Abstract
Extensive reports of pulmonary embolisms, ischaemic stroke and myocardial infarctions caused by coronavirus disease 2019 (COVID-19), as well as a significantly increased long-term risk of cardiovascular diseases in COVID-19 survivors, have highlighted severe deficiencies in our understanding of thromboinflammation and the need for new therapeutic options. Due to the complexity of the immunothrombosis pathophysiology, the efficacy of treatment with conventional anti-thrombotic medication is questioned. Thrombolytics do appear efficacious, but are hindered by severe bleeding risks, limiting their use. Nanomedicine can have profound impact in this context, protecting delicate (bio)pharmaceuticals from degradation en route and enabling delivery in a targeted and on demand manner. We provide an overview of the most promising nanocarrier systems and design strategies that may be adapted to develop nanomedicine for COVID-19-induced thromboinflammation, including dual-therapeutic approaches with antiviral and immunosuppressants. Resultant targeted and side-effect-free treatment may aid greatly in the fight against the ongoing COVID-19 pandemic.
Similar content being viewed by others
Main
The coronavirus disease 2019 (COVID-19) pandemic continues to strain health-care systems globally, with 615.6 million cases and 6.5 million deaths reported worldwide as of September 20221. COVID-19 involves the (re)infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which can result in a variety of symptoms and complications. The impact of this pandemic is likely to be felt for the foreseeable future due to the emergence of new variants of concern such as Delta (B.1.617.2) and Omicron (B.1.1.529) and low vaccination rates in many countries. Particularly detrimental to the mortality associated with SARS-CoV-2 is its tendency to cause a hypercoagulable state, resulting in extensive reports of COVID-19-induced thrombosis2, including incident rates as high as 49% in patients admitted to intensive care units3. Reports include arterial, venous and microvascular thrombosis, most commonly resulting in pulmonary embolism, stroke, deep vein thrombosis (DVT) and myocardial infarction, in order of frequency3. A high correlation between thrombotic markers and patient mortality has also been established, indicating that there is a need to improve current treatment approaches2. Furthermore, a recent study indicated a substantial long-term risk for cardiovascular disease—including thromboembolisms—in patients with COVID-19, even if hospitalization did not occur4. Hence, COVID-19-related thrombosis is likely to remain a major challenge for some time to come.
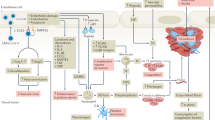
Several aspects of COVID-19-induced thrombosis make it a unique challenge compared with conventional thrombosis. In non-COVID-19-related thrombosis, coagulation is commonly triggered by the exposure of blood to pro-thrombotic stimulants upon rupture of atherosclerotic plaques, resulting in atherothrombosis. These plaques are often a result of poor diet, lack of exercise and/or smoking5. In contrast, COVID-19-related thrombosis occurs relatively frequently in otherwise healthy individuals, suggesting other pathways of activation are to blame6. A common theory is that SARS-CoV-2 can infect vascular endothelial cells, causing damage to vascular walls and instigating a systemic immune response, resulting in immunothrombosis (Fig. 1)7. It should be noted that the pathophysiology in this schematic, particularly the immune response, is simplified, as several of the signaling pathways are poorly understood as of yet. Regardless, more in-depth accounts have been provided in recently published reviews7,8.
Thrombosis appears to be a result of endothelial damage caused by SARS-CoV-2 infection stimulating an excessive immune response. It should be noted that the pathways involved are simplified, as they are highly complex and poorly understood as of yet. Following this immune response, formation of a thrombus is stimulated by upregulation of tissue factor (shown in yellow), and activation of platelets. It should be noted that several other coagulation markers are also involved, including von Willebrand factor, factor VIII and tumour necrosis factor-α. Finally, upregulation of plasminogen activator inhibitor-1 (PAI-1) also prevents breakdown of the clot by inhibiting the endogenous thrombolytic pathway. Figure created with BioRender.com.
The current consensus is that the immune response is enacted by three components, namely, systemic inflammation, activation of the complement system and formation of neutrophil extracellular traps (NETs), through a process known as NETosis. Inflammation and complement activation are known to contribute to conventional thrombosis, but the involvement of NETosis appears to be unique to the COVID-19 pathophysiology (Box 1). Together, these processes lead to upregulation of three components—activated platelets, tissue factor and thrombin—which are connected in a positive feedback loop and contribute to thrombogenesis9,10. Thrombin facilitates cleavage of fibrinogen to form fibrin, which combined with activated platelets and red blood cells (RBCs) forms a thrombus. Hence, their upregulation explains the thrombogenicity of the immune response. Furthermore, breakdown of the blood clots (thrombolysis) is also hindered. Normally, clot degradation is facilitated by cleavage of fibrin to D-dimer by plasmin, as stimulated by tissue plasminogen activator (tPA). However, clinical studies of patients with COVID-19 indicate that thrombolysis is hindered by upregulation of plasminogen activator inhibitor-1, resulting in the thrombolytic system becoming overwhelmed11.
Prospective treatment of COVID-19-induced thrombosis
Current clinical treatment of COVID-19-induced thrombosis relies primarily on low-molecular-weight heparin (LMWH) as an anticoagulant12. LMWH inhibits thrombin activation and subsequent fibrin production, as well as reducing inflammation. Unfortunately, clinical results do not show a consistent benefit, with thrombotic events still occurring despite anticoagulant treatment13,14. Furthermore, anticoagulants are inefficient at removing pre-existing blood clots. Therefore, alternative treatment options need to be considered to achieve recanalization of (partially) occluded vessels. This process must be fast to limit tissue damage, which occurs rapidly upon occlusion of a blood vessel15. Surgical interventions such as percutaneous transluminal coronary angioplasty or mechanical thrombectomy may be sufficiently rapid, but remain highly invasive and are limited by the location of the thrombus16. In particular, thrombi causing pulmonary embolisms, as frequently observed in patients with COVID-19, are complex to reach and are thus rarely removed in such manner. Therefore, calls have been made for administration of thrombolytics as clot-busting therapeutics to treat COVID-19 symptoms17.
