Abstract
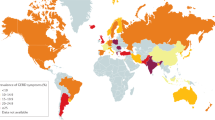
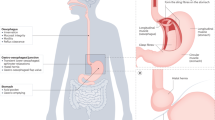
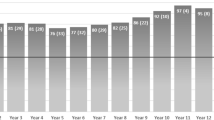
Achalasia is a rare disorder of the oesophageal smooth muscle characterized by impaired relaxation of the lower oesophageal sphincter (LES) and absent or spastic contractions in the oesophageal body. The key pathophysiological mechanism is loss of inhibitory nerve function that probably results from an autoimmune attack targeting oesophageal myenteric nerves through cell-mediated and, possibly, antibody-mediated mechanisms. Achalasia incidence and prevalence increase with age, but the disorder can affect all ages and both sexes. Cardinal symptoms consist of dysphagia, regurgitation, chest pain and weight loss. Several years can pass between symptom onset and an achalasia diagnosis. Evaluation starts with endoscopy to rule out structural causes, followed by high-resolution manometry and/or barium radiography. Functional lumen imaging probe can provide complementary evidence. Achalasia subtypes have management and prognostic implications. Although symptom questionnaires are not useful for diagnosis, the Eckardt score is a simple symptom scoring scale that helps to quantify symptom response to therapy. Oral pharmacotherapy is not particularly effective. Botulinum toxin injection into the LES can temporize symptoms and function as a bridge to definitive therapy. Pneumatic dilation, per-oral endoscopic myotomy and laparoscopic Heller myotomy can provide durable symptom benefit. End-stage achalasia with a dilated, non-functioning oesophagus may require oesophagectomy or enteral feeding into the stomach. Long-term complications can, rarely, include oesophageal cancer, but surveillance recommendations have not been established.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pandolfino, J. E. & Gawron, A. J. Achalasia: a systematic review. JAMA 313, 1841–1852 (2015).
Vaezi, M. F. et al. ACG clinical guidelines: diagnosis and management of achalasia. Am. J. Gastroenterol. 115, 1393–1411 (2020).
Yadlapati, R. et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol. Motil. 33, e14058 (2021).
Gergely, M. et al. Duration of symptoms and manometric parameters offer clues to diagnosis of pseudoachalasia. Neurogastroenterol. Motil. 33, e13965 (2021).
Furuzawa-Carballeda, J. et al. Achalasia — an autoimmune inflammatory disease: a cross-sectional study. J. Immunol. Res. 2015, 729217 (2015).
De Giorgio, R. et al. Esophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am. J. Gastroenterol. 94, 2357–2362 (1999).
Kilic, A. et al. Variations in inflammation and nerve fiber loss reflect different subsets of achalasia patients. J. Surg. Res. 143, 177–182 (2007).
Moses, P. L. et al. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut 52, 629–636 (2003).
Gaber, C. E. et al. Autoimmune and viral risk factors are associated with achalasia: a case-control study. Neurogastroenterol. Motil. https://doi.org/10.1111/nmo.14312 (2021).
Naik, R. D. et al. Association of achalasia with active Varicella zoster virus infection of the esophagus. Gastroenterology 161, 719–721 e2 (2021).
Facco, M. et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am. J. Gastroenterol. 103, 1598–1609 (2008).
Sodikoff, J. B. et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol. Motil. 28, 139–145 (2016).
Reddy, C. A. et al. Per-oral endoscopic myotomy biopsies of achalasia patients reveal Schwann cell depletion in the muscularis propria. Clin. Gastroenterol. Hepatol. 19, 1294–1295 (2021).
Sato, H. et al. Epidemiological analysis of achalasia in Japan using a large-scale claims database. J. Gastroenterol. 54, 621–627 (2019).
van Hoeij, F. B. et al. Incidence and costs of achalasia in The Netherlands. Neurogastroenterol. Motil. https://doi.org/10.1111/nmo.13195 (2018).
Harvey, P. R. et al. Incidence, morbidity and mortality of patients with achalasia in England: findings from a study of nationwide hospital and primary care data. Gut 68, 790–795 (2019).
Sadowski, D. C. et al. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol. Motil. 22, e256–e261 (2010).
Samo, S. et al. Incidence and prevalence of achalasia in central Chicago, 2004-2014, since the widespread use of high-resolution manometry. Clin. Gastroenterol. Hepatol. 15, 366–373 (2017).
Duffield, J. A. et al. Incidence of achalasia in South Australia based on esophageal manometry findings. Clin. Gastroenterol. Hepatol. 15, 360–365 (2017).
