Perel, P. & Miranda, J. J. Forty years since Alma-Ata: do we need a new model for noncommunicable diseases? J. Glob. Health https://doi.org/10.7189/jogh.09.010316 (2019).
Nicholson, K., Almirall, J. & Fortin, M. The measurement of multimorbidity. Health Psychol. 38, 783–790 (2019).
Harrison, C. et al. Comorbidity versus multimorbidity: why it matters. J. Comorb. 11, 2633556521993993 (2021).
Boyd, C. M. & Fortin, M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public. Health Rev. 32, 451–474 (2010).
Tugwell, P. & Knottnerus, J. A. Multimorbidity and comorbidity are now separate MESH headings. J. Clin. Epidemiol. 105, vi–viii (2019).
Menotti, A. et al. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: the FINE study (Finland, Italy, Netherlands, Elderly). J. Clin. Epidemiol. 54, 680–686 (2001).
Vogeli, C. et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J. Gen. Intern. Med. 22 (Suppl. 3), 391–395 (2007).
Fortin, M. et al. Psychological distress and multimorbidity in primary care. Ann. Fam. Med. 4, 417–422 (2006).
Fortin, M. et al. Multimorbidity and quality of life in primary care: a systematic review. Health Qual. Life Outcomes 2, 51 (2004).
Bayliss, E. A., Bayliss, M. S., Ware, J. E. Jr. & Steiner, J. F. Predicting declines in physical function in persons with multiple chronic medical conditions: what we can learn from the medical problem list. Health Qual. Life Out. 2, 47 (2004).
Marengoni, A. et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev. 10, 430–439 (2011).
Townsend, A., Hunt, K. & Wyke, S. Managing multiple morbidity in mid-life: a qualitative study of attitudes to drug use. BMJ 327, 837 (2003).
Ryan, A., Wallace, E., O’Hara, P. & Smith, S. M. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual. Life Outcomes 13, 168 (2015).
Lehnert, T. et al. Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med. Care Res. Rev. 68, 387–420 (2011).
Read, J. R., Sharpe, L., Modini, M. & Dear, B. F. Multimorbidity and depression: a systematic review and meta-analysis. J. Affect. Disord. 221, 36–46 (2017).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012). This study extracted data on 40 conditions from a database of 1.7 million people across Scotland, and highlighted the differences in prevalence of multimorbidity across age groups, sex and socioeconomic status.
Boyd, C. M. et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 294, 716–724 (2005). This early review suggests that adhering to clinical practice guidelines developed for individual conditions may have undesirable effects if applied in older people with several comorbidities; this was later confirmed by Hughes et al. (2013).
Mair, F. S. & May, C. R. Thinking about the burden of treatment. BMJ 349, g6680 (2014). This editorial introduces the concept of treatment burden and highlights the enormous demands that patients and their carers are put under by the health-care system.
Noel, P. H., Frueh, B. C., Larme, A. C. & Pugh, J. A. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expect. 8, 54–63 (2005). This early qualitative study highlights the concerns of patients with multimorbidity who report functional difficulties, negative effects on well-being and relationships and challenges managing multiple medicines.
Bower, P. et al. Multimorbidity, service organization and clinical decision making in primary care: a qualitative study. Fam. Pract. 28, 579–587 (2011).
Smith, S. M., O’Kelly, S. & O’Dowd, T. GPs’ and pharmacists’ experiences of managing multimorbidity: a ‘Pandora’s box’. Br. J. Gen. Pract. 60, 285–294 (2010).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Ryan, B. L. et al. Beyond the grey tsunami: a population-based study of multimorbidity in Ontario. Can. J. Public Health 109, 845–854 (2018).
Fortin, M., Stewart, M., Poitras, M.-E., Almirall, J. & Maddocks, H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann. Fam. Med. 10, 142–151 (2012).
Miranda, J. J. et al. Multimorbidity at sea level and high-altitude urban and rural settings: the CRONICAS cohort study. J. Comorb. 9, 2235042X19875297 (2019).
Miranda, J. J. et al. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat. Med. 25, 1667–1679 (2019). This review explores the long-term impacts of adverse exposures in early life, many of them more common in LMICs than in high-income settings, including the exposure to repeated infections and chronic undernutrition in childhood, among others, and how these environmental, social, and biological penalties in early life impair reaching full development of individuals, and thus provides a more nuanced understanding of the rising incidence of non-communicable diseases, including multimorbidity, in LMICs.
Pesantes, M. A. et al. Family support and diabetes: patient’s experiences from a public hospital in Peru. Qual. Health Res. 28, 1871–1882 (2018).
Pesantes, M. A., Tetens, A., Valle, A. D. & Miranda, J. J. “It is not easy living with this illness”: a syndemic approach to medication adherence and lifestyle change among low-income diabetes patients in Lima. Peru. Hum. Org. 78, 85–96 (2019).
Jan, S. et al. Action to address the household economic burden of non-communicable diseases. Lancet 391, 2047–2058 (2018).
Macinko, J., Andrade, F. C. D., Nunes, B. P. & Guanais, F. C. Primary care and multimorbidity in six Latin American and Caribbean countries. Rev. Panam. Salud Publica 43, e8 (2019).
Aebischer Perone, S. et al. Report of the WHO independent high-level commission on NCDs: where is the focus on addressing inequalities? BMJ Glob. Health https://doi.org/10.1136/bmjgh-2020-002820 (2020).
Chikumbu, E. et al. Experiences of multimorbidity in urban and rural Malawi: an interview study of burdens of treatment and lack of treatment. PLoS Glob. Public Health 2, e0000139 (2022).
Iaccarino, G. et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian Society of Hypertension. Hypertension 76, 366–372 (2020).
McQueenie, R. et al. Multimorbidity, polypharmacy, and COVID-19 infection within the UK Biobank cohort. PLoS ONE 15, e0238091 (2020).
Mair, F. S., Foster, H. M. & Nicholl, B. I. Multimorbidity and the COVID-19 pandemic – an urgent call to action. J. Comorb. 10, 2235042X20961676 (2020).
Emami, A., Javanmardi, F., Pirbonyeh, N. & Akbari, A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 8, e35 (2020).
Pati, S., Mahapatra, P., Kanungo, S., Uddin, A. & Sahoo, K. C. Managing multimorbidity (multiple chronic diseases) amid COVID-19 pandemic: a community based study from Odisha, India. Front. Public Health https://doi.org/10.3389/fpubh.2020.584408 (2021).
Burgess, R. COVID-19 mental-health responses neglect social realities. Nature https://doi.org/10.1038/d41586-020-01313-9 (2020).
Pesantes, A. et al. Los retos del cuidado de las personas con diabetes durante el estado de emergencia nacional por la COVID-19 en Lima, Perú: recomendaciones para la atención primaria. Rev. Peru. Med. Exp. Salud Publica 37, 541–546 (2020).
The Academy of Medical Sciences. Multimorbidity: a priority for global health research (The Academy of Medical Sciences, 2018). This report focuses on important research areas within multimorbidity, including pending agendas in LMICs.
The Lancet. Making more of multimorbidity: an emerging priority. Lancet 391, 1637 (2018).
Kruk, M. E. et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob. Health 6, e1196–e1252 (2018).
Fortin, M. “It-that-must-not-be-named”: addressing patient discomfort with the term multimorbidity. Can. Fam. Physician 64, 881–882 (2018).
World Health Organization. Multimorbidity: Technical Series on Safer Primary Care (WHO, 2016).
Griffith, L. E. et al. Multimorbidity frameworks impact prevalence and relationships with patient-important outcomes. J. Am. Geriatr. Soc. 67, 1632–1640 (2019).
Stirland, L. E. et al. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ 368, m160 (2020). This review demonstrates that 35 different multimorbidity indices are available with different components and outcomes and provides a guide to choosing a relevant multimorbidity index.
Aubert, C. E. et al. Best definitions of multimorbidity to identify patients with high health care resource utilization. Mayo Clin. Proc. Innov. Qual. Outcomes 4, 40–49 (2020).
Harrison, C., Britt, H., Miller, G. & Henderson, J. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open 4, e004694 (2014).
