Abstract
Background
Comorbidities such as obesity, hypertension, and diabetes are associated with COVID-19 development and severity, probably due to immune dysregulation; however, the mechanisms underlying these associations are not clear. The immune signatures of hypertensive patients with obesity with COVID-19 may provide new insight into the mechanisms of immune dysregulation and progression to severe disease in these patients.
Methods
Hypertensive patients were selected prospectively from a multicenter registry of adults hospitalized with COVID-19 and stratified according to obesity (BMI ≥ 30 kg/m²). Clinical data including baseline characteristics, complications, treatment, and 46 immune markers were compared between groups. Logistic regression was performed to identify variables associated with the risk of COVID-19 progression in each group.
Results
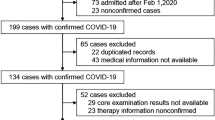
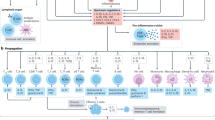
The sample comprised 213 patients (89 with and 124 without obesity). The clinical profiles of patients with and without obesity differed, suggesting potential interactions with COVID-19 severity. Relative to patients without obesity, patients with obesity were younger and fewer had cardiac disease and myocardial injury. Patients with obesity had higher EGF, GCSF, GMCSF, interleukin (IL)-1ra, IL-5, IL-7, IL-8, IL-15, IL-1β, MCP 1, and VEGF levels, total lymphocyte counts, and CD8+ CD38+ mean fluorescence intensity (MFI), and lower NK-NKG2A MFI and percentage of CD8+ CD38+ T cells. Significant correlations between cytokine and immune cell expression were observed in both groups. Five variables best predicted progression to severe COVID-19 in patients with obesity: diabetes, the EGF, IL-10, and IL-13 levels, and the percentage of CD8+ HLA-DR+ CD38+ cells. Three variables were predictive for patients without obesity: myocardial injury and the percentages of B lymphocytes and HLA-DR+ CD38+ cells.
Conclusion
Our findings suggest that clinical and immune variables and obesity interact synergistically to increase the COVID-19 progression risk. The immune signatures of hypertensive patients with and without obesity severe COVID-19 highlight differences in immune dysregulation mechanisms, with potential therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
World HO. World health organization. (2020). Who corona disease (covid-19) dashboard. World health organization. Available at https://covid19.Who.Int 2022; (accessed October 25, 2022).
Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in covid-19. New England J Med. 2020;382:e102.
Gao M, Wang Q, Piernas C, Astbury NM, Jebb SA, Holmes MV, et al. Associations between body composition, fat distribution and metabolic consequences of excess adiposity with severe covid-19 outcomes: Observational study and mendelian randomisation analysis. Int J Obesity. 2022;46:943–50.
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (covid-19). JAMA Cardiol. 2020;5:811–8.
Liu PP, Blet A, Smyth D, Li H. The science underlying covid-19: Implications for the cardiovascular system. Circulation. 2020;142:68–78.
Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and covid‐19: A global perspective on the epidemiology and biological relationships. Obesity Rev. 2020;21:e13128.
Földi M, Farkas N, Kiss S, Dembrovszky F, Szakács Z, Balaskó M, et al. Visceral adiposity elevates the risk of critical condition in covid‐19: A systematic review and meta‐analysis. Obesity. 2021;29:521–8.
Piroth L, Cottenet J, Mariet A-S, Bonniaud P, Blot M, Tubert-Bitter P, et al. Comparison of the characteristics, morbidity, and mortality of covid-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respiratory Med. 2021;9:251–9.
Sattar N, McInnes IB, McMurray JJ. Obesity is a risk factor for severe covid-19 infection: Multiple potential mechanisms. Circulation. 2020;142:4–6.
Blot M, Masson D, Nguyen M, Bourredjem A, Binquet C, Piroth L. Are adipokines the missing link between obesity, immune response, and outcomes in severe covid-19? Int J Obesity. 2021;45:2126–31.
Asghar A, Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cellular Immunol. 2017;315:18–26.
Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Current Pharmaceutical Design. 2008;14:1225–30.