Gaber, C. E. et al. Epidemiologic and economic burden of achalasia in the United States. Clin. Gastroenterol. Hepatol. 20, 342–352.e5. (2021).
Roman, S. et al. High-resolution manometry improves the diagnosis of esophageal motility disorders in patients with dysphagia: a randomized multicenter study. Am. J. Gastroenterol. 111, 372–80. (2016).
Wadhwa, V. et al. Changing trends in age, gender, racial distribution and inpatient burden of achalasia. Gastroenterol. Res. 10, 70–77 (2017).
Gregersen, H. & Lo, K. M. Pathophysiology and treatment of achalasia in a muscle mechanical perspective. Ann. N. Y. Acad. Sci. 1434, 173–184 (2018).
Behar, J. & Biancani, P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology 105, 111–118 (1993).
Sifrim, D., Janssens, J. & Vantrappen, G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology 106, 875–882 (1994).
Fornari, F. et al. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol. Motil. 21, 718–e41 (2009).
Savojardo, D. et al. Multiple rapid swallowing in idiopathic achalasia: evidence for patients’ heterogeneity. Neurogastroenterol. Motil. 19, 263–269 (2007).
Kushnir, V., Sayuk, G. S. & Gyawali, C. P. Multiple rapid swallow responses segregate achalasia subtypes on high-resolution manometry. Neurogastroenterol. Motil. 24, 1069–e561 (2012).
Murray, J. A. et al. The effects of recombinant human hemoglobin on esophageal motor functions in humans. Gastroenterology 109, 1241–1248 (1995).
Aggestrup, S. et al. Lack of vasoactive intestinal polypeptide nerves in esophageal achalasia. Gastroenterology 84, 924–927 (1983).
Mearin, F. et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur. J. Clin. Invest. 23, 724–728 (1993).
Yamato, S., Saha, J. K. & Goyal, R. K. Role of nitric oxide in lower esophageal sphincter relaxation to swallowing. Life Sci. 50, 1263–1272 (1992).
Dodds, W. J. et al. Paradoxical lower esophageal sphincter contraction induced by cholecystokinin-octapeptide in patients with achalasia. Gastroenterology 80, 327–333 (1981).
Cohen, S., Fisher, R. & Tuch, A. The site of denervation in achalasia. Gut 13, 556–558 (1972).
Booy, J. D. et al. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis. Esophagus 25, 209–213 (2012).
King, D. et al. Achalasia is associated with atopy in patients younger than 40 years of age. Am. J. Gastroenterol. 116, 416–419 (2021).
Romero-Hernández, F. et al. Autoimmune comorbidity in achalasia patients. J. Gastroenterol. Hepatol. 33, 203–208 (2018).
Sara, C. et al. Clinical correlation and disease phenotype in patients with esophageal achalasia and comorbid autoimmune diseases. Dis. Esophagus 34, doaa072 (2021).
Becker, J. et al. Comprehensive epidemiological and genotype-phenotype analyses in a large European sample with idiopathic achalasia. Eur. J. Gastroenterol. Hepatol. 28, 689–695 (2016).
Becker, J. et al. The HLA-DQβ1 insertion is a strong achalasia risk factor and displays a geospatial north-south gradient among Europeans. Eur. J. Hum. Genet. 24, 1228–1231 (2016).
Furuzawa-Carballeda, J. et al. An original Eurasian haplotype, HLA-DRB1*14:54-DQB1*05:03, influences the susceptibility to idiopathic achalasia. PLoS ONE 13, e0201676 (2018).
Vackova, Z. et al. First genotype-phenotype study reveals HLA-DQβ1 insertion heterogeneity in high-resolution manometry achalasia subtypes. United European Gastroenterol. J. 7, 45–51 (2019).
Goldblum, J. R., Rice, T. W. & Richter, J. E. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology 111, 648–654 (1996).
Villanacci, V. et al. An immunohistochemical study of the myenteric plexus in idiopathic achalasia. J. Clin. Gastroenterol. 44, 407–410 (2010).
Clark, S. B. et al. The nature of the myenteric infiltrate in achalasia: an immunohistochemical analysis. Am. J. Surg. Pathol. 24, 1153–1158 (2000).
Spechler, S. J., Konda, V. & Souza, R. Can eosinophilic esophagitis cause achalasia and other esophageal motility disorders? Am. J. Gastroenterol. 113, 1594–1599 (2018).