Gaspar, A. & Miranda, J. Burden of treatment as a measure of healthcare quality: an innovative approach to addressing global inequities in multimorbidity. PLoS Glob. Public. Health 2, e0000484 (2022).
Tran, V.-T., Barnes, C., Montori, V. M., Falissard, B. & Ravaud, P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med. 13, 115 (2015).
Storeng, S. H., Vinjerui, K. H., Sund, E. R. & Krokstad, S. Associations between complex multimorbidity, activities of daily living and mortality among older Norwegians. A prospective cohort study: the HUNT study, Norway. BMC Geriatr. 20, 21 (2020).
Ramond-Roquin, A., Haggerty, J., Lambert, M., Almirall, J. & Fortin, M. Different multimorbidity measures result in varying estimated levels of physical quality of life in individuals with multimorbidity: a cross-sectional study in the general population. Biomed. Res. Int. 2016, 7845438 (2016).
Ho, I. S.-S. et al. Examining variation in the measurement of multimorbidity in research: a systematic review of 566 studies. Lancet Public Health 6, e587–e597 (2021).
Lee, E. S. et al. Systematic review on the instruments used for measuring the association of the level of multimorbidity and clinically important outcomes. BMJ Open 11, e041219 (2021).
Johnston, M. C., Crilly, M., Black, C., Prescott, G. J. & Mercer, S. W. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur. J. Public. Health 29, 182–189 (2019).
Huntley, A. L., Johnson, R., Purdy, S., Valderas, J. M. & Salisbury, C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann. Fam. Med. 10, 134–141 (2012).
Boyd, C. M. et al. Framework for evaluating disease severity measures in older adults with comorbidity. J. Gerontol. A-Biol. 62, 286–295 (2007).
Yurkovich, M., Avina-Zubieta, J. A., Thomas, J., Gorenchtein, M. & Lacaille, D. A systematic review identifies valid comorbidity indices derived from administrative health data. J. Clin. Epidemiol. 68, 3–14 (2015).
Suls, J. et al. Measuring multimorbidity: selecting the right instrument for the purpose and the data source. Med. Care 59, 743–756 (2021).
Ho, I. S.-S. et al. Measuring multimorbidity in research: a Delphi consensus study. BMJ Med. https://doi.org/10.1136/bmjmed-2022-000247 (2022).
Nguyen, H. et al. Prevalence of multimorbidity in community settings: a systematic review and meta-analysis of observational studies. J. Comorb. 9, 2235042X19870934 (2019).
Hien, H. et al. Prevalence and patterns of multimorbidity among the elderly in Burkina Faso: cross-sectional study. Trop. Med. Int. Health 19, 1328–1333 (2014).
Pati, S., Swain, S., Hussain, M. A., Kadam, S. & Salisbury, C. Prevalence, correlates, and outcomes of multimorbidity among patients attending primary care in Odisha, India. Ann. Fam. Med. 13, 446–450 (2015).
Vadrevu, L., Kumar, V. & Kanjilal, B. Rising challenge of multiple morbidities among the rural poor in India – a case of the Sundarbans in West Bengal. Int. J. Med. Sci. Public Health https://doi.org/10.5455/ijmsph.2016.25082015129 (2016).
Huaquía-Díaz, A. M., Chalán-Dávila, T. S., Carrillo-Larco, R. M. & Bernabe-Ortiz, A. Multimorbidity in Latin America and the Caribbean: a systematic review and meta-analysis. BMJ Open 11, e050409 (2021).
Asogwa, O. A. et al. Multimorbidity of non-communicable diseases in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open 12, e049133 (2022).
Abebe, F., Schneider, M., Asrat, B. & Ambaw, F. Multimorbidity of chronic non-communicable diseases in low-and middle-income countries: a scoping review. J. Comorb. 10, 2235042X20961919 (2020).
Violan, C. et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS ONE 9, e102149 (2014).
Armocida, B. et al. Burden of non-communicable diseases among adolescents aged 10–24 years in the EU, 1990–2019: a systematic analysis of the Global Burden of Diseases Study 2019. Lancet Child Adolesc. Health https://doi.org/10.1016/s2352-4642(22)00073-6 (2022).
Pathirana, T. I. & Jackson, C. A. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust. N. Z. J. Public Health 42, 186–194 (2018).
Alimohammadian, M. et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. BMJ Open. 7, e013548 (2017).
Pati, S., Swain, S., Knottnerus, J. A., Metsemakers, J. F. M. & van den Akker, M. Magnitude and determinants of multimorbidity and health care utilization among patients attending public versus private primary care: a cross-sectional study from Odisha, India. Int. J. Equity Health 19, 57 (2020).
Whitty, C. J. & Watt, F. M. Map clusters of diseases to tackle multimorbidity. Nature 579, 494–496 (2020).
Prados-Torres, A., Calderon-Larranaga, A., Hancco-Saavedra, J., Poblador-Plou, B. & van den Akker, M. Multimorbidity patterns: a systematic review. J. Clin. Epidemiol. 67, 254–266 (2014).
Busija, L., Lim, K., Szoeke, C., Sanders, K. M. & McCabe, M. P. Do replicable profiles of multimorbidity exist? Systematic review and synthesis. Eur. J. Epidemiol. https://doi.org/10.1007/s10654-019-00568-5 (2019).
Ng, S. K., Tawiah, R., Sawyer, M. & Scuffham, P. Patterns of multimorbid health conditions: a systematic review of analytical methods and comparison analysis. Int. J. Epidemiol. 47, 1687–1704 (2018).
Tang, L. H. et al. The association between clusters of chronic conditions and psychological well-being in younger and older people–a cross-sectional, population-based study from the Lolland–Falster health study, Denmark. J. Comorb. 10, 2235042X20981185 (2020).
Sheridan, P. E., Mair, C. A. & Quiñones, A. R. Associations between prevalent multimorbidity combinations and prospective disability and self-rated health among older adults in Europe. BMC Geriatr. 19, 198 (2019).
Fisher, K. et al. Functional limitations in people with multimorbidity and the association with mental health conditions: baseline data from the Canadian Longitudinal Study on Aging (CLSA). PLoS ONE 16, e0255907 (2021).
Schousboe, J. T. et al. Depressive symptoms and total healthcare costs: roles of functional limitations and multimorbidity. J. Am. Geriatr. Soc. 67, 1596–1603 (2019).
Poblador-Plou, B. et al. Similar multimorbidity patterns in primary care patients from two European regions: results of a factor analysis. PLoS ONE 9, e100375 (2014).
Bayes-Marin, I. et al. Multimorbidity patterns in low-middle and high income regions: a multiregion latent class analysis using ATHLOS harmonised cohorts. BMJ Open. 10, e034441 (2020).
Roso-Llorach, A. et al. Comparative analysis of methods for identifying multimorbidity patterns: a study of ‘real-world’ data. BMJ Open 8, e018986 (2018).
Malecki, S. L. et al. A genetic model for multimorbidity in young adults. Genet. Med. https://doi.org/10.1038/s41436-019-0603-1 (2019).
Quinones, A. R. et al. Tracking multimorbidity changes in racial/ethnic diverse populations over time: issues and considerations. J. Gerontol. A. Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glz028 (2019).
Vetrano, D. L. et al. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat. Commun. 11, 3223 (2020).
Quiñones, A. R. et al. Racial and ethnic differences in multimorbidity changes over time. Med. Care 59, 402–409 (2021).
Bisquera, A. et al. Identifying longitudinal clusters of multimorbidity in an urban setting: a population-based cross-sectional study. Lancet Reg. Health Eur. https://doi.org/10.1016/j.lanepe.2021.100047 (2021).
Cezard, G., McHale, C. T., Sullivan, F., Bowles, J. K. F. & Keenan, K. Studying trajectories of multimorbidity: a systematic scoping review of longitudinal approaches and evidence. BMJ Open. 11, e048485 (2021).
Bica, T., Castelló, R., Toussaint, L. L. & Montesó‐Curto, P. Depression as a risk factor of organic diseases: an international integrative review. J. Nurs. Scholarsh. 49, 389–399 (2017).