Thrombolytics, such as tPA or urokinase, stimulate plasmin and subsequently the cleavage of fibrin, resulting in degradation of the thrombus. Hence, several thrombolytics (streptokinase, urokinase and alteplase) have been approved for the treatment of pulmonary embolism18, with the latter also being approved for the treatment of acute ischaemic stroke19. Clinical reports indicate that administration of thrombolytics is also effective in the context of patients with COVID-19, as they may assist the overwhelmed thrombolytic system20,21,22. Unfortunately, thrombolytic treatment has limitations, such as the premature degradation of the thrombolytic enzymes by systemic proteolytic enzymes. Most damaging, however, is their substantial haemorrhagic risk23. To monitor for such disastrous bleeding effects, administration occurs only in hospitals, prolonging the time to treatment and increasing treatment costs. Therefore, there is a critical need to improve the delivery of thrombolytics and reduce their side effects, potentially enabling on-the-spot treatment of patients with COVID-19 experiencing acute thrombotic events.
Nanomedicine for delivery of thrombolytics
Nanotechnology has been vital in the ongoing fight against SARS-CoV-2 owing to the successful development and approval of two lipid nanoparticle-based messenger RNA vaccines24. Furthermore, nanomedicine is also a promising strategy towards improving thrombolytic treatment via the delivery of thrombolytics with nanoparticles. Although such an approach is yet to be developed and tested for COVID-19-induced thrombosis due to the novelty of the disease, it has gathered widespread preclinical attention for the treatment of conventional atherothrombosis. Here, incorporation of the thrombolytic agent into nanoparticles enhances the effective dosage by increasing the circulation time and providing protection against premature degradation by systemic enzymes25. Thus, overall dosage may be reduced, mitigating the haemorrhagic risk. In addition, nanoparticles function as a scaffold to introduce further functionality, such as active targeting and responsive drug release without compromising loading capacity, further improving efficacy and reducing side effects26,27. Preclinical studies in rodents and some large animals such as canines have shown promising results; however, transition to clinical trials has been hampered by safety concerns and poor scalability, which is partially due to complex design. Therefore, this Review aims to highlight design approaches within this field with high translatability, and which may be adapted to the treatment of COVID-19-induced thrombosis.
Thus far, a wide range of nanoparticle systems has been developed for the treatment of acute thrombosis, which can largely be divided into liposomal, polymeric, inorganic and cell-derived nanoparticles (Fig. 2). Of these, liposomes and polymeric nanoparticles have seen the most use. Liposomes have gained widespread interest throughout many disease areas, due to their commercial success, ease of production and ability to incorporate a wide range of therapeutics28,29. Polymeric nanoparticles, which have also seen commercial success in the form of poly(lactic-co-glycolic acid) (PLGA) nanoparticles, display similar relative ease of production, while providing greater control over drug release than liposomes30, which are prone to leakage and non-specific release due to degradation28. Such solid-core polymeric nanoparticles are limited to surface loading, which reduces their loading capacity and exposes the thrombolytic payload to enzymatic degradation. Hence, polymersomes are of interest, although these vesicle structures provide limited benefits over liposomes yet show increased complexity of production31. Inorganic nanoparticles have also become popular, mainly due to their inherent multifunctionality, which enables production of highly sophisticated nanocarriers without increasing the synthesis complexity. Such functionality includes hyperthermia induced by near-infrared radiation32 or magnetic stimulation33,34, and even accumulation at the site of thrombosis via magnetic guidance33. Mesoporous inorganic nanomaterials are of particular interest due to their increased drug-loading capacity compared with their solid counterparts. Unfortunately, many inorganic materials require complex surface modification to mitigate toxicity of the raw material, which may still pose a safety risk upon clearance and degradation in the body35.
Strategies with high relevance to the treatment of COVID-19-induced thrombosis are shown. a, A comparison of the nanoparticle types, with benefits and limitations provided as a consideration for the design of clinically relevant thrombolytic nanomedicine. Thrombolytics are depicted as red ovals to indicate the relevant loading approaches. As depicted, the cell-derived vesicles contain endogenous surface receptors and ligands naturally present after production. b–d, Targeted delivery (b) and responsive delivery (c) may also be utilized to further improve efficacy and specificity of thrombolytic therapy for COVID-19-induced thrombosis, which degrades blood clots through stimulation of the thrombolytic pathway (d). Figure created with BioRender.com.
In contrast, cell-derived nanoparticles have gained considerable interest due to their high biocompatibility. This approach is particularly interesting for the treatment of acute thrombosis, as vesicles derived from RBCs or platelets can be used to generate nanoparticles displaying endogenous thrombus-binding proteins, enabling a natural affinity for blood clots27,36. Such an approach also drastically improves the pharmacokinetics associated with nanoparticle systems. Compared with non-cloaked nanoparticles, RBC-derived and platelet-membrane-derived nanoparticles showed 3 and 1.5 times longer circulation times, respectively, which may be highly beneficial to extend the thrombolytic effect36. Chen et al.36 indicated this may be due to reduced elimination of these membrane-derived vesicles through the reticuloendothelial system, as macrophage uptake was decreased by 69.0% (RBC cloaked) and 70.2% (platelet cloaked). Unfortunately, the production of such endogenously derived vesicles at large scale is still challenging, limiting their translational potential37.
As an alternative to mimicking the membrane physiology of endogenous cells, Colasuonno et al.25 aimed to instead mimic their physical structure by utilizing more scalable polymeric nanoparticles. Specifically, RBCs were mimicked, as these cells have a long circulation time and can pass through even the smallest capillaries despite their large size due to their soft, discoidal shape. Thus, the authors produced soft, discoidal tPA-conjugated polymeric nanostructures25. These nanostructures showed superior thrombolytic efficacy in vivo over spherical nanoparticles, reducing more blood clots and to a greater extent. This was conceivably due to improved adhesion to the blood clots combined with longer circulation times25. Liver and kidney accumulation of the discoidal nanoconstructs was comparatively lower, indicating elimination via the reticuloendothelial system is affected by the shape of the nanoparticles25. Due to these promising results, together with its scalability, this approach may have high translational potential, although its efficacy in COVID-19 models remains to be tested.
Finally, a vital consideration in the design of nanomedicine for COVID-19-induced thrombosis is their ability to stimulate NETosis. Several papers have reported nanoparticle-induced NETosis, which may aggravate the thrombogenic nature of the SARS-CoV-2 infection. Thus far, nanoparticle-induced NETosis has been reported for nanoparticles based on silver38, gold39,40, iron oxide41,42, manganese oxide41, graphene oxide43, cationic lipids44, polystyrene45 and nanodiamonds45, although further research may reveal other materials exhibiting similar behaviour. There is as-of-yet contention over whether this is caused by the nanoparticles themselves or their dissolution products46. Several studies have reported that smaller nanoparticles (<100 nm) are more prone to inducing NETosis than larger nanoparticles, supposedly due to their increased surface-area-to-volume ratio39,45. Hence, NETosis may be mitigated by increasing the particle size. Surface functionalization with biocompatible layers of human serum albumin or dextran has also been shown to reduce the NET-generating properties of iron oxide nanoparticles42, providing another strategy for minimizing NETosis. Interestingly, several papers have indicated that the addition of polyethylene glycol—which is normally seen as highly biocompatible—does not reduce NETosis40, and may even aggravate it41. Due to these variabilities, it is suggested that nanomedicines designed to treat COVID-19-induced thrombosis are optimized and tested pre-clinically for their ability to generate NETosis, to avoid potential pro-thrombotic effects.