Jin, H. et al. Activated eosinophils are present in esophageal muscle in patients with achalasia of the esophagus. Med. Sci. Monit. 24, 2377–2383 (2018).
Visaggi, P. et al. Systematic review: esophageal motility patterns in patients with eosinophilic esophagitis. Dig. Liver Dis. https://doi.org/10.1016/j.dld.2022.01.003 (2022).
Nelson, M. et al. Lower esophageal sphincter muscle of patients with achalasia exhibits profound mast cell degranulation. Neurogastroenterol. Motil. 33, e14055 (2021).
Zarate, N. et al. Intramuscular interstitial cells of Cajal associated with mast cells survive nitrergic nerves in achalasia. Neurogastroenterol. Motil. 18, 556–568 (2006).
Ruiz-de-Leon, A. et al. Myenteric antiplexus antibodies and class II HLA in achalasia. Dig. Dis. Sci. 47, 15–19 (2002).
Storch, W. B. et al. Autoantibodies to Auerbach’s plexus in achalasia. Cell Mol. Biol. 41, 1033–1038 (1995).
Verne, G. N., Sallustio, J. E. & Eaker, E. Y. Anti-myenteric neuronal antibodies in patients with achalasia. A prospective study. Dig. Dis. Sci. 42, 307–313 (1997).
Robertson, C. S., Martin, B. A. & Atkinson, M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut 34, 299–302 (1993).
Castagliuolo, I. et al. Esophageal achalasia: is the herpes simplex virus really innocent? J. Gastrointest. Surg. 8, 24–30 (2004).
Birgisson, S. et al. Achalasia is not associated with measles or known herpes and human papilloma viruses. Dig. Dis. Sci. 42, 300–306 (1997).
Boeckxstaens, G. E. Achalasia: virus-induced euthanasia of neurons? Am. J. Gastroenterol. 103, 1610–1612 (2008).
Boeckxstaens, G. E., Zaninotto, G. & Richter, J. E. Achalasia. Lancet 383, 83–93 (2014).
Wong, R. K. et al. Significant DQw1 association in achalasia. Dig. Dis. Sci. 34, 349–52. (1989).
Kilpatrick, Z. M. & Milles, S. S. Achalasia in mother and daughter. Gastroenterology 62, 1042–1046 (1972).
Monnig, P. J. Familial achalasia in children. Ann. Thorac. Surg. 49, 1019–1022 (1990).
Brooks, B. P. et al. Genotypic heterogeneity and clinical phenotype in triple A syndrome: a review of the NIH experience 2000-2005. Clin. Genet. 68, 215–221 (2005).
Koehler, K. et al. Mutations in GMPPA cause a glycosylation disorder characterized by intellectual disability and autonomic dysfunction. Am. J. Hum. Genet. 93, 727–734 (2013).
Goldblum, J. R. et al. Achalasia. A morphologic study of 42 resected specimens. Am. J. Surg. Pathol. 18, 327–337 (1994).
Faussone-Pellegrini, M. S. & Cortesini, C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J. Submicrosc. Cytol. 17, 673–685 (1985).
Bonora, E. et al. INPP4B overexpression and c-KIT downregulation in human achalasia. Neurogastroenterol. Motil. 30, e13346 (2018).
Tustumi, F. et al. Achalasia: a syndrome. Neurogastroenterol. Motil. 33, e14089 (2021).
Kahrilas, P. J. & Boeckxstaens, G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 145, 954–965 (2013).
Mittal, R. K., Hong, S. J. & Bhargava, V. Longitudinal muscle dysfunction in achalasia esophagus and its relevance. J. Neurogastroenterol. Motil. 19, 126–136 (2013).
Kim, T. H. et al. Esophageal contractions in type 3 achalasia esophagus: simultaneous or peristaltic? Am. J. Physiol. Gastrointest. Liver Physiol. 310, G689–G695 (2016).
Bruley des Varannes, S. et al. Serum from achalasia patients alters neurochemical coding in the myenteric plexus and nitric oxide mediated motor response in normal human fundus. Gut 55, 319–326 (2006).
Vaezi, M. F., Pandolfino, J. E. & Vela, M. F. ACG clinical guideline: diagnosis and management of achalasia. Am. J. Gastroenterol. 108, 1238–1249 (2013). quiz 1250.
Koppman, J. S. et al. Esophageal motility disorders in the morbidly obese population. Surg. Endosc. 21, 761–764 (2007).