Bauer, G. R. et al. Intersectionality in quantitative research: a systematic review of its emergence and applications of theory and methods. SSM-Popul. health 14, 100798 (2021).
Gold, S. M. et al. Comorbid depression in medical diseases. Nat. Rev. Dis. Prim. 6, 69 (2020).
Jani, B. D. et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK Biobank cohort. BMC Med. 17, 74 (2019).
Salisbury, C., Johnson, L., Purdy, S., Valderas, J. M. & Montgomery, A. A. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br. J. Gen. Pract. 61, e12–e21 (2011).
Frølich, A., Ghith, N., Schiøtz, M., Jacobsen, R. & Stockmarr, A. Multimorbidity, healthcare utilization and socioeconomic status: a register-based study in Denmark. PLoS ONE 14, e0214183 (2019).
Shippee, N. D., Shah, N. D., May, C. R., Mair, F. S. & Montori, V. M. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J. Clin. Epidemiol. 65, 1041–1051 (2012).
May, C. R. et al. Rethinking the patient: using burden of treatment theory to understand the changing dynamics of illness. BMC Health Serv. Res. 14, 281 (2014).
Jaspers, L. et al. The global impact of non-communicable diseases on households and impoverishment: a systematic review. Eur. J. Epidemiol. 30, 163–188 (2015).
Larkin, J., Foley, L., Smith, S. M., Harrington, P. & Clyne, B. The experience of financial burden for people with multimorbidity: a systematic review of qualitative research. Health Expect. 24, 282–295 (2021).
Thrush, A. & Hyder, A. A. The neglected burden of caregiving in low- and middle-income countries. Disabil. Health J. 7, 262–272 (2014).
Pesantes, M. A., Brandt, L. R., Ipince, A., Miranda, J. J. & Diez-Canseco, F. An exploration into caring for a stroke-survivor in Lima, Peru: emotional impact, stress factors, coping mechanisms and unmet needs of informal caregivers. eNeurologicalSci 6, 33–50 (2017).
Piette, J. D. & Kerr, E. A. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 29, 725–731 (2006).
Sturmberg, J. P., Bennett, J. M., Martin, C. M. & Picard, M. ‘Multimorbidity’ as the manifestation of network disturbances. J. Eval. Clin. Pract. 23, 199–208 (2017).
Wetterling, T. Pathogenesis of multimorbidity–what is known? Z. Gerontol. Geriatr. 54, 590–596 (2021).
Singer, L., Green, M., Rowe, F., Ben-Shlomo, Y. & Morrissey, K. Social determinants of multimorbidity and multiple functional limitations among the ageing population of England, 2002–2015. SSM-Popul. Health 8, 100413 (2019).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Barnes, P. J. Mechanisms of development of multimorbidity in the elderly. Eur. Respir. J. 45, 790–806 (2015).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Ermogenous, C., Green, C., Jackson, T., Ferguson, M. & Lord, J. M. Treating age-related multimorbidity: the drug discovery challenge. Drug Discov. Today 25, 1403–1415 (2020).
Yousefzadeh, M. et al. DNA damage – how and why we age? Elife https://doi.org/10.7554/eLife.62852 (2021).
Jakovljević, M., Reiner, Z., Milicić, D. & Crncević, Z. Comorbidity, multimorbidity and personalized psychosomatic medicine: epigenetics rolling on the horizon. Psychiatr. Danub. 22, 184–189 (2010).
Takeshima, H. & Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Oncol. 3, 7 (2019).
Stylianou, E. Epigenetics of chronic inflammatory diseases. J. Inflamm. Res. 12, 1–14 (2019).
Barnes, P. J. Senescence in COPD and its comorbidities. Annu. Rev. Physiol. 79, 517–539 (2017).
Casagrande, S. & Hau, M. Telomere attrition: metabolic regulation and signalling function? Biol. Lett. 15, 20180885 (2019).
Simons, M. J. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 24, 191–196 (2015).
Young, A. J. The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Philos. T. R. Soc. B Biol. Sci. 373, 20160452 (2018).
Niedzwiedz, C. L., Katikireddi, S. V., Pell, J. P. & Smith, D. J. Sex differences in the association between salivary telomere length and multimorbidity within the US Health & Retirement Study. Age Ageing 48, 703–710 (2019).
Bernabeu-Wittel, M. et al. Oxidative stress, telomere shortening, and apoptosis associated to sarcopenia and frailty in patients with multimorbidity. J. Clin. Med. https://doi.org/10.3390/jcm9082669 (2020).
Okamoto, K. & Seimiya, H. Revisiting telomere shortening in cancer. Cells https://doi.org/10.3390/cells8020107 (2019).
Chakravarti, D. et al. Telomere dysfunction instigates inflammation in inflammatory bowel disease. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2024853118 (2021).
Wang, M. Telomere shortening promotes kidney fibrosis. Nat. Rev. Nephrol. 17, 368 (2021).
Levstek, T., Kozjek, E., Dolžan, V. & Trebušak Podkrajšek, K. Telomere attrition in neurodegenerative disorders. Front. Cell Neurosci. 14, 219 (2020).
Höhn, A., Tramutola, A. & Cascella, R. Proteostasis failure in neurodegenerative diseases: focus on oxidative stress. Oxid. Med. Cell Longev. 2020, 5497046 (2020).
Farr, J. N. & Almeida, M. The spectrum of fundamental basic science discoveries contributing to organismal aging. J. Bone Min. Res. 33, 1568–1584 (2018).
Guerville, F. et al. Revisiting the hallmarks of aging to identify markers of biological age. J. Prev. Alzheimers Dis. 7, 56–64 (2020).
Guo, C., Sun, L., Chen, X. & Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 8, 2003 (2013).
Zhang, H., Menzies, K. J. & Auwerx, J. The role of mitochondria in stem cell fate and aging. Development https://doi.org/10.1242/dev.143420 (2018).
Gallage, S. & Gil, J. Mitochondrial dysfunction meets senescence. Trends Biochem. Sci. 41, 207–209 (2016).
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Schroth, J. & Henson, S. M. Mitochondrial dysfunction accelerates ageing. Immunometabolism 2, e200035 (2020).
Kirkwood, T. B. L. Understanding ageing from an evolutionary perspective. J. Intern. Med. 263, 117–127 (2008).
Correia-Melo, C., Hewitt, G. & Passos, J. F. Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev. Healthspan 3, 1 (2014).
Khosla, S., Farr, J. N., Tchkonia, T. & Kirkland, J. L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 16, 263–275 (2020).
Tchkonia, T., Palmer, A. K. & Kirkland, J. L. New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J. Clin. Endocrinol. Metab. 106, e1481–e1487 (2021).
Oh, J., Lee, Y. D. & Wagers, A. J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20, 870–880 (2014).
Mora, A. L. & Rojas, M. Adult stem cells for chronic lung diseases. Respirology 18, 1041–1046 (2013).
Fraser, H. C. et al. Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell https://doi.org/10.1111/acel.13524 (2022).
Shiels, P. G., Stenvinkel, P., Kooman, J. P. & McGuinness, D. Circulating markers of ageing and allostatic load: a slow train coming. Pract. Lab. Med. 7, 49–54 (2017).
Jani, B. D. et al. Risk assessment and predicting outcomes in patients with depressive symptoms: a review of potential role of peripheral blood based biomarkers. Front. Hum. Neurosci. 9, 18 (2015).
Seeman, T. E., McEwen, B. S., Rowe, J. W. & Singer, B. H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl Acad. Sci. USA 98, 4770–4775 (2001).
Guidi, J., Lucente, M., Sonino, N. & Fava, G. A. Allostatic load and its impact on health: a systematic review. Psychother. Psychosom. 90, 11–27 (2021).
Ingram, E. et al. Household and area-level social determinants of multimorbidity: a systematic review. J. Epidemiol. Community Health 75, 232–241 (2021).
Jackson, C. A., Dobson, A., Tooth, L. & Mishra, G. D. Body mass index and socioeconomic position are associated with 9-year trajectories of multimorbidity: a population-based study. Prev. Med. 81, 92–98 (2015).
Mounce, L. T. et al. Predicting incident multimorbidity. Ann. Fam. Med. 16, 322–329 (2018).