Fabbrini E, Cella M, Mccartney SA, Fuchs A, Abumrad NA, Pietka TA, et al. Association between specific adipose tissue cd4+ t-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–74.
Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch Immunol Ther Exp. 2013;61:119–25.
Akhvlediani T, Ali SM, Angus DC, Arabi YM, Ashraf S, Baillie JK, et al. Global outbreak research: Harmony not hegemony. Lancet Infectious Dis. 2020;20:770–2.
Moll-Bernardes R, Fortier SC, Sousa AS, Lopes RD, Vera N, Conde L, et al. Nkg2a expression among cd8 cells is associated with covid-19 progression in hypertensive patients: Insights from the brace corona randomized trial. J Clin Med. 2022;11:3713.
Gupta A, Jayakumar MN, Saleh MA, Kannan M, Halwani R, Qaisar R, et al. Sars-cov-2 infection-induced growth factors play differential roles in covid-19 pathogenesis. Life Sci. 2022;304:120703.
Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (egfr) signaling in sars coronavirus-induced pulmonary fibrosis. Antiviral Res. 2017;143:142–50.
Ahmad F, Kannan M, Ansari AW. Role of sars-cov-2-induced cytokines and growth factors in coagulopathy and thromboembolism. Cytokine Growth Factor Rev. 2022;63:58–68.
Perreau M, Suffiotti M, Marques-Vidal P, Wiedemann A, Levy Y, Laouénan C, et al. The cytokines hgf and cxcl13 predict the severity and the mortality in covid-19 patients. Nat Commun. 2021;12:4888.
Dhar SK, Vishnupriyan K, Damodar S, Gujar S, Das M. Il-6 and il-10 as predictors of disease severity in covid-19 patients: Results from meta-analysis and regression. Heliyon. 2021;7:e06155.
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in covid-19 patients reveals il-6 and il-10 are disease severity predictors. Emerging Microbes Infections. 2020;9:1123–30.
Huang F, Liu X, Sun X, Li Z. Il-10 served as an indicator in severe covid-19 patients. J Med Virol. 2020;93:1233–5.
Moll-Bernardes R, De Sousa AS, Macedo AVS, Lopes RD, Vera N, Maia LCR, et al. Il-10 and il-12 (p70) levels predict the risk of covid-19 progression in hypertensive patients: Insights from the brace-corona trial. Front Cardio Med. 2021;8:1–10.
Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai C-CS, Maverakis E, et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211:2373–83.
Donlan AN, Sutherland TE, Marie C, Preissner S, Bradley BT, Carpenter RM, et al. Il-13 is a driver of covid-19 severity. JCI insight. 2021;6:e150107.
Gibellini L, De Biasi S, Meschiari M, Gozzi L, Paolini A, Borella R, et al. Plasma cytokine atlas reveals the importance of th2 polarization and interferons in predicting covid-19 severity and survival. Front Immunol. 2022;13:1030.
Marone G, Granata F, Pucino V, Pecoraro A, Heffler E, Loffredo S, et al. The intriguing role of interleukin 13 in the pathophysiology of asthma. Front Pharmacol. 2019;10:1387.
Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. Il-13 signaling through the il-13α2 receptor is involved in induction of tgf-β1 production and fibrosis. Nature Med. 2006;12:99–106.
Sasson J, Moreau GB, Petri Jr WA. The role of il-13 and the type 2 immune pathway in covid-19: A review. Ann Allergy, Asthma Immunol. 2023;130:727–32.
Tanaka SI, Isoda F, Ishihara Y, Kimura M, Yamakawa T. T lymphopaenia in relation to body mass index and tnf‐α in human obesity: Adequate weight reduction can be corrective. Clinical Endocrinol. 2001;54:347–54.
Karlsson EA, Marcelin G, Webby RJ, Schultz‐Cherry S. Review on the impact of pregnancy and obesity on influenza virus infection. Influenza Other Respiratory Viruses. 2012;6:449–60.