Miller, J., Khlevner, J. & Rodriguez, L. Upper gastrointestinal functional and motility disorders in children. Pediatr. Clin. North. Am. 68, 1237–1253 (2021).
Franklin, A. L., Petrosyan, M. & Kane, T. D. Childhood achalasia: a comprehensive review of disease, diagnosis and therapeutic management. World J. Gastrointest. Endosc. 6, 105–111 (2014).
Eckardt, V. F., Aignherr, C. & Bernhard, G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 103, 1732–1738 (1992).
Patel, D. A. et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis. Esophagus 30, dow028 (2017).
Urbach, D. R. et al. A measure of disease-specific health-related quality of life for achalasia. Am. J. Gastroenterol. 100, 1668–1676 (2005).
Tsuboi, K. et al. Insights gained from symptom evaluation of esophageal motility disorders: a review of 4,215 patients. Digestion 85, 236–242 (2012).
Chan, W. W., Haroian, L. R. & Gyawali, C. P. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg. Endosc. 25, 2943–2949 (2011).
Iwakiri, K. et al. The appearance of rosette-like esophageal folds (“esophageal rosette”) in the lower esophagus after a deep inspiration is a characteristic endoscopic finding of primary achalasia. J. Gastroenterol. 45, 422–425 (2010).
Gomi, K. et al. New endoscopic classification of the cardiac orifice in esophageal achalasia: champagne glass sign. Dig. Endosc. 28, 645–649 (2016).
Hoversten, P., Otaki, F. & Katzka, D. A. Course of esophageal candidiasis and outcomes of patients at a single center. Clin. Gastroenterol. Hepatol. 17, 200–202.e1 (2019).
Carlson, D. A. et al. Diagnosis of esophageal motility disorders: esophageal pressure topography vs. conventional line tracing. Am. J. Gastroenterol. 110, 967–977 (2015).
Soudagar, A. S., Sayuk, G. S. & Gyawali, C. P. Learners favour high resolution oesophageal manometry with better diagnostic accuracy over conventional line tracings. Gut 61, 798–803 (2012).
Rohof, W. O. et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 144, 718–725 (2013). quiz e13-14.
Pandolfino, J. E. et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 135, 1526–1533 (2008).
Ghosh, S. K. et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G878–G885 (2007).
Kahrilas, P. J. et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 27, 160–174 (2015).
Ponds, F. A. et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol. Motil. https://doi.org/10.1111/nmo.12908 (2017).
Khan, M. A. et al. Is POEM the answer for management of spastic esophageal disorders? A systematic review and meta-analysis. Dig. Dis. Sci. 62, 35–44 (2017).
van Hoeij, F. B., Smout, A. J. & Bredenoord, A. J. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol. Motil. 27, 1310–1316 (2015).
Biasutto, D. et al. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol. Motil. 30, e13293 (2018).
Babaei, A., Shad, S. & Massey, B. T. Motility patterns following esophageal pharmacologic provocation with amyl nitrite or cholecystokinin during high-resolution manometry distinguish idiopathic vs opioid-induced type 3 achalasia. Clin. Gastroenterol. Hepatol. 18, 813–821.e1 (2020).
Babaei, A. et al. Pharmacologic interrogation of patients with esophagogastric junction outflow obstruction using amyl nitrite. Neurogastroenterol. Motil. 31, e13668 (2019).
Porter, R. F. & Gyawali, C. P. Botulinum toxin injection in dysphagia syndromes with preserved esophageal peristalsis and incomplete lower esophageal sphincter relaxation. Neurogastroenterol. Motil. 23, 139–144 (2011).
Gyawali, C. P. et al. ACG clinical guidelines: clinical use of esophageal physiologic testing. Am. J. Gastroenterol. 115, 1412–1428 (2020).
Blonski, W. et al. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am. J. Gastroenterol. 113, 196–203 (2018).
Kostic, S. et al. Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis. Esophagus 18, 96–103 (2005).
Rohof, W. O., Lei, A. & Boeckxstaens, G. E. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am. J. Gastroenterol. 108, 49–55 (2013).
Savarino, E. et al. Use of the functional lumen imaging probe in clinical esophagology. Am. J. Gastroenterol. 115, 1786–1796 (2020).
Carlson, D. A. et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am. J. Gastroenterol. 111, 1726–1735 (2016).
Carlson, D. A. et al. Loss of peristaltic reserve, determined by multiple rapid swallows, is the most frequent esophageal motility abnormality in patients with systemic sclerosis. Clin. Gastroenterol. Hepatol. 14, 1502–1506 (2016).