Kivimäki, M. et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 5, e140–e149 (2020).
Katikireddi, S. V., Skivington, K., Leyland, A. H., Hunt, K. & Mercer, S. W. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the lifecourse: a longitudinal analysis of the Twenty-07 cohort. BMC Med. 15, 152 (2017).
Freisling, H. et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 18, 5 (2020).
Fortin, M. et al. Lifestyle factors and multimorbidity: a cross sectional study. BMC Public Health 14, 686 (2014).
He, L. et al. The prevalence of multimorbidity and its association with physical activity and sleep duration in middle aged and elderly adults: a longitudinal analysis from China. Int. J. Behav. Nutr. Phys. Act. 18, 77 (2021).
Shang, X., Peng, W., Wu, J., He, M. & Zhang, L. Leading determinants for multimorbidity in middle-aged Australian men and women: a nine-year follow-up cohort study. Prev. Med. 141, 106260 (2020). This longitudinal cohort study examined factors that predict the development of multimorbidity and highlighted that individuals with low socioeconomic status and/or those in psychological distress should be targeted for preventive interventions.
Mishra, V. K., Srivastava, S., Muhammad, T. & Murthy, P. V. Population attributable risk for multimorbidity among adult women in India: do smoking tobacco, chewing tobacco and consuming alcohol make a difference? PLoS ONE 16, e0259578 (2021).
Poussin, C. et al. Systems toxicology study reveals reduced impact of heated tobacco product aerosol extract relative to cigarette smoke on premature aging and exacerbation effects in aged aortic cells in vitro. Arch. Toxicol. 95, 3341–3359 (2021).
Werner, C. et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120, 2438–2447 (2009).
Krokstad, S. et al. Multiple lifestyle behaviours and mortality, findings from a large population-based Norwegian cohort study – the HUNT Study. BMC Public Health 17, 58 (2017).
Ding, D., Rogers, K., van der Ploeg, H., Stamatakis, E. & Bauman, A. E. Traditional and emerging lifestyle risk behaviors and all-cause mortality in middle-aged and older adults: evidence from a large population-based australian cohort. PLoS Med. 12, e1001917 (2015).
Sun, J. W. et al. Association between television viewing time and all-cause mortality: a meta-analysis of cohort studies. Am. J. Epidemiol. 182, 908–916 (2015).
Jike, M., Itani, O., Watanabe, N., Buysse, D. J. & Kaneita, Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep. Med. Rev. 39, 25–36 (2018).
Cacioppo, J. T. & Cacioppo, S. Social relationships and health: the toxic effects of perceived social isolation. Soc. Personal. Psychol. Compass 8, 58–72 (2014).
Sindi, S. et al. Sleep disturbances and the speed of multimorbidity development in old age: results from a longitudinal population-based study. BMC Med. 18, 382 (2020).
Ruiz-Castell, M., Makovski, T. T., Bocquet, V. & Stranges, S. Sleep duration and multimorbidity in Luxembourg: results from the European Health Examination Survey in Luxembourg, 2013–2015. BMJ Open 9, e026942 (2019).
Loprinzi, P. D. Sedentary behavior and medical multimorbidity. Physiol. Behav. 151, 395–397 (2015).
Hajek, A., Kretzler, B. & König, H.-H. Multimorbidity, loneliness, and social isolation. A systematic review. Int. J. Environ. Res. Public Health 17, 8688 (2020).
Kim, J. M. et al. Insomnia, depression, and physical disorders in late life: a 2-year longitudinal community study in Koreans. Sleep 32, 1221–1228 (2009).
Gellert, P. et al. Testing the stress-buffering hypothesis of social support in couples coping with early-stage dementia. PLoS ONE 13, e0189849 (2018).
Sinnott, C., Mc Hugh, S., Fitzgerald, A. P., Bradley, C. P. & Kearney, P. M. Psychosocial complexity in multimorbidity: the legacy of adverse childhood experiences. Fam. Pract. 32, 269–275 (2015).
Hanlon, P. et al. Assessing risks of polypharmacy involving medications with anticholinergic properties. Ann. Fam. Med. 18, 148–155 (2020).
Lin, L., Wang, H. H., Lu, C., Chen, W. & Guo, V. Y. Adverse childhood experiences and subsequent chronic diseases among middle-aged or older adults in China and associations with demographic and socioeconomic characteristics. JAMA Netw. Open 4, e2130143 (2021).
Iob, E., Lacey, R. & Steptoe, A. The long-term association of adverse childhood experiences with C-reactive protein and hair cortisol: cumulative risk versus dimensions of adversity. Brain Behav. Immun. 87, 318–328 (2020).
Lang, J. et al. Adverse childhood experiences, epigenetics and telomere length variation in childhood and beyond: a systematic review of the literature. Eur. Child. Adolesc. Psychiatry 29, 1329–1338 (2020).
Herrmann, M., Pusceddu, I., März, W. & Herrmann, W. Telomere biology and age-related diseases. Clin. Chem. Lab. Med. 56, 1210–1222 (2018).
Prior, A., Vestergaard, M., Larsen, K. K. & Fenger-Grøn, M. Association between perceived stress, multimorbidity and primary care health services: a Danish population-based cohort study. BMJ Open 8, e018323 (2018).
Prior, A. et al. Perceived stress, multimorbidity, and risk for hospitalizations for ambulatory care-sensitive conditions: a population-based cohort study. Med. Care 55, 131–139 (2017).
McEwen, B. S. & Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 153, 2093–2101 (1993).
Crimmins, E. M. Social hallmarks of aging: suggestions for geroscience research. Ageing Res. Rev. 63, 101136 (2020).
Newcomer, J. W. Antipsychotic medications: metabolic and cardiovascular risk. J. Clin. Psychiatry 64, 8–13 (2007).
Dalton, S. O. et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch. Intern. Med. 163, 59–64 (2003).
National Institute for Health and Care Excellence. Multimorbidity: clinical assessment and management. NICE https://www.nice.org.uk/guidance/ng56 (2016). This clinical guideline for multimorbidity addresses optimizing care for adults with multimorbidity with a focus on reducing treatment burden and adopting an approach to care that takes account of multimorbidity, including how to identify suitable patients to target and how care can be reoriented.
Fried, L. P. et al. Frailty in older adults evidence for a phenotype. J. Gerontol. Ser. A 56, M146–M157 (2001).
Hanlon, P. et al. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 3, e323–e332 (2018).
Vetrano, D. L. et al. Frailty and multimorbidity: a systematic review and meta-analysis. J. Gerontol. A. Biol. Sci. Med. Sci. 74, 659–666 (2019).
Dugravot, A. et al. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: a 24-year follow-up of the Whitehall II cohort study. Lancet Pub. Health 5, e42–e50 (2020).
American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American Geriatrics Society. J. Am. Geriatr. Soc. 60, 1957–1968 (2012).
Tinetti, M. E. et al. Association of patient priorities – aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: a nonrandomized clinical trial. JAMA Intern. Med. 179, 1688–1697 (2019).
Boyd, C. et al. Decision making for older adults with multiple chronic conditions: executive summary for the American Geriatrics Society guiding principles on the care of older adults with multimorbidity. J. Am. Geriatr. Soc. 67, 665–673 (2019).
Clyne, B. et al. Interventions to address potentially inappropriate prescribing in community-dwelling older adults: a systematic review of randomized controlled trials. J. Am. Geriatr. Soc. 64, 1210–1222 (2016).
Rankin, A. et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD008165.pub4 (2018).
Spencer, E. A., Ford, G. A., Chan, M. S., Perera, R. & Heneghan, C. Biomarkers in the prediction of multimorbidity: scoping review. Preprint at medRxiv https://doi.org/10.1101/2020.11.25.20214999 (2020).
Strandberg, A. Y. et al. Low midlife blood pressure, survival, comorbidity, and health-related quality of life in old age: the Helsinki Businessmen study. J. Hypertens. 32, 1797–1804 (2014).
Ishizaki, T. et al. Association of physical performance and self-rated health with multimorbidity among older adults: results from a nationwide survey in Japan. Arch. Gerontol. Geriat. 84, 103904 (2019).