Targeting of nanoparticles to COVID-19-induced thrombosis
In general, nanoparticles are cleared by the liver and spleen and accumulation at the site of thrombosis is limited, reducing their efficacy and specificity. Micrometre-sized particles may be used instead, as these particles can accumulate in the microvasculature of the lungs, treating localized thrombosis and thromboinflammation. However, caution must be exercised as microparticles >10 μm are able to occlude lung capillaries47. Localized accumulation of nanoparticles may be achieved through decoration of the particles with ligands with affinity for components of the thrombi, which has widely been investigated for treatment of conventional atherothrombosis (Table 1). As these components also play a central role in COVID-19-induced thrombosis, decoration of nanoparticles with such ligands may be highly beneficial. It should be noted that the functional optimization of active targeting is complex; for example, a study showed that only 3.5% of proteins conjugated to a particle had an appropriate orientation for receptor recognition48, and that the ligand surface density can affect targeting too49. Furthermore, the addition of targeting ligands adds complexity; hence, scalability must be considered to ensure high translational potential.
To avoid this added complexity, therapeutics with inherently high affinity for thrombi may be utilized. For instance, the previously discussed discoidal polymeric nanostructures include tPA conjugated to the surface, where tPA’s notable affinity for fibrin and multivalent adhesive interactions have been suggested to contribute to an improved thrombolytic efficacy25. Alternatively, LMWH, which has seen widespread clinical use in the treatment of COVID-19-induced thrombosis, selectively targets P-selectin on activated platelets, and provides effective targeting when applied to nanomedicine50. Fucoidan, a complex polysaccharide derived from algae with anticoagulant properties, displays two orders of magnitude higher affinity for the same target (dissociation constant Kd = 1.2 × 10–9 M)51. This, in combination with its Food and Drug Administration (FDA) approval, sparked the use of fucoidan in several thrombus-targeted nanoparticle systems, including poly(isobutylcyanoacrylate) nanoparticles52, manganese oxide53 and mesoporous silica-coated gold nanorods54.
Small peptides to target fibrin or activated platelets have been utilized by several groups, due to their ease of scalability and low immunogenicity55. However, the greatest targeting efficiency was observed when a synergistic approach combining multiple ligands was applied. For example, decorating liposomes with both platelet- and fibrin-binding peptides enhanced their thrombus-anchorage efficacy compared with using only one of them56. Interestingly, at high (beyond 5 mol%) total ligand density, the clot-anchorage capability decreased, indicating ligand conjugation must be optimized to ensure high targeting affinity57. Combining a fibrin-binding peptide with an activatable cell-penetrating peptide also improved targeting efficacy and especially promoted penetration into the thrombus58. Unfortunately, peptides often have poor proteolytic stability and have in general a lower affinity compared with, for instance, antibodies.
Due to the favourable high affinity of antibodies, several groups have explored the use of antibodies, seeing preclinical success also in large animal models. In a canine model, conjugation of an anti-fibrin monoclonal antibody (Kd = 1.0 × 10–9 M) to a perfluorocarbon nanoparticle resulted in increased accumulation at the thrombus59. In addition, a single-chain antibody was developed for activated αIIb/β3 using phage display60 and poly(2-oxazoline)-based polymer capsules modified with this antibody were found to specifically target activated platelets61. Again, optimization of the conjugation of the ligands onto the nanoparticles is essential, including consideration of (1) the conjugation site of the linker to preserve affinity and warrant correct orientation, and (2) the density of the antibody on the nanoparticle surface62. Although production of antibodies is dearer than small peptides, scalability is not an issue, as evident from their considerable therapeutic market share63.
As previously mentioned, the use of endogenously derived membranes in nanoparticles affords the benefit of inherent targeting capabilities, while also providing extended pharmacokinetic profiles. To this end, platelet and RBC membranes are both of interest; hence, their targeting capabilities were compared in two separate studies27,36. Interestingly, Xu et al.27 found that platelet membranes provided superior thrombus targeting, whereas Chen et al.36 indicated that RBC membranes had higher affinity. This discrepancy can be attributed to the activation status of the platelet membranes, which varied between the studies, their affinity following activated platelets > RBC > inactivated platelets. As 81% of platelet membrane proteins are preserved in the coating, one might expect use of such activated platelet membranes may worsen thrombosis due to the activation of thrombogenesis27. However, despite the presence of adhesion-associated proteins αIIb/β3, CD62p and P-selectin, no effect on aggregation of other platelets was observed27. Nonetheless, it is critical to consider the source of platelet membrane to prevent potential (allogeneic) immune response. Finally, the therapeutic agent must also be considered. For instance, the Gong group utilized RBC-coated nanoparticles, as the incorporated drug (tirofiban) is an antagonist of the platelet αIIb/β3 receptor and could potentially compromise the targeting capability of an activated platelet membrane64.
The vital role of neutrophils in the COVID-19 thrombotic pathophysiology makes this is another target of high interest. A neutrophil-targeting approach is particularly interesting for early treatment, as the thrombus does not need to be formed yet for this targeting to be effective. For instance, Cruz et al.65 recently developed a short peptide sequence (CGEAIPMSIPPEVK) with affinity for neutrophil elastase, which is only expressed by activated neutrophils. Decoration of liposomes with said ligand enabled specific targeting of activated neutrophils, and subsequently promoted accumulation at the site of thrombosis. A dual targeting strategy also including a platelet-targeting peptide (DAEWVDVS) enabled binding to activated platelet–neutrophil complexes in a DVT mouse model65. These complexes are highly prevalent following SARS-CoV-2 infection; therefore, this targeting approach may also enable efficient therapy for COVID-19-induced thrombosis.