Carlson, D. A. et al. Prediction of esophageal retention: a study comparing high-resolution manometry and functional luminal imaging probe panometry. Am. J. Gastroenterol. 116, 2032–2041 (2021).
Carlson, D. A. et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology 149, 1742–1751 (2015).
Carlson, D. A. et al. Classifying esophageal motility by FLIP Panometry: A Study of 722 subjects with manometry. Am. J. Gastroenterol. 116, 2357–2366 (2021).
Ponds, F. A. et al. Diagnostic features of malignancy-associated pseudoachalasia. Aliment. Pharmacol. Ther. 45, 1449–1458 (2017).
Lynch, K. L. et al. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis. Esophagus 30, dox004 (2017).
Lucchinetti, C. F., Kimmel, D. W. & Lennon, V. A. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology 50, 652–657 (1998).
Bredenoord, A. J. et al. Esophagogastric junction outflow obstruction. Neurogastroenterol. Motil. 33, e14193 (2021).
de Oliveira, R. B. et al. The spectrum of esophageal motor disorders in Chagas’ disease. Am. J. Gastroenterol. 90, 1119–1124 (1995).
Dantas, R. O. Management of esophageal dysphagia in Chagas disease. Dysphagia 36, 517–522 (2021).
Ghisa, M. et al. Achalasia and obstructive motor disorders are not uncommon in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 19, 1554–1563 (2021).
Savarino, E. et al. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin. Gastroenterol. Hepatol. 9, 1104–1106 (2011).
Ikeda, H. et al. Diagnosis of congenital esophageal stenosis in adults and treatment with peroral endoscopic myotomy. Ann. Gastroenterol. 34, 493–500 (2021).
Triadafilopoulos, G. et al. Changes in high-resolution manometric diagnosis over time: implications for clinical decision-making. Dis. Esophagus 33, doz094 (2020).
Gelfond, M., Rozen, P. & Gilat, T. Isosorbide dinitrate and nifedipine treatment of achalasia: a clinical, manometric and radionuclide evaluation. Gastroenterology 83, 963–969 (1982).
Bortolotti, M. & Labo, G. Clinical and manometric effects of nifedipine in patients with esophageal achalasia. Gastroenterology 80, 39–44 (1981).
Bortolotti, M. et al. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology 118, 253–257 (2000).
Oude Nijhuis, R. A. B. et al. European guidelines on achalasia: United European Gastroenterology and European Society of Neurogastroenterology and Motility recommendations. United European Gastroenterol. J. 8, 13–33 (2020).
Vaezi, M. F. & Richter, J. E. Diagnosis and management of achalasia. American College of Gastroenterology Practice Parameter Committee. Am. J. Gastroenterol. 94, 3406–3412 (1999).
Bassotti, G. & Annese, V. Review article: pharmacological options in achalasia. Aliment. Pharmacol. Ther. 13, 1391–1396 (1999).
Weusten, B. et al. Endoscopic management of gastrointestinal motility disorders - part 1: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 52, 498–515 (2020).
Weusten, B. et al. Endoscopic management of gastrointestinal motility disorders - part 2: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 52, 600–614 (2020).
Khashab, M. A. et al. ASGE guideline on the management of achalasia. Gastrointest. Endosc. 91, 213–227.e6 (2020).
Annese, V. et al. Controlled trial of botulinum toxin injection versus placebo and pneumatic dilation in achalasia. Gastroenterology 111, 1418–1424 (1996).
van Hoeij, F. B. et al. Complications of botulinum toxin injections for treatment of esophageal motility disorders. Dis. Esophagus 30, dote12491 (2016).
van Hoeij, F. B. et al. Efficacy and safety of pneumatic dilation in achalasia: a systematic review and meta-analysis. Neurogastroenterol. Motil. 31, e13548 (2019).
Boeckxstaens, G. E. et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N. Engl. J. Med. 364, 1807–1816 (2011).
Inoue, H. et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 42, 265–271 (2010).
Modayil, R. J. et al. Peroral endoscopic myotomy: 10-year outcomes from a large, single-center U.S. series with high follow-up completion and comprehensive analysis of long-term efficacy, safety, objective GERD, and endoscopic functional luminal assessment. Gastrointest. Endosc. 94, 930–942 (2021).
Nabi, Z. et al. Comparison of short versus long esophageal myotomy in cases with idiopathic achalasia: a randomized controlled trial. J. Neurogastroenterol. Motil. 27, 63–70 (2021).
