Abstract
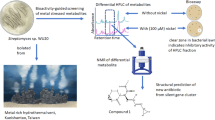
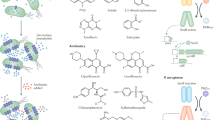
Environmental bacteria, such as Streptomyces spp., produce specialized metabolites that are potent antibiotics and therapeutics. Selected specialized antimicrobials are co-produced and function together synergistically. Co-produced antimicrobials comprise multiple chemical classes and are produced by a wide variety of bacteria in different environmental niches, suggesting that their combined functions are ecologically important. Here, we highlight the exquisite mechanisms that underlie the simultaneous production and functional synergy of 16 sets of co-produced antimicrobials. To date, antibiotic and antifungal discovery has focused mainly on single molecules, but we propose that methods to target co-produced antimicrobials could widen the scope and applications of discovery programs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wright, G. D. Opportunities for natural products in 21st century antibiotic discovery. Nat. Prod. Rep. 34, 694–701 (2017).
Davies, J. & Ryan, K. S. Introducing the parvome: bioactive compounds in the microbial world. ACS Chem. Biol. 7, 252–259 (2012).
van der Meij, A., Worsley, S. F., Hutchings, M. I. & van Wezel, G. P. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 41, 392–416 (2017).
Ho, L. K. & Nodwell, J. R. David and Goliath: chemical perturbation of eukaryotes by bacteria. J. Ind. Microbiol. Biotechnol. 43, 233–248 (2016).
Challis, G. L. & Hopwood, D. A. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl Acad. Sci. USA 100, 14555–14561 (2003).
Krug, D. et al. Discovering the hidden secondary metabolome of Myxococcus xanthus: a study of intraspecific diversity. Appl. Environ. Microbiol. 74, 3058–3068 (2008).
Maansson, M. et al. An integrated metabolomic and genomic mining workflow to uncover the biosynthetic potential of bacteria. Msystems https://doi.org/10.1128/mSystems.00028-15 (2016).
Duncan, K. R. et al. Molecular networking and pattern-based genome mining improves discovery of biosynthetic gene clusters and their products from Salinispora species. Chem. Biol. 22, 460–471 (2015).
Cimermancic, P. et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 158, 412–421 (2014).
Baltz, R. H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 44, 573–588 (2017).
Sharrar, A. M. et al. Bacterial secondary metabolite biosynthetic potential in soil varies with phylum, depth, and vegetation type. Mbio 11, e00416-20 (2020).
Osbourn, A. Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet. 26, 449–457 (2010).
Fischbach, M. A., Walsh, C. T. & Clardy, J. The evolution of gene collectives: how natural selection drives chemical innovation. Proc. Natl Acad. Sci. USA 105, 4601–4608 (2008).
Firn, R. D. & Jones, C. G. The evolution of secondary metabolism—a unifying model. Mol. Microbiol. 37, 989–994 (2000).
Charney, J., Fisher, W. P., Curran, C., Machlowitz, R. A. & Tytell, A. A. Streptogramin, a new antibiotic. Antibiot. Chemother. 3, 1283–1286 (1953).
De Somer, P. & Van Dijck, P. A preliminary report on antibiotic number 899, a streptogramin-like substance. Antibiot. Chemother. 5, 632–639 (1955).
Mast, Y. & Wohlleben, W. Streptogramins—two are better than one! Int. J. Med. Microbiol. 304, 44–50 (2014).
Cocito, C. Antibiotics of the virginiamycin family, inhibitors which contain synergistic components. Microbiol Rev. 43, 145–192 (1979).
Reissier, S. & Cattoir, V. Streptogramins for the treatment of infections caused by Gram-positive pathogens. Expert Rev. Anti Infect. Ther. 19, 587–599 (2021).
Harms, J. M., Schlünzen, F., Fucini, P., Bartels, H. & Yonath, A. Alterations at the peptidyl transferase centre of the ribosome induced by the synergistic action of the streptogramins dalfopristin and quinupristin. BMC Biol. 2, 4 (2004).
Mast, Y. et al. Characterization of the ‘pristinamycin supercluster’ of Streptomyces pristinaespiralis. Microb. Biotechnol. 4, 192–206 (2011).
Pulsawat, N., Kitani, S. & Nihira, T. Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae. Gene 393, 31–42 (2007).
Kawachi, R. et al. Identification of an AfsA homologue (BarX) from Streptomyces virginiae as a pleiotropic regulator controlling autoregulator biosynthesis, virginiamycin biosynthesis and virginiamycin M1 resistance. Mol. Microbiol. 36, 302–313 (2000).
Mochizuki, S. et al. The large linear plasmid pSLA2-L of Streptomyces rochei has an unusually condensed gene organization for secondary metabolism. Mol. Microbiol. 48, 1501–1510 (2003).
Auerbach, T. et al. The structure of ribosome-lankacidin complex reveals ribosomal sites for synergistic antibiotics. Proc. Natl Acad. Sci. USA 107, 1983–1988 (2010).