Cheung, C.-L., Nguyen, U.-S. D., Au, E., Tan, K. C. & Kung, A. W. Association of handgrip strength with chronic diseases and multimorbidity. Age 35, 929–941 (2013).
Taylor, A. W. et al. Multimorbidity–not just an older person’s issue. Results from an Australian biomedical study. BMC Public Health 10, 718 (2010).
Kivimäki, M. et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2, e277–e285 (2017).
Buttery, S. C. et al. Contemporary perspectives in COPD: patient burden, the role of gender and trajectories of multimorbidity. Respirology 26, 419–441 (2021).
Ferreira, G. D. et al. Physiological markers and multimorbidity: a systematic review. J. Comorb. 8, 2235042X18806986 (2018).
Pérez, L. M. et al. Glutathione serum levels and rate of multimorbidity development in older adults. J. Gerontol. A Biol. 75, 1089–1094 (2020).
Schöttker, B., Saum, K.-U., Jansen, E. H., Holleczek, B. & Brenner, H. Associations of metabolic, inflammatory and oxidative stress markers with total morbidity and multi-morbidity in a large cohort of older German adults. Age Ageing 45, 127–135 (2016).
Calderón-Larrañaga, A. et al. Association of homocysteine, methionine, and MTHFR 677C>T polymorphism with rate of cardiovascular multimorbidity development in older adults in Sweden. JAMA Netw. Open 3, e205316 (2020).
Meems, L. M. et al. Low levels of vitamin D are associated with multimorbidity: results from the LifeLines Cohort study. Ann. Med. 47, 474–481 (2015).
Moo, H. et al. The effect of the comorbidity burden on vitamin D levels in geriatric hip fracture. BMC Musculoskel. Dis. 21, 524 (2020).
Foster, H. M. E. et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health 3, e576–e585 (2018).
Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2, 1143–1211 (2012).
Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 30, 427–432 (2001).
Stuckler, D. et al. in Sick Societies: Responding to the Global Challenge of Chronic Disease Ch. 4 (eds Stuckler, D. & Siegel, K.) 87–134 (Oxford Univ. Press, 2011).
Kontis, V. et al. Three public health interventions could save 94 million lives in 25 years: global impact assessment analysis. Circulation 140, 715–725 (2019).
Bernabe-Ortiz, A. et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat. Med. 26, 374–378 (2020).
Warner, K. E. Tobacco control policies and their impacts. Past, present, and future. Ann. Am. Thorac. Soc. 11, 227–230 (2014).
Colchero, M. A., Rivera-Dommarco, J., Popkin, B. M. & Ng, S. W. In Mexico, evidence of sustained consumer response two years after implementing a sugar-sweetened beverage tax. Health Aff. 36, 564–571 (2017).
Corvalán, C., Reyes, M., Garmendia, M. L. & Uauy, R. Structural responses to the obesity and non-communicable diseases epidemic: update on the Chilean law of food labelling and advertising. Obes. Rev. 20, 367–374 (2019).
Bhopal, R. et al. The global society on migration, ethnicity, race and health: why race can’t be ignored even if it causes discomfort. Eur. J. Public. Health 31, 3–4 (2021).
Ben-Shlomo, Y. & Kuh, D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int. J. Epidemiol. 31, 285–293 (2002).
Marmot, M. & Allen, J. J. Social determinants of health equity. Am. J. Public Health 104 (Suppl. 4), 517–519 (2014).
Boyd, C. M. & Kent, D. M. Evidence-based medicine and the hard problem of multimorbidity. J. Gen. Intern. Med. 29, 552–553 (2014).
Guthrie, B., Payne, K., Alderson, P., McMurdo, M. E. T. & Mercer, S. W. Adapting clinical guidelines to take account of multimorbidity. BMJ 345, e6341 (2012).
May, C., Montori, V. M. & Mair, F. S. We need minimally disruptive medicine. BMJ 339, b2803 (2009).
Tinetti, M. E., Bogardus, S. T. Jr. & Agostini, J. V. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N. Engl. J. Med. 351, 2870–2874 (2004).
Hargraves, I. G. & Montori, V. M. Aligning care with patient values and priorities. JAMA Intern. Med. 179, 1697–1698 (2019).
Smith, S. M., Wallace, E., O’Dowd, T. & Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst. Rev. 1, Cd006560 (2021).
Araya, R. et al. Effect of a digital intervention on depressive symptoms in patients with comorbid hypertension or diabetes in Brazil and Peru: two randomized clinical trials. JAMA 325, 1852–1862 (2021).
Possin, K. L. et al. Effect of collaborative dementia care via telephone and internet on quality of life, caregiver well-being, and health care use: the care ecosystem randomized clinical trial. JAMA Intern. Med. 179, 1658–1667 (2019).
Smith, S. M., Wallace, E., Clyne, B., Boland, F. & Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst. Rev. 10, 271 (2021). This systematic review concluded that despite identifying 16 randomized trials evaluating interventions for multimorbidity, there is no clear evidence to inform policy and practice. Future research should consider patient experience of care, optimizing medicines management and targeted patient health behaviours such as exercise.
McDonald, K. M. et al. Care coordination measures atlas update (Agency for Healthcare Research and Quality, 2014).
Dineen-Griffin, S., Garcia-Cardenas, V., Williams, K. & Benrimoj, S. I. Helping patients help themselves: a systematic review of self-management support strategies in primary health care practice. PLoS ONE 14, e0220116 (2019).
McGrattan, M., Ryan, C., Barry, H. E. & Hughes, C. M. Interventions to improve medicines management for people with dementia: a systematic review. Drugs Aging 34, 907–916 (2017).
Ellis, G. et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst. Rev. 9, Cd006211 (2017).
Ferrat, E. et al. Efficacy of nurse-led and general practitioner-led comprehensive geriatric assessment in primary care: protocol of a pragmatic three-arm cluster randomised controlled trial (CEpiA study). BMJ Open 8, e020597 (2018).
McCarthy, C. et al. GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE study): a cluster randomised controlled trial. PLoS Med. 19, e1003862 (2022).
Mercer, S. W. et al. The CARE Plus study – a whole-system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: exploratory cluster randomised controlled trial and cost-utility analysis. BMC Med. 14, 88 (2016).
Onder, G. et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin. Exp. Res. https://doi.org/10.1007/s40520-022-02094-z (2022).
Muth, C. et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. J. Intern. Med. 285, 272–288 (2019).
Sinnott, C., Mc Hugh, S., Browne, J. & Bradley, C. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open 3, e003610 (2013).
Noël, P. H. et al. The challenges of multimorbidity from the patient perspective. J. Gen. Int. Med. 22, 419–424 (2007).
Reeve, E. et al. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging 30, 793–807 (2013).
Doherty, A. J. et al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open https://doi.org/10.3399/bjgpopen20X101096 (2020).
Buffel du Vaure, C., Dechartres, A., Battin, C., Ravaud, P. & Boutron, I. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials.gov: a systematic review of registration details. BMJ Open 6, e012265 (2016).
Van Spall, H. G., Toren, A., Kiss, A. & Fowler, R. A. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 297, 1233–1240 (2007).
Lee, P. Y., Alexander, K. P., Hammill, B. G., Pasquali, S. K. & Peterson, E. D. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA 286, 708–713 (2001).
He, J., Morales, D. R. & Guthrie, B. Exclusion rates in randomized controlled trials of treatments for physical conditions: a systematic review. Trials 21, 228 (2020).
Hanlon, P. et al. Observed and expected serious adverse event rates in randomised clinical trials for hypertension: an observational study comparing trials that do and do not focus on older people. Lancet Healthy Longev. 2, e398–e406 (2021).
Boyd, C. M., Vollenweider, D. & Puhan, M. A. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS ONE 7, e41601 (2012).
O’Hare, A. M. et al. Interpreting treatment effects from clinical trials in the context of real-world risk information: end-stage renal disease prevention in older adults. JAMA Intern. Med. 174, 391–397 (2014).
Li, L., Geraghty, O. C., Mehta, Z. & Rothwell, P. M. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 390, 490–499 (2017).