Delliturri, A. et al. A Narrative Review of update in per oral endoscopic myotomy (POEM) and endoscopic esophageal surgery. Ann. Transl. Med. 9, 909 (2021).
Inoue, H. et al. Per-oral endoscopic myotomy: a series of 500 patients. J. Am. Coll. Surg. 221, 256–264 (2015).
Akintoye, E. et al. Peroral endoscopic myotomy: a meta-analysis. Endoscopy 48, 1059–1068 (2016).
Onimaru, M. et al. Long-term clinical results of per-oral endoscopic myotomy (POEM) for achalasia: first report of more than 10-year patient experience as assessed with a questionnaire-based survey. Endosc. Int. Open 9, E409–E416 (2021).
Repici, A. et al. GERD after per-oral endoscopic myotomy as compared with Heller’s myotomy with fundoplication: a systematic review with meta-analysis. Gastrointest. Endosc. 87, 934–943.e18 (2018).
Karyampudi, A. et al. Gastroesophageal reflux after per-oral endoscopic myotomy is frequently asymptomatic, but leads to more severe esophagitis: a case-control study. United European Gastroenterol. J. 9, 63–71 (2021).
Patti, M. G., Tamburini, A. & Pellegrini, C. A. Cardiomyotomy. Semin. Laparosc. Surg. 6, 186–193 (1999).
Milone, M. et al. Robotic versus laparoscopic approach to treat symptomatic achalasia: systematic review with meta-analysis. Dis. Esophagus 32, 1–8 (2019).
Richards, W. O. et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann. Surg. 240, 405–412; discussion 412–415 (2004).
Torres-Villalobos, G. et al. Dor vs Toupet fundoplication after laparoscopic Heller myotomy: long-term randomized controlled trial evaluated by high-resolution manometry. J. Gastrointest. Surg. 22, 13–22 (2018).
Ortiz, A. et al. Very long-term objective evaluation of Heller myotomy plus posterior partial fundoplication in patients with achalasia of the cardia. Ann. Surg. 247, 258–264 (2008).
Zaninotto, G. et al. Four hundred laparoscopic myotomies for esophageal achalasia: a single centre experience. Ann. Surg. 248, 986–993 (2008).
Gossage, J. A. et al. Surveillance endoscopy at five or more years after cardiomyotomy for achalasia. Ann. Surg. 259, 464–468 (2014).
Chrystoja, C. C. et al. Achalasia-specific quality of life after pneumatic dilation or laparoscopic Heller myotomy with partial fundoplication: a multicenter, randomized clinical trial. Am. J. Gastroenterol. 111, 1536–1545 (2016).
Ponds, F. A. et al. Effect of peroral endoscopic myotomy vs pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: a randomized clinical trial. JAMA 322, 134–144 (2019).
Sloan, J. A. et al. Treatment experience with a novel 30-mm hydrostatic balloon in esophageal dysmotility: a multicenter retrospective analysis. Gastrointest. Endosc. 92, 1251–1257 (2020).
Schnurre, L. et al. Short-term outcome after singular hydraulic EsoFLIP dilation in patients with achalasia: a feasibility study. Neurogastroenterol. Motil. 32, e13864 (2020).
Jung, H. K. et al. 2019 Seoul consensus on esophageal achalasia guidelines. J. Neurogastroenterol. Motil. 26, 180–203 (2020).
Werner, Y. B. et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N. Engl. J. Med. 381, 2219–2229 (2019).
Pratap, N. et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J. Neurogastroenterol. Motil. 17, 48–53 (2011).
Salvador, R. et al. Effects of laparoscopic myotomy on the esophageal motility pattern of esophageal achalasia as measured by high-resolution manometry. Surg. Endosc. 31, 3510–3518 (2017).
Kumbhari, V. et al. Peroral endoscopic myotomy (POEM) vs laparoscopic Heller myotomy (LHM) for the treatment of type III achalasia in 75 patients: a multicenter comparative study. Endosc. Int. Open 3, E195–E201 (2015).
Borges, A. A. et al. Pneumatic dilation versus laparoscopic Heller myotomy for the treatment of achalasia: variables related to a good response. Dis. Esophagus 27, 18–23 (2014).
Fernandez-Ananin, S. et al. What to do when Heller’s myotomy fails? Pneumatic dilatation, laparoscopic remyotomy or peroral endoscopic myotomy: a systematic review. J. Minim. Access. Surg. 14, 177–184 (2018).
van Hoeij, F. B. et al. Management of recurrent symptoms after per-oral endoscopic myotomy in achalasia. Gastrointest. Endosc. 87, 95–101 (2018).