Belousoff, M. J. et al. Crystal structure of the synergistic antibiotic pair, lankamycin and lankacidin, in complex with the large ribosomal subunit. Proc. Natl Acad. Sci. USA 108, 2717–2722 (2011).
Yamamoto, S., He, Y., Arakawa, K. & Kinashi, H. Gamma-butyrolactone-dependent expression of the Streptomyces antibiotic regulatory protein gene srrY plays a central role in the regulatory cascade leading to lankacidin and lankamycin production in Streptomyces rochei. J. Bacteriol. 190, 1308–1316 (2008).
Mrak, P. et al. Discovery of the actinoplanic acid pathway in Streptomyces rapamycinicus reveals a genetically conserved synergism with rapamycin. J. Biol. Chem. 293, 19982–19995 (2018).
McLean, T. C., Wilkinson, B., Hutchings, M. I. & Devine, R. Dissolution of the disparate: co-ordinate regulation in antibiotic biosynthesis. Antibiotics https://doi.org/10.3390/antibiotics8020083 (2019).
Daniel-Ivad, M., Pimentel-Elardo, S. & Nodwell, J. R. Control of specialized metabolism by signaling and transcriptional regulation: opportunities for new platforms for drug discovery? Annu. Rev. Microbiol. 72, 25–48 (2018).
Imada, A., Kintaka, K., Nakao, M. & Shinagawa, S. Bulgecin, a bacterial metabolite which in concert with beta-lactam antibiotics causes bulge formation. J. Antibiot. 35, 1400–1403 (1982).
Templin, M. F., Edwards, D. H. & Höltje, J. V. A murein hydrolase is the specific target of bulgecin in Escherichia coli. J. Biol. Chem. 267, 20039–20043 (1992).
Tomoshige, S. et al. Total syntheses of bulgecins A, B, and C and their bactericidal potentiation of the β-lactam antibiotics. ACS Infect. Dis. 4, 860–867 (2018).
Horsman, M. E. et al. Whole-genome shotgun sequencing of two β-proteobacterial species in search of the bulgecin biosynthetic cluster. ACS Chem. Biol. 12, 2552–2557 (2017).
Wu, Y. & Seyedsayamdost, M. R. Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem. Biol. 24, 1437–1444 (2017).
Minato, Y. et al. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 9, 1003 (2018).
Mandler, M. D. et al. Novobiocin enhances polymyxin activity by stimulating lipopolysaccharide transport. J. Am. Chem. Soc. 140, 6749–6753 (2018).
Dixon, S. J. et al. Significant conservation of synthetic lethal genetic interaction networks between distantly related eukaryotes. Proc. Natl Acad. Sci. USA 105, 16653–16658 (2008).
Mori, H. et al. Identification of essential genes and synthetic lethal gene combinations in Escherichia coli K-12. Methods Mol. Biol. 1279, 45–65 (2015).
Wiedemann, I. et al. The mode of action of the lantibiotic lacticin 3147—a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61, 285–296 (2006).
Arnison, P. G. et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 (2013).
Martin, N. I. et al. Structural characterization of lacticin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry 43, 3049–3056 (2004).
Booth, M. C. et al. Structural analysis and proteolytic activation of Enterococcus faecalis cytolysin, a novel lantibiotic. Mol. Microbiol. 21, 1175–1184 (1996).
Oman, T. J. et al. Haloduracin α binds the peptidoglycan precursor lipid II with 2:1 stoichiometry. J. Am. Chem. Soc. 133, 17544–17547 (2011).
Rea, M. C. et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl Acad. Sci. USA 107, 9352–9357 (2010).
Mathur, H. et al. Insights into the mode of action of the sactibiotic thuricin CD. Front. Microbiol. 8, 696 (2017).
Nissen-Meyer, J., Oppegård, C., Rogne, P., Haugen, H. S. & Kristiansen, P. E. Structure and mode-of-action of the two-peptide (class-IIb) bacteriocins. Probiotics Antimicrob. Proteins 2, 52–60 (2010).
McCafferty, D. G., Cudic, P., Yu, M. K., Behenna, D. C. & Kruger, R. Synergy and duality in peptide antibiotic mechanisms. Curr. Opin. Chem. Biol. 3, 672–680 (1999).
Garneau, S., Martin, N. I. & Vederas, J. C. Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84, 577–592 (2002).
Cai, W., Matthew, S., Chen, Q. Y., Paul, V. J. & Luesch, H. Discovery of new A- and B-type laxaphycins with synergistic anticancer activity. Bioorg. Med. Chem. 26, 2310–2319 (2018).
Frankmölle, W. P. et al. Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. I. Isolation and biological properties. J. Antibiot. 45, 1451–1457 (1992).
Heinilä, L. M. P. et al. Shared PKS module in biosynthesis of synergistic laxaphycins. Front. Microbiol. 11, 578878 (2020).
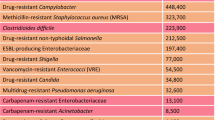
Surette, M. D. & Wright, G. D. Lessons from the environmental antibiotic resistome. Annu. Rev. Microbiol. 71, 309–329 (2017).