Guyatt, G. H. et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J. Clin. Epidemiol. 64, 1303–1310 (2011).
Uhlig, K. et al. A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J. Gen. Intern. Med. 29, 670–679 (2014).
Demain, S. et al. Living with, managing and minimising treatment burden in long term conditions: a systematic review of qualitative research. PLoS ONE 10, e0125457 (2015).
Eton, D. T. et al. Deriving and validating a brief measure of treatment burden to assess person-centered healthcare quality in primary care: a multi-method study. BMC Fam. Pract. 21, 221 (2020).
Tran, V. T. et al. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 12, 109 (2014).
Duncan, P. et al. Development and validation of the multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open 8, e019413 (2018).
Gallacher, K. I., May, C. R., Langhorne, P. & Mair, F. S. A conceptual model of treatment burden and patient capacity in stroke. BMC Fam. Pract. 19, 9 (2018).
Boehmer, K. R. et al. Patient capacity and constraints in the experience of chronic disease: a qualitative systematic review and thematic synthesis. BMC Fam. Pract. 17, 127 (2016).
Smith, S. M. et al. A core outcome set for multimorbidity research (COSmm). Ann. Fam. Med. 16, 132–138 (2018).
Bayliss, E. A. et al. Using electronic health record data to measure care quality for individuals with multiple chronic medical conditions. J. Am. Geriatr. Soc. 64, 1839–1844 (2016).
Hurst, J. R. et al. Critical review of multimorbidity outcome measures suitable for low-income and middle-income country settings: perspectives from the Global Alliance for Chronic Diseases (GACD) researchers. BMJ Open 10, e037079 (2020).
Boehnke, J. R. et al. Development of a core outcome set for multimorbidity trials in low/middle-income countries (COSMOS): study protocol. BMJ Open 12, e051810 (2022).
Boyd, C. M. et al. Healthcare task difficulty among older adults with multimorbidity. Med. Care 52 (Suppl. 3), S118–S125 (2014).
Van Merode, T., Van De Ven, K. & Van Den Akker, M. Patients with multimorbidity and their treatment burden in different daily life domains: a qualitative study in primary care in the Netherlands and Belgium. J. Comorb. 8, 9–15 (2018).
Price, M. L., Surr, C. A., Gough, B. & Ashley, L. Experiences and support needs of informal caregivers of people with multimorbidity: a scoping literature review. Psychol. Health 35, 36–69 (2020).
Amer Nordin, A., Mohd Hairi, F., Choo, W. Y. & Hairi, N. N. Care recipient multimorbidity and health impacts on informal caregivers: a systematic review. Gerontologist 59, e611–e628 (2019).
Ploeg, J. et al. Managing multiple chronic conditions in the community: a Canadian qualitative study of the experiences of older adults, family caregivers and healthcare providers. BMC Geriatr. 17, 40 (2017).
Newbould, J. et al. Experiences of care planning in England: interviews with patients with long term conditions. BMC Fam. Pract. 13, 71 (2012).
Aubert, C. E. et al. Multimorbidity and healthcare resource utilization in Switzerland: a multicentre cohort study. BMC Health Serv. Res. 19, 708 (2019).
Zhao, Q. et al. Health-related quality of life and health service use among multimorbid middle-aged and older-aged adults in China: a cross-sectional study in Shandong Province. Int. J. Environ. Res. Public Health 17, 9261 (2020).
Pati, S. et al. Non communicable disease multimorbidity and associated health care utilization and expenditures in India: cross-sectional study. BMC Health Serv. Res. 14, 451 (2014).
Soley-Bori, M. et al. Impact of multimorbidity on healthcare costs and utilisation: a systematic review of the UK literature. Br. J. Gen. Pract. 71, e39–e46 (2021).
McPhail, S. M. Multimorbidity in chronic disease: impact on health care resources and costs. Risk Manag. Healthc. Policy 9, 143 (2016).
Wolff, J. L. & Boyd, C. M. A look at person- and family-centered care among older adults: results from a national survey [corrected]. J. Gen. Intern. Med. 30, 1497–1504 (2015).
Swartz, J. A. & Jantz, I. Association between nonspecific severe psychological distress as an indicator of serious mental illness and increasing levels of medical multimorbidity. Am. J. Public Health 104, 2350–2358 (2014).
Stanley, J. & Sarfati, D. The new measuring multimorbidity index predicted mortality better than Charlson and Elixhauser indices among the general population. J. Clin. Epidemiol. 92, 99–110 (2017).
Olaya, B. et al. Latent class analysis of multimorbidity patterns and associated outcomes in Spanish older adults: a prospective cohort study. BMC Geriatr. 17, 186 (2017).
Constantinou, P. et al. Two morbidity indices developed in a nationwide population permitted performant outcome-specific severity adjustment. J. Clin. Epidemiol. 103, 60–70 (2018).
Wei, M. Y., Kawachi, I., Okereke, O. I. & Mukamal, K. J. Diverse cumulative impact of chronic diseases on physical health-related quality of life: implications for a measure of multimorbidity. Am. J. Epidemiol. 184, 357–365 (2016).
Fortin, M., Almirall, J. & Nicholson, K. Development of a research tool to document self-reported chronic conditions in primary care. J. Comorb. 7, 117–123 (2017).
Wei, M. Y. & Mukamal, K. J. Multimorbidity, mortality, and long-term physical functioning in 3 prospective cohorts of community-dwelling adults. Am. J. Epidemiol. 187, 103–112 (2018).
van der Aa, M. J., van den Broeke, J. R., Stronks, K. & Plochg, T. Patients with multimorbidity and their experiences with the healthcare process: a scoping review. J. Comorb. 7, 11–21 (2017).
Matima, R., Murphy, K., Levitt, N. S., BeLue, R. & Oni, T. A qualitative study on the experiences and perspectives of public sector patients in Cape Town in managing the workload of demands of HIV and type 2 diabetes multimorbidity. PLoS ONE 13, e0194191 (2018).
Michelson, H., Bolund, C. & Brandberg, Y. Multiple chronic health problems are negatively associated with health related quality of life (HRQoL) irrespective of age. Qual. Life. Res. 9, 1093–1104 (2000).
Fortin, M. et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual. Life. Res. 15, 83–91 (2006).
Makovski, T. T., Schmitz, S., Zeegers, M. P., Stranges, S. & van den Akker, M. Multimorbidity and quality of life: systematic literature review and meta-analysis. Ageing Res. Rev. 53, 100903 (2019).
Lawson, K. D. et al. Double trouble: the impact of multimorbidity and deprivation on preference-weighted health related quality of life a cross sectional analysis of the Scottish Health Survey. Int. J. Equity Health 12, 67 (2013).
Brettschneider, C. et al. Relative impact of multimorbid chronic conditions on health-related quality of life – results from the MultiCare cohort study. PLoS ONE 8, e66742 (2013).
Singer, M., Bulled, N., Ostrach, B. & Mendenhall, E. Syndemics and the biosocial conception of health. Lancet 389, 941–950 (2017).
Poitras, M. E., Maltais, M. E., Bestard-Denommé, L., Stewart, M. & Fortin, M. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv. Res. 18, 446 (2018).
Bricca, A. et al. Effect of in-person delivered behavioural interventions in people with multimorbidity: systematic review and meta-analysis. Int. J. Behav. Med. https://doi.org/10.1007/s12529-022-10092-8 (2022).
Pedersen, B. K. & Saltin, B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 25 (Suppl. 3), 1–72 (2015).
Bricca, A. et al. Benefits and harms of exercise therapy in people with multimorbidity: a systematic review and meta-analysis of randomised controlled trials. Ageing Res. Rev. 63, 101166 (2020). This systematic review identified 23 randomized trials and concluded that exercise therapy appears to be safe and to have a beneficial effect on physical and psychosocial health in people with multimorbidity.
Jäger, M. et al. Putting the pieces together: a qualitative study exploring perspectives on self-management and exercise behavior among people living with multimorbidity, healthcare professionals, relatives, and patient advocates. J. Multimorb. Comorb. https://doi.org/10.1177/26335565221100172 (2022).