Giulini, L., Dubecz, A. & Stein, H. J. Laparoscopic Heller myotomy after failed POEM and multiple balloon dilatations: better late than never [German]. Chirurg 88, 303–306 (2017).
Panchanatheeswaran, K. et al. Laparoscopic Heller’s cardiomyotomy: a viable treatment option for sigmoid oesophagus. Interact. Cardiovasc. Thorac. Surg. 16, 49–54 (2013).
Tyberg, A. et al. Peroral endoscopic myotomy as salvation technique post-Heller: international experience. Dig. Endosc. 30, 52–56 (2018).
Tang, X. et al. Feasibility and safety of peroral endoscopic myotomy for achalasia after failed endoscopic interventions. Dis. Esophagus 30, dote.12457 (2017).
Ling, T., Guo, H. & Zou, X. Effect of peroral endoscopic myotomy in achalasia patients with failure of prior pneumatic dilation: a prospective case-control study. J. Gastroenterol. Hepatol. 29, 1609–1613 (2014).
Aiolfi, A. et al. Esophageal resection for end-stage achalasia. Am. Surg. 84, 506–511 (2018).
Molena, D. & Yang, S. C. Surgical management of end-stage achalasia. Semin. Thorac. Cardiovasc. Surg. 24, 19–26 (2012).
Eckardt, V. F., Hoischen, T. & Bernhard, G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur. J. Gastroenterol. Hepatol. 20, 956–960 (2008).
Zaninotto, G. et al. The 2018 ISDE achalasia guidelines. Dis. Esophagus https://doi.org/10.1093/dote/doy071 (2018).
Palanivelu, C. et al. Laparoscopic transhiatal esophagectomy for ‘sigmoid’ megaesophagus following failed cardiomyotomy: experience of 11 patients. Dig. Dis. Sci. 53, 1513–1518 (2008).
Glatz, S. M. & Richardson, J. D. Esophagectomy for end stage achalasia. J. Gastrointest. Surg. 11, 1134–1137 (2007).
Eckardt, V. F. Clinical presentations and complications of achalasia. Gastrointest. Endosc. Clin. N. Am. 11, 281–292 (2001).
Taft, T. H. et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol. Motil. 30, e13287 (2018).
Cisternas, D. et al. Fair reliability of eckardt scores in achalasia and non-achalasia patients: psychometric properties of the eckardt spanish version in a multicentric study. Neurogastroenterol. Motil. 32, e13827 (2020).
Vantrappen, G. & Hellemans, J. Treatment of achalasia and related motor disorders. Gastroenterology 79, 144–154 (1980).
Dakkak, M. & Bennett, J. R. A new dysphagia score with objective validation. J. Clin. Gastroenterol. 14, 99–100 (1992).
Taft, T. H. et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol. Motil. 28, 1854–1860 (2016).
Cisternas, D. et al. The Brief Esophageal Dysphagia Questionnaire shows better discriminative capacity for clinical and manometric findings than the Eckardt score: results from a multicenter study. Neurogastroenterol. Motil. 34, e14228 (2021).
Goldacre, M., Benians, R. & Goldacre, R. Esophageal achalasia diagnosed in people previously diagnosed with an eating disorder: epidemiological study using record-linkage. Int. J. Eat. Disord. 54, 2015–2018 (2021).
Carlson, D. A. et al. Esophageal hypervigilance and visceral anxiety are contributors to symptom severity among patients evaluated with high-resolution esophageal manometry. Am. J. Gastroenterol. 115, 367–375 (2020).
Loosen, S. H. et al. Achalasia is associated with a higher incidence of depression in outpatients in Germany. PLoS ONE 16, e0250503 (2021).
Ross, D., Richter, J. & Velanovich, V. Health-related quality of life and physiological measurements in achalasia. Dis. Esophagus 30, dote.12494 (2017).
Zhong, C. et al. Quality of life following peroral endoscopic myotomy for esophageal achalasia: a systematic review and meta-analysis. Ann. Thorac. Cardiovasc. Surg. 26, 113–124 (2020).
Slone, S. et al. Accuracy of achalasia quality of life and eckardt scores for assessment of clinical improvement post treatment for achalasia. Dis. Esophagus 34, doaa080 (2021).
Smits, M. et al. Pediatric achalasia in the Netherlands: incidence, clinical course, and quality of life. J. Pediatr. 169, 110–5.e3 (2016).
