Abstract
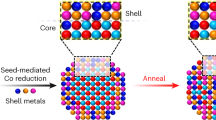
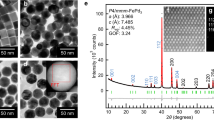
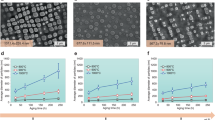
Understanding the mixing behaviour of elements in a multielement material is important to control its structure and property. When the size of a multielement material is decreased to the nanoscale, the miscibility of elements in the nanomaterial often changes from its bulk counterpart. However, there is a lack of comprehensive and quantitative experimental insight into this process. Here we explored how the miscibility of Au and Rh evolves in nanoparticles of sizes varying from 4 to 1 nm and composition changing from 15% Au to 85% Au. We found that the two immiscible elements exhibit a phase-separation-to-alloy transition in nanoparticles with decreased size and become completely miscible in sub-2 nm particles across the entire compositional range. Quantitative electron microscopy analysis and theoretical calculations were used to show that the observed immiscibility-to-miscibility transition is dictated by particle size, composition and possible surface adsorbates present under the synthesis conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information, and are available from the corresponding author on reasonable request.
Code availability
The source codes of the algorithm for the HAADF-STEM image analysis are available via Zenodo at https://doi.org/10.5281/zenodo.10510952.
References
Xie, C. L. et al. Tandem catalysis for CO2 hydrogenation to C2-C4 hydrocarbons. Nano Lett. 17, 3798–3802 (2017).
Lewis, R. J. et al. Highly efficient catalytic production of oximes from ketones using in situ-generated H2O2. Science 376, 615–620 (2022).
Yang, T. et al. Ultrahigh-strength and ductile superlattice alloys with nanoscale disordered interfaces. Science 369, 427–432 (2020).
Shi, P. J. et al. Hierarchical crack buffering triples ductility in eutectic herringbone high-entropy alloys. Science 373, 912–918 (2021).
He, Q. F. et al. A highly distorted ultraelastic chemically complex Elinvar alloy. Nature 602, 251–257 (2022).
Oh, N. R. et al. Double-heterojunction nanorod light-responsive LEDs for display applications. Science 355, 616–619 (2017).
Ibrar, M. & Skrabalak, S. E. Designer plasmonic nanostructures for unclonable anticounterfeit tags. Small Struct. 2, 2100043 (2021).
Jiang, B. B. et al. High-entropy-stabilized chalcogenides with high thermoelectric performance. Science 371, 830–834 (2021).
Jiang, B. B. et al. High figure-of-merit and power generation in high-entropy GeTe-based thermoelectrics. Science 377, 208–213 (2022).
Pendharkar, M. et al. Parity-preserving and magnetic field-resilient superconductivity in InSb nanowires with Sn shells. Science 372, 508–511 (2021).
Okamoto, H., Schlesinger, M. E. & Mueller, E. M. (eds) Binary Alloy Phase Diagrams (ASM International, 2016).
Loffler, T. et al. Discovery of a multinary noble metal-free oxygen reduction catalyst. Adv. Energy Mater. 8, 1802269 (2018).
Kusada, K. et al. Nonequilibrium flow-synthesis of solid-solution alloy nanoparticles: from immiscible binary to high-entropy alloys. J. Phys. Chem. C 125, 458–463 (2021).
Chen, Y. F. et al. Synthesis of monodisperse high entropy alloy nanocatalysts from core@shell nanoparticles. Nanoscale Horiz. 6, 231–237 (2021).
Wang, C. Y. et al. Facet-dependent deposition of highly strained alloyed shells on intermetallic nanoparticles for enhanced electrocatalysis. Nano Lett. 17, 5526–5532 (2017).
Chen, P. C. et al. Polyelemental nanoparticle libraries. Science 352, 1565–1569 (2016).
Chen, P. C. et al. Interface and heterostructure design in polyelemental nanoparticles. Science 363, 959–964 (2019).
Chen, P. C. et al. Chain-end functionalized polymers for the controlled synthesis of sub-2 nm particles. J. Am. Chem. Soc. 142, 7350–7355 (2020).
Fenton, J. L., Steimle, B. C. & Schaak, R. E. Tunable intraparticle frameworks for creating complex heterostructured nanoparticle libraries. Science 360, 513–517 (2018).
Steimle, B. C., Fenton, J. L. & Schaak, R. E. Rational construction of a scalable heterostructured nanorod megalibrary. Science 367, 418–424 (2020).
Piccolo, L. et al. Understanding and controlling the structure and segregation behaviour of AuRh nanocatalysts. Sci. Rep. 6, 35226 (2016).
Chen, P.-C. et al. Chemical and structural evolution of AgCu catalysts in electrochemical CO2 reduction. J. Am. Chem. Soc. 145, 10116–10125 (2023).
Wang, B. et al. General synthesis of high-entropy alloy and ceramic nanoparticles in nanoseconds. Nat. Synth. 1, 138–146 (2022).
Yang, C. L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Feng, G. et al. Sub-2 nm ultrasmall high-entropy alloy nanoparticles for extremely superior electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 143, 17117–17127 (2021).
Buendia, F., Vargas, J. A., Johnston, R. L. & Beltran, M. R. Study of the stability of small AuRh clusters found by a genetic algorithm methodology. Comput. Theor. Chem. 1119, 51–58 (2017).
Rahm, J. M. & Erhart, P. Understanding chemical ordering in bimetallic nanoparticles from atomic-scale simulations: the competition between bulk, surface, and strain. J. Phys. Chem. C 122, 28439–28445 (2018).
Christensen, A., Stoltze, P. & Norskov, J. K. Size dependence of phase-separation in small bimetallic clusters. J. Phys. Condens. Matter 7, 1047–1057 (1995).