Responsive nanomedicines for COVID-19-induced thrombosis
Targeted therapy of thrombosis may also be achieved through development of responsive nanocarriers, which selectively release thrombolytics on demand. A wide range of stimuli have been studied for this purpose thus far, although not all approaches appear to be applicable to COVID-19-induced thrombosis. For instance, nanomedicines responsive to external stimuli such as magnetic fields and ultrasound rely on input from a trained professional. This complicates treatment; hence, the development of drug delivery systems with responsiveness to internal stimuli appears more conducive to the required rapid treatment of COVID-19-induced thrombosis. In addition, despite the success of shear- and pH-responsive nanomedicine in acute ischaemic stroke models66,67, such approaches should also be disregarded due to the absence of these stimuli in pulmonary embolisms, which is the most common thrombotic complication in patients with COVID-19 at 87% (ref. 3). Various other approaches do appear to be highly relevant to COVID-19-induced thrombosis, and are therefore outlined below.
Enzyme-induced drug release is a widely used paradigm as it is highly selective and efficient68. Examples of enzymes that are upregulated in COVID-19-induced thrombosis that may be exploited for this purpose include secreted phospholipase-A2 (sPLA2)69 and thrombin9. sPLA2 is produced by activated platelets and inflammatory cells70, and can cleave the SN-2 ester bonds in glycerophospholipids. Hence, Pawlowski et al.57 developed streptokinase-loaded liposomes, which exhibited disruption of the liposomal bilayer upon sPLA2-induced cleavage, followed by burst release of the thrombolytic agent. This nanoparticle system enabled thrombolysis with an efficacy comparable to free streptokinase in a carotid arterial thrombosis mouse model, while negating the bleeding complications of the free drug. Furthermore, this simple nanocarrier system holds considerable clinical potential, due to the scalability and previous regulatory approval of liposomes. However, as liposomes tend to suffer from non-specific leakage of the payload, release of the thrombolytic was observed even in the absence of sPLA2 (ref. 57). In addition, sPLA2 is involved not only in stimulation of thrombogenesis by COVID-19 but also in other aspects of COVID-19 pathophysiology69. Hence, further in vivo studies in COVID-19 animal models are required to determine whether such an approach results in drug release specific to the site of thrombosis, or whether systemic release and subsequent bleeding might be observed.
Thrombin, in contrast, is highly specific to acute thrombosis; thus, off-target drug release from thrombin-sensitive nanoparticles may be less likely. Thrombin stimulates cleavage of fibrinogen to fibrin; hence, thrombin sensitivity can be introduced through incorporation of peptides mimicking its binding site on fibrinogen. Gallwitz et al.71 performed an in-depth study to identify the preferred sequences, with LTPRGWRL showing the highest cleavage efficiency. Thus, several nanoparticle systems have used this or similar peptides to deliver thrombolytics in response to the presence of thrombin. For instance, Xu et al.26 conjugated recombinant tissue plasminogen activator (rtPA) to the surface of their platelet membrane nanovesicles through a thrombin-cleavable peptide. Beside the highly specific responsive release observed, this cleavage also revealed a cell-penetrating peptide motif, increasing penetration into the thrombus26. Unfortunately, surface loading as applied here probably has limited loading capacity and introduces susceptibility to premature degradation by systemic enzymes. Hence, a core-loaded system, such as proposed by Zhang et al.53, may be more promising. Here, urokinase was loaded into the pores of mesoporous manganese dioxide (MnO2), and conjugation of fucoidan through a thrombin-cleavable peptide to the surface prevented release while simultaneously providing targeting. This system displayed impressive in vivo thrombolytic capabilities and exhibited a low haemorrhagic risk53.
Interestingly, the MnO2 particles also acted as hydrogen peroxide (H2O2) scavengers, which may be highly beneficial in the treatment of COVID-19-induced thrombosis. An increase in reactive oxygen species (ROS), such as H2O2, is a chemical biomarker of thrombosis, as ROS promote platelet activation and inflammation72. Upregulated ROS levels were also reported in patients with COVID-19, contributing to many aspects of the pathophysiology. Therefore treatment with antioxidants has been identified as a promising approach against COVID-19 symptoms73. The ability of the MnO2 nanoparticles to reduce H2O2 levels by up to two-thirds compared with a control in vitro may therefore be of great benefit53. Alternatively, Mei et al.74 developed tPA-loaded polymeric nanoparticles, which contained 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl (4-amino-TEMPO) as H2O2 scavengers. This enabled a reduction of ROS levels in rat brains after ischaemic stroke to a level comparable to healthy brains. The protective effect of this was profound, as seen by a haemorrhagic propensity indistinguishable from the saline control74. Although the ischaemic stroke model used here may not accurately mimic COVID-19-induced thrombosis, these results and the reported impact of ROS in COVID-19 pathophysiology warrant further testing of such systems in COVID-19 models. Thus, a system that delivers thrombolytic agents as well as provides ROS scavenging may enable treatment of COVID-19-induced thrombosis while simultaneously relieving COVID-19-related oxidative stress.
Immunosuppressant and antiviral nanomedicines
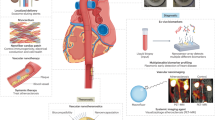
An alternative or complementary approach to the use of thrombolytics for the treatment of COVID-19-induced thrombosis may be the administration of therapeutics that suppress infection or the subsequent immune response. This has been an approach of great interest in the general treatment of COVID-19, with various clinical trials underway. This includes the immunosuppressants tocilizumab75, a complement activation inhibitor, and recombinant human deoxyribonuclease (rhDNase)76, a NETosis inhibitor, which have reached Phase III and Phase II, respectively. Antiviral agents have seen even greater success, with molnupiravir and paxlovid receiving regulatory approval77,78. Several virucidal, virus-trapping and immunosuppressant nanomedicines have been developed and tested for treatment against COVID-19 (Fig. 3). The effect of these nanomedicines in reducing infection and the linked immune response may also translate into a lower thrombosis risk by limiting the underlying stimulatory pathways.
a, Virucidal nanoparticles associate with the virus through interaction of the positive nanoparticle surface (red) with the negatively charged virus particles, following by stimulation of lysis of the virus envelope. b, Virus-trapping nanoparticles competitively bind to SARS-CoV-2 via the ACE2 receptors (blue) present on their surface, preventing infection of endothelial cells. c, Immune-suppressing nanoparticles are surface-loaded with rhDNase (yellow), which breaks down the DNA strands (purple strands) to mitigate excessive NETosis. Thus, through all three approaches, the excessive immune response to SARS-CoV-2 infection may be limited, potentially aiding in preventing COVID-19-induced thrombosis. Figure created with BioRender.com.