Rutter, H. et al. Balancing upstream and downstream measures to tackle the obesity epidemic: a position statement from the European Association for the Study of Obesity. Obes. Facts 10, 61–63 (2017).
O’Donnell, C. et al. We should consider prevention burden in our approach to tackling NCDs. BMJ Opinion https://blogs.bmj.com/bmj/2020/09/20/we-should-consider-prevention-burden-in-our-approach-to-tackling-ncds/ (2020).
Vaportzis, E., Clausen, M. G. & Gow, A. J. Older adults perceptions of technology and barriers to interacting with tablet computers: a focus group study. Front. Psychol. 8, 1687 (2017).
Lennon, M. R. et al. Readiness for delivering digital health at scale: lessons from a longitudinal qualitative evaluation of a national digital health innovation program in the United Kingdom. J. Med. Internet Res. 19, e42 (2017).
Watts, G. COVID-19 and the digital divide in the UK. Lancet Digit. Health 2, e395–e396 (2020).
Jameson, J. L. & Longo, D. L. Precision medicine–personalized, problematic, and promising. N. Engl. J. Med. 372, 2229–2234 (2015).
Dobson, J. Co-production helps ensure that new technology succeeds. BMJ 366, l4833 (2019).
Australian Centre for the Medical Home. What is a Medical Home? Australian Centre for the Medical Home http://medicalhome.org.au/what-is-a-medical-home/ (2022).
Primary Care Collaborative. Defining the medical home. PCPCC https://www.pcpcc.org/about/medical-home (2021).
Patient’s Medical Home. Family practice teams. Patient’s Medical Home https://patientsmedicalhome.ca/vision/physicians/ (2021).
Fortin, M. & Stewart, M. Implementing patient-centred integrated care for multiple chronic conditions: evidence-informed framework. Can. Fam. Physician 67, 235–238 (2021). This evidence-informed framework is rooted in specific literature about patient-centred care and is intended for guiding interventions in patients with multimorbidity.
Wagner, E. H. Chronic disease management: what will it take to improve care for chronic illness? Eff. Clin. Pract. 1, 2–4 (1998).
The King’s Fund. What is social prescribing? The King’s fund https://www.kingsfund.org.uk/publications/social-prescribing (2020).
Husk, K., Elston, J., Gradinger, F., Callaghan, L. & Asthana, S. Social prescribing: where is the evidence? Br. J. Gen. Pract. 69, 6–7 (2019).
Mercer, S. W. et al. Effectiveness of community-links practitioners in areas of high socioeconomic deprivation. Ann. Fam. Med. 17, 518–525 (2019).
Kiely, B. et al. Link workers providing social prescribing and health and social care coordination for people with multimorbidity in socially deprived areas (the LinkMM trial): protocol for a pragmatic randomised controlled trial. BMJ Open 11, e041809 (2021).
Pereira Gray, D. J., Sidaway-Lee, K., White, E., Thorne, A. & Evans, P. H. Continuity of care with doctors – a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open 8, e021161 (2018).
van Walraven, C., Oake, N., Jennings, A. & Forster, A. J. The association between continuity of care and outcomes: a systematic and critical review. J. Eval. Clin. Pract. 16, 947–956 (2010).
Salisbury, C. et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 392, 41–50 (2018).
Boult, C. et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch. Intern. Med. 171, 460–466 (2011).
Muth, C. et al. Effectiveness of a complex intervention on prioritising multimedication in multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open 8, e017740 (2018).
Mann, C. et al. Can implementation failure or intervention failure explain the result of the 3D multimorbidity trial in general practice: mixed-methods process evaluation. BMJ Open 9, e031438 (2019).
Basto-Abreu, A. et al. Multimorbidity matters in low and middle-income countries. J. Multimorb. Comorb. https://doi.org/10.1177/26335565221106074 (2022).
Nicholson, K. et al. Prevalence, characteristics, and patterns of patients with multimorbidity in primary care: a retrospective cohort analysis in Canada. Br. J. Gen. Pract. 69, e647–e656 (2019).
Excoffier, S., Herzig, L., N’Goran, A. A., Déruaz-Luyet, A. & Haller, D. M. Prevalence of multimorbidity in general practice: a cross-sectional study within the Swiss Sentinel Surveillance System (Sentinella). BMJ Open 8, e019616 (2018).
van den Akker, M., Buntinx, F., Metsemakers, J. F., Roos, S. & Knottnerus, J. A. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J. Clin. Epidemiol. 51, 367–375 (1998).
Corallo, B., Proser, M. & Nocon, R. Comparing rates of multiple chronic conditions at primary care and mental health visits to community health centers versus private practice providers. J. Ambul. Care Manag. 43, 136–147 (2020).
Prazeres, F. & Santiago, L. Prevalence of multimorbidity in the adult population attending primary care in Portugal: a cross-sectional study. BMJ Open 5, e009287 (2015).
Uijen, A. A. & van de Lisdonk, E. H. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur. J. Gen. Pract. 14 (Suppl. 1), 28–32 (2008).
Britt, H. C., Harrison, C. M., Miller, G. C. & Knox, S. A. Prevalence and patterns of multimorbidity in Australia. Med. J. Aust. 189, 72–77 (2008).
C, R. & Jeemon, P. Prevalence and patterns of multi-morbidity in the productive age group of 30–69 years: a cross-sectional study in Pathanamthitta District, Kerala. Wellcome Open Res. 5, 233 (2020).
Keetile, M., Navaneetham, K. & Letamo, G. Prevalence and correlates of multimorbidity among adults in Botswana: a cross-sectional study. PLoS ONE 15, e0239334 (2020).
Nunes, B. P. et al. Multimorbidity: the Brazilian longitudinal study of aging (ELSI-Brazil). Rev. Saude Publica 52 (Suppl. 2), 10s (2018).
Rzewuska, M. et al. Epidemiology of multimorbidity within the Brazilian adult general population: evidence from the 2013 national health survey (PNS 2013). PLoS ONE 12, e0171813 (2017).
Hughes, L. D., McMurdo, M. E. & Guthrie, B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 42, 62–69 (2013).
Groot, V. D., Beckerman, H., Lankhorst, G. J. & Bouter, L. M. How to measure comorbidity: a critical review of available methods. J. Clin. Epidemiol. 56, 221–229 (2003).
Diederichs, C., Berger, K. & Bartels, D. B. The measurement of multiple chronic diseases–a systematic review on existing multimorbidity indices. J. Gerontol. A. Biol. Sci. Med. Sci. 66, 301–311 (2011).
Le Reste, J. Y. et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J. Am. Med. Dir. Assoc. 14, 319–325 (2013).
National Institute for Health and Care Excellence. Common mental health problems: identification and pathways to care. NICE https://www.nice.org.uk/guidance/cg123 (2011).
Pati, S., Hussain, M. A., Chauhan, A. S., Mallick, D. & Nayak, S. Patient navigation pathway and barriers to treatment seeking in cancer in India: a qualitative inquiry. Cancer Epidemiol. 37, 973–978 (2013).
Foo, K. M., Sundram, M. & Legido-Quigley, H. Facilitators and barriers of managing patients with multiple chronic conditions in the community: a qualitative study. BMC Public Health 20, 273 (2020).
Taype-Rondan, A., Lazo-Porras, M., Moscoso-Porras, M., Moreano-Sáenz, M. & Miranda, J. J. Inadequate glycaemic control in LMIC: health system failures in Peru. Br. J. Gen. Pract. 66, 197 (2016).
Banerjee, A., Hurst, J., Fottrell, E. & Miranda, J. J. Multimorbidity: not just for the west. Glob. Heart 15, 45 (2020).
Seiglie, J. A., Nambiar, D., Beran, D. & Miranda, J. J. To tackle diabetes, science and health systems must take into account social context. Nat. Med. 27, 193–195 (2021).
Bernabe-Ortiz, A., Diez-Canseco, F., Vásquez, A. & Miranda, J. J. Disability, caregiver’s dependency and patterns of access to rehabilitation care: results from a national representative study in Peru. Disabil. Rehabil. 38, 582–588 (2016).
Rubinstein, A. et al. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 4, 52–63 (2016).