Newberry, C. et al. Achalasia patients are at nutritional risk regardless of presenting weight category. Dig. Dis. Sci. 63, 1243–1249 (2018).
Chino, O. et al. Clinicopathological studies of esophageal carcinoma in achalasia: analyses of carcinogenesis using histological and immunohistochemical procedures. Anticancer. Res. 20, 3717–3722 (2000).
Leeuwenburgh, I. et al. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am. J. Gastroenterol. 105, 2144–2149 (2010).
Tustumi, F. et al. Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis. Dis. Esophagus 30, dox072 (2017).
Sandler, R. S. et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 274, 1359–1362 (1995).
Zagari, R. M. et al. Risk of squamous cell carcinoma and adenocarcinoma of the esophagus in patients with achalasia: a long-term prospective cohort study in Italy. Am. J. Gastroenterol. 116, 289–295 (2021).
Markar, S. R. et al. Incidence and risk factors for esophageal cancer following achalasia treatment: national population-based case-control study. Dis. Esophagus 32, doy106 (2019).
Tassi, V. et al. Incidence and risk factors for the development of epidermoid carcinoma in oesophageal achalasia. Eur. J. Cardiothorac. Surg. 55, 956–963 (2019).
Wu, X. Y. et al. The etiology of achalasia: an immune-dominant disease. J. Dig. Dis. 22, 126–135 (2021).
El Kafsi, J. et al. Management of achalasia in the UK, do we need new guidelines? Ann. Med. Surg. 12, 32–36 (2016).
Werner, Y. B. et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut 65, 899–906 (2016).
Acknowledgements
The authors thank the anonymous patient for their contribution in Box 2.
Author information
Authors and Affiliations
Contributions
Introduction (E.S., C.P.G. and S.B.); Epidemiology (S.R.); Mechanisms/pathophysiology (D.S.); Diagnosis/screening/prevention (C.P.G.); Management (E.S. and S.K.T.); Quality of life (S.R.); Outlook (J.T.); Overview of the Primer (E.S.).
Corresponding author
Ethics declarations
Competing interests
E.S. has served as speaker for AbbVie, AGPharma, Alfasigma, EG Stada Group, Fresenius Kabi, Grifols, Janssen, Innovamedica, Malesci, Medtronic, Novartis, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda and Unifarco; has served as consultant for Alfasigma, Amgen, Biogen, Bristol-Myers Squibb, Celltrion, Diadema Farmaceutici, Falk, Fresenius Kabi, Janssen, Merck & Co., Reckitt Benckiser, Regeneron, Sanofi, Shire, SILA, Sofar, Synformulas GmbH, Takeda and Unifarco; and has received research support from Reckitt Benckiser, SILA, Sofar and Unifarco. S.B. has served as speaker for Medtronic. S.R. has served as consultant for Reckitt Benckiser and Dr Falk Pharma; and has received research support from Medtronic and Diversatek Healthcare. D.S. has served as consultant for Reckitt Benckiser UK, Jinshan Technology China and Alfasigma Italy. J.T. has served on the speaker bureau for Abbott, Mylan and Takeda; has served as consultant for Arena, Bayer, Falk, Takeda and Truvion pharmaceuticals; and has received research support from Shire, Sofar and Takeda. S.K.T. has served as a consultant for Medtronic. C.P.G. has served as speaker for Medtronic, Takeda and Johnson&Johnson, and has served as consultant for Medtronic, Diversatek, Takeda and Ironwood.
Peer review
Peer review information
Nature Reviews Disease Primers thanks I. Gockel; Z. Nabi; M. Patti; Y. Shimamura, who co-reviewed with Y. Fujiyoshi; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Savarino, E., Bhatia, S., Roman, S. et al. Achalasia. Nat Rev Dis Primers 8, 28 (2022). https://doi.org/10.1038/s41572-022-00356-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-022-00356-8
This article is cited by
-
Achalasia alters physiological networks depending on sex
Scientific Reports (2024)
-
Myasthenia gravis with achalasia secondary to thymoma: a case report and literature review
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)
-
Phosphodiesterase 5 (PDE-5) inhibitors (sildenafil, tadalafil, and vardenafil) effects on esophageal motility: a systematic review
BMC Gastroenterology (2023)
-
Ethnic Differences in Clinical Presentations and Esophageal High-Resolution Manometry Findings in Patients with Achalasia
Dysphagia (2023)
-
Mixed Esophageal Disease (MED): A New Concept
Digestive Diseases and Sciences (2023)