Virucidal nanoparticles have seen much interest to prevent infection and replication of the virus in the body79. Besides loading antiviral therapeutics into nanoparticles, the use of nanomaterials with inherent virucidal properties is an interesting approach. To this end, nanoparticles based on silver80,81, polylysine82 and glycyrrhizic acid83 have been investigated. These nanoparticles may bind to SARS-CoV-2, disrupting its integrity and subsequently preventing infection of endothelial cells and replication (Fig. 3a). In vivo testing of their efficacy has thus far been limited to the glycyrrhizic acid nanoparticles, which decreased infection and improved survival in a SARS-CoV-2 mouse model83. Interestingly, both silver and polylysine nanoparticles showed a positive correlation between their antiviral activity and their surface charge80,82. This was rationalized by an improved interaction with the viral particles, which display a negative charge at physiological pH. Furthermore, silver nanoparticles displayed size-dependent virucidal properties with 10 nm nanoparticles exhibiting optimal SARS-CoV-2 inhibition, whereas 100 nm nanoparticles were entirely ineffective80,81. Once optimized, all nanoparticles displayed exceptional capacities to suppress viral replication, making the use of these nanomaterials a promising approach for the prevention of COVID-19-induced thrombosis. However, despite promising biocompatibility when in their nanoparticulate form, the cytotoxicity related to silver and glycyrrhizic acid, and potential stimulation of NETosis may complicate their use81,83.
Alternatively, several groups have utilized angiotensin-converting enzyme-2 (ACE2)-expressing membranes to produce nanoconstructs that competitively bind and trap SARS-CoV-2, lightening the viral load (Fig. 3b)84,85,86,87. ACE2 plays a critical role in SARS-CoV-2 binding and infection, and is highly expressed in kidney cells (HEK293), macrophages (THP-1) and lung spheroid cells (LSCs). Hence membranes derived from these cells provide excellent inherent targeting, binding and trapping of SARS-CoV-284,85,86,87. Li et al.87 showed that LSCs express higher levels of ACE2 than HEK293 cells, resulting in improved SARS-CoV-2 neutralization by LSC-derived nanoparticles, thus making this approach preferable. However, use of macrophage-derived vesicles may be the most appropriate, as Tan et al.84 showed that said nanoparticles not only reduced the viral load but also prevented NETosis by inhibiting proinflammatory factors. As upon infection the immune system’s overactivation contributes heavily to the severity of COVID-19 symptoms, such an approach may be highly beneficial in the prevention of COVID-19-induced thrombosis88. Furthermore, nanostructures formed from these endogenously derived membranes display high biocompatibility, and can be core-loaded with a therapeutic84. Complex large-scale production of cell-derived nanoparticles does still constitute a substantial hurdle to their translational potential. However, it is expected that this obstacle may be overcome in the future as more efficient methods are established.
Also of consideration is the route of administration of these antiviral nanoparticles, with inhalable formulations gathering notable interest for the treatment of COVID-19 due to the localized delivery directly to the primary site of infection89. In contrast, intravenous injection, as is often used to administer nanomedicine, results in systemic delivery and often undesirable accumulation in the liver. Hence, Li et al.87 designed their LSC-derived nanovesicles to be nebulized and inhaled. Viral clearance was subsequently improved substantially in mice infected with SARS-CoV-2 mimicking viruses. Accumulation in the lungs could be observed even after 72 hours, indicating localized delivery and prolonged protection87. This is consistent with in-human studies of inhaled nanoparticles, which have indicated that accumulation of nanoparticles in the lungs is followed by translocation to the bloodstream via passive diffusion for an extended period of time90. In contrast, the intravenously administered HEK293- and THP-1-derived nanoparticles appeared to be entirely localized to the liver after only 24 hours84,86. Further studies should be performed to ensure adequate elimination and biocompatibility. Regardless, inhalable antiviral nanomedicine appears to be a promising approach towards the localized treatment of COVID-19.
As an alternative approach to limiting the immunothrombotic response to SARS-CoV-2 infection, the effect of NETosis may also be mitigated by degrading the extracellular trap components. This area of research has thus far been led by the Park group, who utilized PLGA–dopamine91 and melanin-like nanoparticles92 to deliver rhDNase. rhDNase breaks down the DNA fibres present in NETs, hence reducing the thrombogenicity related to NETosis (Fig. 3c). Park et al. were able to deliver said therapeutic with a higher stability compared with free rhDNase; thus, this approach enabled significantly improved reductions in NETosis levels in blood samples of patients with COVID-19 and a sepsis mouse model91,92. A previous study showed that reducing NETosis in an identical sepsis model reduced thrombosis93; hence, it is expected that these particles may exhibit a similar effect.
Despite these promising results, such antiviral or immunosuppressant approaches are limited to the prevention of thrombogenesis similar to LMWH, and can therefore not effectively remove pre-existing thrombi. Hence, the development of dual-therapeutic nanomedicine based on these antiviral or immunosuppressant nanoparticles co-loaded with a thrombolytic is suggested as a promising approach towards the holistic treatment of COVID-19-induced thrombosis. It should be noted that such dual-therapeutic strategies do introduce additional challenges. The loading of therapeutics must be well optimized, to ensure both therapeutic effects are achieved at adequate efficacy while avoiding toxicity. This increased complexity may only be acceptable if the scalability of synthesis is ensured, which is an obstacle many nanomaterials are currently limited by. Regardless, this appears to be an avenue of research with the potential to greatly improve the treatment of COVID-19-induced thrombosis if further investigated.
Outlook
The development of nanomedicine to treat COVID-19-induced thrombosis may have a great impact on improving patient outcomes worldwide. Current challenges to reaching clinical uptake lie primarily in the novelty of the virus, a lack of testing in COVID-19-specific animal models, as well as the translatability of current nanomedicines. Our understanding of the immunothrombotic pathophysiology stimulated by SARS-CoV-2 infection is still limited, but it appears to vary drastically from conventional thrombosis. This probably means that conventional thrombosis animal models are non-representative; hence, COVID-19 models or models of immunothrombosis, such as sepsis models, should be utilized instead. Although sepsis models are relatively easy to establish and operate, their relevance to COVID-19-induced pathophysiology of immunothrombosis remains to be confirmed94. COVID-19 models introduce higher complexity, as conventionally utilized animals such as mice and canines show innate insusceptibility to SARS-CoV-2 infection and can therefore not be used95,96. However, highly representative immunothrombosis in response to SARS-CoV-2 has been observed in minks, Roborovski dwarf hamsters and rhesus macaques95. Despite the added complexity, these models ensure higher accuracy in determining the clinical potential of nanomedicine for the treatment of COVID-19-induced thrombosis and should therefore be explored.