Greene E, MacIver NJ Targeting t cell (oxidative) metabolism to improve immunity to viral infection in the context of obesity. Front Immunol. 2022;13:1025495.
Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: Recent epidemiological, laboratory, and clinical evidence. Current Hypertens Rep. 2016;18:1–9.
Ruibal P, Oestereich L, Lüdtke A, Becker-Ziaja B, Wozniak DM, Kerber R, et al. Unique human immune signature of ebola virus disease in guinea. Nature. 2016;533:100–4.
Hua S, Lecuroux C, Saez-Cirion A, Pancino G, Girault I, Versmisse P, et al. Potential role for hiv-specific cd38−/hla-dr+ cd8+ t cells in viral suppression and cytotoxicity in hiv controllers. PloS one. 2014;9:e101920.
Fox A, Hoa LNM, Horby P, van Doorn HR, Trung NV, Ha NH, et al. Severe pandemic h1n1 2009 infection is associated with transient nk and t deficiency and aberrant cd8 responses. PloS one. 2012;7:e31535.
Zhou R, To KK-W, Wong Y-C, Liu L, Zhou B, Li X, et al. Acute sars-cov-2 infection impairs dendritic cell and t cell responses. Immunity. 2020;53:864–77.e865.
Zeng Q, Li Y-Z, Dong S-Y, Chen Z-T, Gao X-Y, Zhang H, et al. Dynamic sars-cov-2-specific immunity in critically ill patients with hypertension. Front Immunol. 2020;11:596684.
Majure DT, Gruberg L, Saba SG, Kvasnovsky C, Hirsch JS, Jauhar R, et al. Usefulness of elevated troponin to predict death in patients with covid-19 and myocardial injury. Am J Cardiol. 2021;138:100–6.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with covid-19 in wuhan, china. JAMA Cardiol. 2020;5:802–10.
Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with covid-19 infection. J Am College Cardiol. 2020;76:533–46.
Moll-Bernardes R, Mattos JD, Schaustz EB, Sousa AS, Ferreira JR, Tortelly MB, et al. Troponin in covid-19: To measure or not to measure? Insights from a prospective cohort study. J Clinical Med. 2022;11:5951.
Sosa-Hernandez VA, Torres-Ruiz J, Cervantes-Diaz R, Romero-Ramirez S, Paez-Franco JC, Meza-Sanchez DE, et al. B cell subsets as severity-associated signatures in covid-19 patients. Front Immunol. 2020;11:611004.
Juno JA, Tan H-X, Lee WS, Reynaldi A, Kelly HG, Wragg K, et al. Humoral and circulating follicular helper t cell responses in recovered patients with covid-19. Nature Med. 2020;26:1428–34.
Acknowledgements
We are very grateful to the staff and research assistants at the D’Or Institute for Research and Education and Rede D’Or hospitals who dedicated their time to support this study.
Funding
This work was supported by intramural grants from the D’Or Institute for Research and Education, FAPERJ (nos. E-26/210.155/2020, E-26/010.000149/2020, E-26/210.191/2020, and E-26/210.253/2020, SEI-260003/002709/2020 and SEI-260003/002718/2020, CAPES, FINEP, and the Serrapilheira Institute).
Author information
Authors and Affiliations
Contributions
RMB, ASS, FAB, RRL and EM contributed to the design of the study and the statistical analysis plan. JRF, MBT, ALP, ACBSF FVOT, AXB, ROS, MMNR recruited patients. NV, LC, MJCC and EM were responsible for biomarker analysis. SF and FAMS performed cellular analysis. EBS, JCPS an ARKS were involved in clinical data collection. RMB and RRL conducted the data analyses. All authors contributed to the interpretation of the analyses and the presentation of the results. RMB and EM were responsible for the drafting the manuscript. All authors contributed to reviewing and editing the manuscript, and all authors have agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moll-Bernardes, R., Ferreira, J.R., Sousa, A.S. et al. Impact of the immune profiles of hypertensive patients with and without obesity on COVID-19 severity. Int J Obes 48, 254–262 (2024). https://doi.org/10.1038/s41366-023-01407-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01407-0