Bernabe-Ortiz, A., Pauschardt, J., Diez-Canseco, F. & Miranda, J. J. Sustainability of mHealth effects on cardiometabolic risk factors: five-year results of a randomized clinical trial. J. Med. Internet Res. 22, e14595 (2020).
Miranda, J. J. et al. Role of mHealth in overcoming the occurrence of post-stroke depression. Acta Neurol. Scand. 137, 12–19 (2018).
Victor, R. G. et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N. Engl. J. Med. 378, 1291–1301 (2018).
Gamero-Vega, G. et al. Research on faith-based interventions and faith-placed health interventions: current situation and perspectives in Latin America [Spanish]. Gac. Sanit. 32, 315–317 (2018).
Miranda, J. J., Taype-Rondan, A., Bazalar-Palacios, J., Bernabe-Ortiz, A. & Ariely, D. The effect of a priest-led intervention on the choice and preference of soda beverages: a cluster-randomized controlled trial in catholic parishes. Ann. Behav. Med. 54, 436–446 (2020).
Dalencour, M. et al. The role of faith-based organizations in the depression care of African Americans and Hispanics in Los Angeles. Psychiatr. Serv. 68, 368–374 (2017).
Beran, D. et al. Rethinking research processes to strengthen co-production in low and middle income countries. BMJ 372, m4785 (2021).
Lazo-Porras, M. et al. Lessons learned about co-creation: developing a complex intervention in rural Peru. Glob. Health Action. 13, 1754016 (2020).
Gitlin, L. N., Winter, L., Dennis, M. P., Hodgson, N. & Hauck, W. W. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA 304, 983–991 (2010).
World Economic Forum. Health systems leapfrogging in emerging economies: from concept to scale-up and system transformation. World Economic Forum http://www3.weforum.org/docs/WEF_Health_Systems_Leapfrogging_Emerging_Economies.pdf (2015).
Acknowledgements
S.T.S. is currently funded by a program grant from Region Zealand (Exercise First), and two grants from the European Union’s Horizon 2020 research and innovation programme, one from the European Research Council (MOBILIZE; grant agreement no. 801790) and the other under grant agreement no. 945377 (ESCAPE). F.S.M. undertakes multimorbidity research funded by the Wellcome Trust, National Institute for Health and Care Research (NIHR202020); UKRI (NIHR203986, NIHR202644); and MRC (MR/T037849/1, MR/T03775X/1). M.F. was funded by the Canadian Institutes of Health Research. B.P.N. receives research grants from the Research Support Foundation of Rio Grande do Sul, Brazil (grants 19/2551-0001231-4, 19/2551-0001704-9 and 21/2551-0000066-0 – Programa Pesquisa para o SUS: gestão compartilhada em saúde - PPSUS) related to projects on multimorbidity, and is a member of the Brazilian Group of Studies on Multimorbidity (GBEM). C.M.B. is funded by the National Institutes of Health, K24 AG056578, 1P30AG066587 and R24 AG064025. J.J.M. acknowledges having received support from the Alliance for Health Policy and Systems Research (HQHSR1206660), Biotechnology and Biological Sciences Research Council (BB/T009004/1), Bernard Lown Scholars in Cardiovascular Health Program at Harvard T.H. Chan School of Public Health (BLSCHP-1902), Bloomberg Philanthropies (via University of North Carolina at Chapel Hill School of Public Health), FONDECYT via CIENCIACTIVA/CONCYTEC, British Council, British Embassy and the Newton-Paulet Fund (223-2018, 224-2018), DFID/MRC/Wellcome Global Health Trials (MR/M007405/1), Fogarty International Center (R21TW009982, D71TW010877, R21TW011740), Grand Challenges Canada (0335-04), International Development Research Center Canada (IDRC 106887, 108167), Inter-American Institute for Global Change Research (IAI CRN3036), Medical Research Council (MR/P008984/1, MR/P024408/1, MR/P02386X/1), National Cancer Institute (1P20CA217231), National Heart, Lung and Blood Institute (HHSN268200900033C, 5U01HL114180, 1UM1HL134590), National Institute of Mental Health (1U19MH098780), Swiss National Science Foundation (40P740-160366), UKRI GCRF/Newton Fund (EP/V043102/1), Wellcome (074833/Z/04/Z, 093541/Z/10/Z, 103994/Z/14/Z, 107435/Z/15/Z, 205177/Z/16/Z, 214185/Z/18/Z, 218743/Z/19/Z) and the World Diabetes Foundation (WDF15-1224). B.G. was supported by Legal and General PLC (research grant to establish the independent Advanced Care Research Centre at University of Edinburgh). S.M.S. was supported by a Health Research Board Collaborative Doctoral Award in Multimorbidity (HRB CDA-2013-008, Prof. Susan Smith). The authors acknowledge J. Larkin, HRB Collaborative Doctoral Award PhD Scholar (HRB CDA-2013-008, Prof. Susan Smith), who supported us in the preparation of the manuscript by retrieving, organizing and inserting references via EndNote.
Author information
Authors and Affiliations
Contributions
Introduction (all); Epidemiology (all); Mechanisms/pathophysiology (all); Diagnosis, screening and prevention (all); Management (all); Quality of life (all); Outlook (all); Overview of Primer (all).
Corresponding author
Ethics declarations
Competing interests
C.M.B. is a co-author of a chapter for UpToDate on Multiple Chronic Conditions for which she receives royalties. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks Peter J. Bower, Kathryn Ann Fisher, Graziano Onder and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Treatment burden
-
The workload associated with managing treatments and health-care recommendations and the impact of this on an individual and their supporters.
- Genomic instability
-
An increased tendency for DNA mutations (changes) and other genetic changes to occur during cell division.
- Telomere attrition
-
Accrual of DNA damage that affects part of the chromosome known as telomeres.
- Proteostasis
-
Regulation of cell proteins.
- Mitochondrial dysfunction
-
Problems with mitochondrial energy production.
- Deregulated nutrient sensing
-
Problems with the processes affecting nutrition that can affect metabolism.
- Cellular senescence
-
Accumulation of unrepaired damage to cells and limitations in repair functions, which may be exacerbated by oxidative stress.
- Stem cell exhaustion
-
Depletion of stem cell numbers and the regeneration potential of tissues.
- Frailty
-
A state of reduced resilience and increased vulnerability to stressors secondary to deterioration in function across several physiological systems.
- Allostasis
-
The adaptive physiological response activated when homeostasis is disrupted during acute stress.
- Condition burden
-
Also called disease burden. The effect of the condition on an individual, for example, on symptoms, activities of daily living or quality of life.
- Care coordination
-
The deliberate organization of patient care activities.
- Self-management support
-
Interventions that equip patients with skills to actively participate and take responsibility in the management of their chronic condition, namely, action plans, goal-setting worksheets, problem-solving, motivational interviewing, reflective listening and selection of effective educational material.
- Medicines management
-
The process through which a medicine is prescribed to optimize health-care delivery and patient outcomes.
- Biographical disruptions
-
A sociological concept referring to a break in social and cultural experience and self-identity.
- Social prescribing
-
A process through which clinicians can refer patients for community support from local, non-clinical services.
Rights and permissions
About this article
Cite this article
Skou, S.T., Mair, F.S., Fortin, M. et al. Multimorbidity. Nat Rev Dis Primers 8, 48 (2022). https://doi.org/10.1038/s41572-022-00376-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-022-00376-4
This article is cited by
-
Determinants of multimorbidity in older adults in Iran: a cross-sectional study using latent class analysis on the Bushehr Elderly Health (BEH) program
BMC Geriatrics (2024)
-
Homecare workers - an untapped resource in preventing emergency department visits among older individuals? A qualitative interview study from Sweden
BMC Geriatrics (2024)
-
Multimorbidity, healthy lifestyle, and the risk of cognitive impairment in Chinese older adults: a longitudinal cohort study
BMC Public Health (2024)
-
Correlational analysis of sarcopenia and multimorbidity among older inpatients
BMC Musculoskeletal Disorders (2024)
-
Trajectories network analysis of chronic diseases among middle-aged and older adults: evidence from the China Health and Retirement Longitudinal Study (CHARLS)
BMC Public Health (2024)