In general, clinical adoption of nanomedicine for cardiovascular diseases has remained lower than other disease areas, such as cancer, due to a higher translational hurdle. In particular, concerns regarding safety and cost have limited clinical trials of nanomedicine97. Nanomedicine for COVID-19-induced thrombosis will probably face the same hurdles; thus, advances in this field should be informed by translatable design to minimize these challenges going forward. Primarily, this includes the choice of nanomaterial utilized. Regulatory-approved nanomaterials utilized in other areas, such as liposomes, PLGA nanoparticles and iron oxide nanoparticles, may at this time produce faster translatable systems. However, these systems can suffer drawbacks regarding payload loading and leakage; hence, focusing on improving scalability and assessing the safety of alternative nanomaterials, which negate these issues, may be more beneficial in the long term. Sophisticated approaches such as post-modifications introducing targeting of thrombi have delivered promising preclinical results. However, the balance between sophistication and ease of synthesis of such design approaches must be considered to ensure both safety and scalability. This includes both the choice of targeting ligand (Table 1) and the design of stimuli-responsive systems, which—despite the encouraging results—require further research to optimize current approaches or investigate alternative opportunities as to afford clinically relevant targeted nanomaterials.
Besides the challenges involved in the design of nanomedicine for COVID-19-induced thrombosis, this area also provides exciting opportunities for the development of highly powerful, yet translatable nanomedicines. Here, the application of multifunctional nanomaterials appears particularly promising. For instance, H2O2-scavenging nanoparticles were earmarked as these enable simultaneous anti-inflammatory effects while delivering thrombolytic therapeutics, which is particularly relevant in the treatment of COVID-19. Virucidal or virus-trapping nanoparticles are also of interest, due to their proven efficacy against SARS-CoV-2 infection. Finally, the use of immunosuppressants to reduce NETosis has been highlighted as a promising strategy to prevent thrombogenesis. In particular, co-delivery of thrombolytics in antiviral or immunosuppressant nanoparticles may be promising to remove existing blood clots as well as prevent further COVID-19-induced thrombogenesis. Formulation of inhalable nanomedicine may also ensure localized delivery directly to the site of infection. Despite the added challenges related to such a strategy, it is expected that such nanomedicines may aid greatly in treating the unique pathophysiology of COVID-19-induced thrombosis, justifying further research in this exciting field to fill critical gaps.
References
Dong, E., Du, H. & Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 20, 533–534 (2020).
Wichmann, D. et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 173, 268–277 (2020).
Klok, F. A. et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 191, 148–150 (2020).
Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590 (2022).
Virani, S. S. et al. Heart disease and stroke statistics—2021 update. Circulation 143, e254–e743 (2021).
Wilcox, T., Smilowitz, N. & Berger, J. Age and sex differences in incident thrombosis in patients hospitalized with COVID-19. J. Am. Coll. Cardiol. 77, 1826 (2021).
Chen, A.-T., Wang, C.-Y., Zhu, W.-L. & Chen, W. Coagulation disorders and thrombosis in COVID-19 patients and a possible mechanism involving endothelial cells: a review. Aging Dis. 13, 144–156 (2022).
Behzadifard, M. & Soleimani, M. NETosis and SARS-COV-2 infection related thrombosis: a narrative review. Thromb. J. 20, 13 (2022).
Campello, E. et al. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. 59, 1323–1330 (2021).
Skendros, P. et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 130, 6151–6157 (2020).
Goshua, G. et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 7, e575–e582 (2020).
Thachil, J. et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 18, 1023–1026 (2020).
Sholzberg, M. et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. Brit. Med. J. 375, n2400 (2021).
The REMAP-CAP, ACTIV-4a & ATTACC Investigators Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N. Engl. J. Med. 385, 777–789 (2021)..
Kalogeris, T., Baines, C. P., Krenz, M. & Korthuis, R. J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317 (2012).
Blanc, R. et al. Recent advances in devices for mechanical thrombectomy. Expert Rev. Med. Devices 17, 697–706 (2020).
Whyte, C. S., Morrow, G. B., Mitchell, J. L., Chowdary, P. & Mutch, N. J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 18, 1548–1555 (2020).
Ucar, E. Y. Update on thrombolytic therapy in acute pulmonary thromboembolism. Eurasian J. Med. 51, 186–190 (2019).
Warach, S. J., Dula, A. N. & Milling, T. J. Tenecteplase thrombolysis for acute ischemic stroke. Stroke 51, 3440–3451 (2020).
Wang, J. et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. 18, 1752–1755 (2020).
Kosanovic, D. et al. Recombinant tissue plasminogen activator treatment for COVID-19 associated ARDS and acute cor pulmonale. Int. J. Infect. Dis. 104, 108–110 (2021).
Barrett, C. D. et al. Study of alteplase for respiratory failure in SARS-CoV-2 COVID-19: a vanguard multicenter, rapidly adaptive, pragmatic, randomized controlled trial. Chest 161, 710–727 (2022).
Rothschild, D. P., Goldstein, J. A. & Bowers, T. R. Low-dose systemic thrombolytic therapy for treatment of submassive pulmonary embolism: clinical efficacy but attendant hemorrhagic risks. Catheter. Cardiovasc. Interv. 93, 506–510 (2019).
Huang, X. et al. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. 17, 1027–1037 (2022).
Colasuonno, M. et al. Erythrocyte-inspired discoidal polymeric nanoconstructs carrying tissue plasminogen activator for the enhanced lysis of blood clots. ACS Nano 12, 12224–12237 (2018).
Xu, J. et al. Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano 13, 8577–8588 (2019).
Xu, J. et al. Engineered nanoplatelets for targeted delivery of plasminogen activators to reverse thrombus in multiple mouse thrombosis models. Adv. Mater. 32, 1905145 (2020).
Russell, L. M., Hultz, M. & Searson, P. C. Leakage kinetics of the liposomal chemotherapeutic agent doxil: the role of dissolution, protonation, and passive transport, and implications for mechanism of action. J. Control. Release 269, 171–176 (2018).
Kim, J.-Y., Kim, J.-K., Park, J.-S., Byun, Y. & Kim, C.-K. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 30, 5751–5756 (2009).
Zhang, W., Mehta, A., Tong, Z., Esser, L. & Voelcker, N. H. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv. Sci. 8, 2003937 (2021).
Matoori, S. & Leroux, J.-C. Twenty-five years of polymersomes: lost in translation? Mater. Horiz. 7, 1297–1309 (2020).