Fevre, M., Le Bouar, Y. & Finel, A. Thermodynamics of phase-separating nanoalloys: single particles and particle assemblies. Phys. Rev. B 97, 195404 (2018).
Srivastava, C., Chithra, S., Malviya, K. D., Sinha, S. K. & Chattopadhyay, K. Size dependent microstructure for Ag-Ni nanoparticles. Acta Mater. 59, 6501–6509 (2011).
Kusada, K., Yamauchi, M., Kobayashi, H., Kitagawa, H. & Kubota, Y. Hydrogen-storage properties of solid-solution alloys of immiscible neighboring elements with Pd. J. Am. Chem. Soc. 132, 15896–15898 (2010).
Zhang, Q. et al. Selective control of fcc and hcp crystal structures in Au-Ru solid-solution alloy nanoparticles. Nat. Commun. 9, 510 (2018).
Zhang, H., Wang, L., Lu, L. & Toshima, N. Preparation and catalytic activity for aerobic glucose oxidation of crown jewel structured Pt/Au bimetallic nanoclusters. Sci. Rep. 6, 30752 (2016).
Toshima, N. & Hirakawa, K. Polymer-protected bimetallic nanocluster catalysts having core/shell structure for accelerated electron transfer in visible-light-induced hydrogen generation. Polym. J. 31, 1127–1132 (1999).
Toshima, N. & Yonezawa, T. Bimetallic nanoparticles-novel materials for chemical and physical applications. New J. Chem. 22, 1179–1201 (1998).
Meischein, M. et al. Elemental (im-)miscibility determines phase formation of multinary nanoparticles co-sputtered in ionic liquids. Nanoscale Adv. 4, 3855–3869 (2022).
Rajeeva, B. B. et al. Accumulation-driven unified spatiotemporal synthesis and structuring of immiscible metallic nanoalloys. Matter 1, 1606–1617 (2019).
Feng, J. C. et al. Unconventional alloys confined in nanoparticles: building blocks for new matter. Matter 3, 1646–1663 (2020).
Qi, W. H. & Wang, M. P. Size effect on the cohesive energy of nanoparticle. J. Mater. Sci. Lett. 21, 1743–1745 (2002).
Xiong, S. Y., Qi, W. H., Huang, B. Y. & Wang, M. P. Size-, shape- and composition-dependent alloying ability of bimetallic nanoparticles. ChemPhysChem 12, 1317–1324 (2011).
Qi, W. H., Huang, B. Y. & Wang, M. P. Size and shape-dependent formation enthalpy of binary alloy nanoparticles. Phys. B 404, 1761–1765 (2009).
Sneed, B. T., Young, A. P. & Tsung, C. K. Building up strain in colloidal metal nanoparticle catalysts. Nanoscale 7, 12248–12265 (2015).
Ferrando, R. Structure and Properties of Nanoalloys (Elsevier, 2016).
Chen, P. C. et al. Revealing the phase separation behavior of thermodynamically immiscible elements in a nanoparticle. Nano Lett. 21, 6684–6689 (2021).
Sohlberg, K., Pennycook, T. J., Zhou, W. & Pennycook, S. J. Insights into the physical chemistry of materials from advances in HAADF-STEM. Phys. Chem. Chem. Phys. 17, 3982–4006 (2015).
Vanzan, M., Jones, R. M., Corni, S., D’Agosta, R. & Baletto, F. Exploring AuRh nanoalloys: a computational perspective on the formation and physical properties. ChemPhysChem 23, e202200035 (2022).
Valizadeh, Z. & Abbaspour, M. Surface energy, relative stability, and structural properties of Au-Pt, Au-Rh, Au-Cu, and Au-Pd nanoclusters created in inert-gas condensation process using MD simulation. J. Phys. Chem. Solids 144, 109480 (2020).
Koch, C. T. Determination of Core Structure Periodicity and Point Defect Density along Dislocations. PhD thesis, Arizona State Univ. (2002).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
This work was supported by Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences & Biosciences Division, US Department of Energy (DOE), under Contract DE-AC02-05CH11231, FWP CH030201 (Catalysis Research Program). Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, US DOE, under contract no. DE-AC02-05CH11231. This work made use of the TEM facilities at the Cornell Center for Materials Research (CCMR), which are supported through the National Science Foundation Materials Research Science and Engineering Center (NSF MRSEC) program (DMR-1719875). P.-C.C. acknowledges support from Kavli ENSI Heising-Simons Fellowship. J.J. acknowledges fellowship support from Suzhou Industrial Park. Y.Y. acknowledges support from Miller Fellowship. C.A.M. acknowledges the National Defense Science and Engineering Graduate (NDSEG) fellowship and the Kavli ENSI Graduate Student Fellowship for financial support. This research used resources of the National Energy Research Scientific Computing Center, a US DOE Office of Science User Facility supported by the Office of Science of the US DOE under contract no. DE-AC02-05CH11231 using NERSC award BES-ERCAP0024004.
Author information
Authors and Affiliations
Contributions
P.-C.C. and P.Y. conceived the ideas and designed the experiments. P.-C.C. performed the nanoparticle synthesis and conducted the electron microscopy characterization. M.G. designed the quantitative analysis algorithm. P.-C.C. and M.G. analysed the electron microscopy characterization data and performed the STEM image simulation. C.A.M. and K.A.P. performed the DFT calculations and theoretical analysis. C.S., J.J., Y.Y. and A.L.M. discussed the experimental results. P.-C.C., M.G. and P.Y. wrote the manuscript with editorial input from the other authors. P.Y. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Hiroshi Kitagawa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28 and Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, PC., Gao, M., McCandler, C.A. et al. Complete miscibility of immiscible elements at the nanometre scale. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01626-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01626-0