Wang, X. et al. Near-infrared triggered release of uPA from nanospheres for localized hyperthermia-enhanced thrombolysis. Adv. Funct. Mater. 27, 1701824 (2017).
Wang, S. et al. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci. Adv. 6, eaaz8204 (2020).
Voros, E. et al. TPA immobilization on iron oxide nanocubes and localized magnetic hyperthermia accelerate blood clot lysis. Adv. Funct. Mater. 25, 1709–1718 (2015).
Yang, G., Phua, S. Z. F., Bindra, A. K. & Zhao, Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 31, 1805730 (2019).
Chen, K. et al. Intrinsic biotaxi solution based on blood cell membrane cloaking enables fullerenol thrombolysis in vivo. ACS Appl. Mater. Interfaces 12, 14958–14970 (2020).
Wang, J., Chen, D. & Ho, E. A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Control. Release 329, 894–906 (2021).
Kang, H., Seo, J., Yang, E.-J. & Choi, I.-H. Silver nanoparticles induce neutrophil extracellular traps via activation of PAD and neutrophil elastase. Biomolecules 11, 317 (2021).
Yang, Y. et al. Gold nanoparticles synergize with bacterial lipopolysaccharide to enhance class A scavenger receptor dependent particle uptake in neutrophils and augment neutrophil extracellular traps formation. Ecotoxicol. Environ. Saf. 211, 111900 (2021).
Bartneck, M., Keul, H. A., Zwadlo-Klarwasser, G. & Groll, J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 10, 59–63 (2010).
Snoderly, H. T. et al. PEGylation of metal oxide nanoparticles modulates neutrophil extracellular trap formation. Biosensors 12, 123 (2022).
Bilyy, R. et al. Inert coats of magnetic nanoparticles prevent formation of occlusive intravascular co-aggregates with neutrophil extracellular traps. Front. Immunol. 9, 2266 (2018).
Mukherjee, S. P. et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 10, 1180–1188 (2018).
Hwang, T.-L., Aljuffali, I. A., Hung, C.-F., Chen, C.-H. & Fang, J.-Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs). Chem. Biol. Interact. 235, 106–114 (2015).
Muñoz, L. E. et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc. Natl Acad. Sci. USA 113, E5856–E5865 (2016).
Yang, H. et al. Nanomaterial exposure induced neutrophil extracellular traps: a new target in inflammation and innate immunity. J. Immunol. Res. 2019, 3560180 (2019).
Kutscher, H. L. et al. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J. Control. Release 143, 31–37 (2010).
Herda, L. M., Hristov, D. R., Lo Giudice, M. C., Polo, E. & Dawson, K. A. Mapping of molecular structure of the nanoscale surface in bionanoparticles. J. Am. Chem. Soc. 139, 111–114 (2017).
Faria, M. et al. Minimum information reporting in bio-nano experimental literature. Nat. Nanotechnol. 13, 777–785 (2018).
Lu, T.-Y. et al. Dual-targeting glycol chitosan/heparin-decorated polypyrrole nanoparticle for augmented photothermal thrombolytic therapy. ACS Appl. Mater. Interfaces 13, 10287–10300 (2021).
Bachelet, L. et al. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. Biochim. Biophys. Acta 1790, 141–146 (2009).
Juenet, M. et al. Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials 156, 204–216 (2018).
Zhang, H. et al. Thrombus-targeted nanoparticles for thrombin-triggered thrombolysis and local inflammatory microenvironment regulation. J. Control. Release 339, 195–207 (2021).
Chang, L.-H. et al. Thrombus-specific theranostic nanocomposite for codelivery of thrombolytic drug, algae-derived anticoagulant and NIR fluorescent contrast agent. Acta Biomater. 134, 686–701 (2021).
Apostolopoulos, V. et al. A global review on short peptides: frontiers and perspectives. Molecules 26, 430 (2021).
Sun, M. et al. Combination targeting of ‘platelets + fibrin’ enhances clot anchorage efficiency of nanoparticles for vascular drug delivery. Nanoscale 12, 21255–21270 (2020).
Pawlowski, C. L. et al. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials 128, 94–108 (2017).
Yang, A. et al. Thrombin-responsive engineered nanoexcavator with full-thickness infiltration capability for pharmaceutical-free deep venous thrombosis theranostics. Biomater. Sci. 8, 4545–4558 (2020).
Marsh, J. N. et al. A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine 6, 605–615 (2011).
Schwarz, M. et al. Conformation-specific blockade of the integrin GPIIb/IIIa. Circ. Res. 99, 25–33 (2006).
Bates, A. & Power, C. A. David vs. Goliath: the structure, function, and clinical prospects of antibody fragments. Antibodies 8, 28 (2019).
Yong, K. W., Yuen, D., Chen, M. Z. & Johnston, A. P. R. Engineering the orientation, density, and flexibility of single-domain antibodies on nanoparticles to improve cell targeting. ACS Appl. Mater. Interfaces 12, 5593–5600 (2020).
Lu, R.-M. et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27, 1 (2020).
Zhao, Y. et al. Biomimetic fibrin-targeted and H2O2-responsive nanocarriers for thrombus therapy. Nano Today 35, 100986 (2020).
Cruz, M. A. et al. Nanomedicine platform for targeting activated neutrophils and neutrophil–platelet complexes using an α1-antitrypsin-derived peptide motif. Nat. Nanotechnol. 17, 1004–1014 (2022).
Korin, N. et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 337, 738–742 (2012).
Marosfoi, M. G. et al. Shear-activated nanoparticle aggregates combined with temporary endovascular bypass to treat large vessel occlusion. Stroke 46, 3507–3513 (2015).
Wang, Y., Shim, M. S., Levinson, N. S., Sung, H.-W. & Xia, Y. Stimuli-responsive materials for controlled release of theranostic agents. Adv. Funct. Mater. 24, 4206–4220 (2014).
Snider, J. M. et al. Group IIA secreted phospholipase A2 is associated with the pathobiology leading to COVID-19 mortality. J. Clin. Invest. 131, e149236 (2021).
Takahashi, S. et al. Phospholipase A2 expression in coronary thrombus is increased in patients with recurrent cardiac events after acute myocardial infarction. Int. J. Cardiol. 168, 4214–4221 (2013).
Gallwitz, M., Enoksson, M., Thorpe, M. & Hellman, L. The extended cleavage specificity of human thrombin. PLoS ONE 7, e31756 (2012).
Freedman, J. E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 28, s11–s16 (2008).
Laforge, M. et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 20, 515–516 (2020).
Mei, T. et al. Encapsulation of tissue plasminogen activator in pH-sensitive self-assembled antioxidant nanoparticles for ischemic stroke treatment—synergistic effect of thrombolysis and antioxidant. Biomaterials 215, 119209 (2019).
Genentech Inc. Efficacy of tocilizumab on patients with COVID-19. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT04356937 (2020).
Simmons, J. Phase 2 trial using rhDNase to reduce mortality in COVID-19 patients with respiratory failure (DAMPENCOVID). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04445285 (2020).
Syed, Y. Y. Molnupiravir: first approval. Drugs 82, 455–460 (2022).
Lamb, Y. N. Nirmatrelvir plus ritonavir: first approval. Drugs 82, 585–591 (2022).
Peplow, M. Nanotechnology offers alternative ways to fight COVID-19 pandemic with antivirals. Nat. Biotechnol. 39, 1172–1174 (2021).
He, Q. et al. Antiviral properties of silver nanoparticles against SARS-CoV-2: effects of surface coating and particle size. Nanomaterials 12, 990 (2022).
Jeremiah, S. S., Miyakawa, K., Morita, T., Yamaoka, Y. & Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 533, 195–200 (2020).
Stagi, L. et al. Effective SARS-CoV-2 antiviral activity of hyperbranched polylysine nanopolymers. Nanoscale 13, 16465–16476 (2021).
Zhao, Z. et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces 13, 20995–21006 (2021).
Tan, Q. et al. Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19. J. Nanobiotechnol. 19, 173 (2021).
Ma, X. et al. HACE2-exosome-based nano-bait for concurrent SARS-CoV-2 trapping and antioxidant therapy. ACS Appl. Mater. Interfaces 14, 4882–4891 (2022).
Wang, C. et al. Membrane nanoparticles derived from ACE2-rich cells block SARS-CoV-2 infection. ACS Nano 15, 6340–6351 (2021).
Li, Z. et al. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat. Nanotechnol. 16, 942–951 (2021).
Peddapalli, A. et al. Demystifying excess immune response in COVID-19 to reposition an orphan drug for down-regulation of NF-κB: a systematic review. Viruses 13, 378 (2021).
Eedara, B. B. et al. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics 13, 1077 (2021).
Miller, M. R. et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11, 4542–4552 (2017).
Lee, Y. Y. et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials 267, 120389 (2021).
Park, H. H. et al. Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv. Sci. 7, 2001940 (2020).
Alsabani, M. et al. Reduction of NETosis by targeting CXCR1/2 reduces thrombosis, lung injury, and mortality in experimental human and murine sepsis. Br. J. Anaesth. 128, 283–293 (2022).
Beristain-Covarrubias, N. et al. Understanding infection-induced thrombosis: lessons learned from animal models. Front. Immunol. 10, 2569 (2019).
Shou, S. et al. Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front. Microbiol. 12, 626553 (2021).
Shi, J. et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–1020 (2020).
Cicha, I. et al. From design to the clinic: practical guidelines for translating cardiovascular nanomedicine. Cardiovasc. Res. 114, 1714–1727 (2018).
Bai, S. et al. Multimodal and multifunctional nanoparticles with platelet targeting ability and phase transition efficiency for the molecular imaging and thrombolysis of coronary microthrombi. Biomater. Sci. 8, 5047–5060 (2020).
Huang, Y. et al. An activated-platelet-sensitive nanocarrier enables targeted delivery of tissue plasminogen activator for effective thrombolytic therapy. J. Control. Release 300, 1–12 (2019).
Sánchez-Cortés, J. & Mrksich, M. The platelet integrin alphaIIbbeta3 binds to the RGD and AGD motifs in fibrinogen. Chem. Biol. 16, 990–1000 (2009).
Absar, S., Nahar, K., Kwon, Y. M. & Ahsan, F. Thrombus-targeted nanocarrier attenuates bleeding complications associated with conventional thrombolytic therapy. Pharm. Res. 30, 1663–1676 (2013).
Zhong, Y. et al. Low-intensity focused ultrasound-responsive phase-transitional nanoparticles for thrombolysis without vascular damage: a synergistic nonpharmaceutical strategy. ACS Nano 13, 3387–3403 (2019).
Chen, H.-A., Ma, Y.-H., Hsu, T.-Y. & Chen, J.-P. Preparation of peptide and recombinant tissue plasminogen activator conjugated poly(lactic-co-glycolic acid) (PLGA) magnetic nanoparticles for dual targeted thrombolytic therapy. Int. J. Mol. Sci. 21, 2690 (2020).
Singh, M. P. et al. Reprogramming the rapid clearance of thrombolytics by nanoparticle encapsulation and anchoring to circulating red blood cells. J. Control. Release 329, 148–161 (2021).
Kawata, H. et al. A new drug delivery system for intravenous coronary thrombolysis with thrombus targeting and stealth activity recoverable by ultrasound. J. Am. Coll. Cardiol. 60, 2550–2557 (2012).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Veras, F. P. et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 217, e20201129 (2020).
Arcanjo, A. et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci. Rep. 10, 19630 (2020).
Kaiser, R. et al. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight 6, e150862 (2021).
Wu, M. et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc. Natl Acad. Sci. USA 117, 28336–28343 (2020).
Liao, M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
Zuo, Y. et al. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. JCI Insight 6, e150111 (2021).
Englert, H. et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. eBioMedicine 67, 103382 (2021).
Semeraro, F. et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 118, 1952–1961 (2011).
Girard, P. et al. Deep venous thrombosis in patients with acute pulmonary embolism: prevalence, risk factors, and clinical significance. Chest 128, 1593–1600 (2005).
Helms, J. et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 46, 1089–1098 (2020).
Chernysh, I. N. et al. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci. Rep. 10, 5112 (2020).
Guan, W.-j et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
Acknowledgements
P.R. thanks the Australian Institute of Nuclear Science and Engineering (AINSE) for a postgraduate research award. L.E. thanks AINSE for an early-career research grant and the CASS Foundation for a science/medicine grant (10021). C.E.H. thanks the National Health and Medical Research Council (NHMRC) of Australia for a Senior Research Fellowship (award number GNT1154270).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Wei Tao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Russell, P., Esser, L., Hagemeyer, C.E. et al. The potential impact of nanomedicine on COVID-19-induced thrombosis. Nat. Nanotechnol. 18, 11–22 (2023). https://doi.org/10.1038/s41565-022-01270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01270-6
This article is cited by
-
A review of metallic nanostructures against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Toxicology and Environmental Health Sciences (2023)