Reading, C. & Cole, M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 11, 852–857 (1977).
Jensen, S. E. & Paradkar, A. S. Biosynthesis and molecular genetics of clavulanic acid. Antonie Van Leeuwenhoek 75, 125–133 (1999).
Doroghazi, J. R. & Buckley, D. H. Intraspecies comparison of Streptomyces pratensis genomes reveals high levels of recombination and gene conservation between strains of disparate geographic origin. BMC Genomics 15, 970 (2014).
Jensen, S. E. Biosynthesis of clavam metabolites. J. Ind. Microbiol. Biotechnol. 39, 1407–1419 (2012).
Maeda, K. et al. Isolation and structure of a beta-lactamase inhibitor from Streptomyces. J. Antibiot. 30, 770–772 (1977).
Hood, J. D., Box, S. J. & Verrall, M. S. Olivanic acids, a family of beta-lactam antibiotics with beta-lactamase inhibitory properties produced by Streptomyces species. II. Isolation and characterisation of the olivanic acids MM 4550, MM 13902 and MM 17880 from Streptomyces olivaceus. J. Antibiot. 32, 295–304 (1979).
Li, R., Lloyd, E. P., Moshos, K. A. & Townsend, C. A. Identification and characterization of the carbapenem MM 4550 and its gene cluster in Streptomyces argenteolus ATCC 11009. ChemBioChem 15, 320–331 (2014).
Wu, P. et al. An unusual protector-protégé strategy for the biosynthesis of purine nucleoside antibiotics. Cell Chem. Biol. 24, 171–181 (2017).
Xia, Y. et al. Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem. Biol. 24, 1479–1489 (2017).
Wencewicz, T. A. Crossroads of antibiotic resistance and biosynthesis. J. Mol. Biol. 431, 3370–3399 (2019).
Park, J. et al. Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat. Chem. Biol. 13, 730–736 (2017).
Arp, J. et al. Synergistic activity of cosecreted natural products from amoebae-associated bacteria. Proc. Natl Acad. Sci. USA 115, 3758–3763 (2018).
Shishido, T. K. et al. Antifungal activity improved by coproduction of cyclodextrins and anabaenolysins in Cyanobacteria. Proc. Natl Acad. Sci. USA 112, 13669–13674 (2015).
MacNair, C. R. et al. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 9, 458 (2018).
Lee, M. D. et al. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Farmaco 56, 81–85 (2001).
Yarlagadda, V., Medina, R. & Wright, G. D. Venturicidin A, a membrane-active natural product inhibitor of ATP synthase potentiates aminoglycoside antibiotics. Sci. Rep. 10, 8134 (2020).
Yin, Y., Zhang, H., Olman, V. & Xu, Y. Genomic arrangement of bacterial operons is constrained by biological pathways encoded in the genome. Proc. Natl Acad. Sci. USA 107, 6310–6315 (2010).
Pang, T. Y. & Lercher, M. J. Supra-operonic clusters of functionally related genes (SOCs) are a source of horizontal gene co-transfers. Sci. Rep. 7, 40294 (2017).
Alanjary, M. & Medema, M. H. Mining bacterial genomes to reveal secret synergy. J. Biol. Chem. 293, 19996–19997 (2018).
Blin, K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87 (2019).
Fischbach, M. A. & Clardy, J. One pathway, many products. Nat. Chem. Biol. 3, 353–355 (2007).
Leão, P. N. et al. Synergistic allelochemicals from a freshwater cyanobacterium. Proc. Natl Acad. Sci. USA 107, 11183–11188 (2010).
Guo, X., Liu, X., Pan, J. & Yang, H. Synergistic algicidal effect and mechanism of two diketopiperazines produced by Chryseobacterium sp. strain GLY-1106 on the harmful bloom-forming Microcystis aeruginosa. Sci. Rep. 5, 14720 (2015).
Walker, M. C. et al. Precursor peptide-targeted mining of more than one hundred thousand genomes expands the lanthipeptide natural product family. BMC Genomics 21, 387 (2020).
Zhao, X. & van der Donk, W. A. Structural characterization and bioactivity analysis of the two-component lantibiotic Flv system from a ruminant bacterium. Cell Chem. Biol. 23, 246–256 (2016).
Gu, W., Sardar, D., Pierce, E. & Schmidt, E. W. Roads to rome: role of multiple cassettes in cyanobactin RiPP biosynthesis. J. Am. Chem. Soc. 140, 16213–16221 (2018).
Martins, J. & Vasconcelos, V. Cyanobactins from cyanobacteria: current genetic and chemical state of knowledge. Mar. Drugs 13, 6910–6946 (2015).
Tan, S., Moore, G. & Nodwell, J. Put a bow on it: knotted antibiotics take center stage. Antibiotics https://doi.org/10.3390/antibiotics8030117 (2019).
Tyers, M. & Wright, G. D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 17, 141–155 (2019).
Guideline on Clinical Development of Fixed Combination Medicinal Products EMA/CHMP/158268/2017 (European Medicines Agency, 2017).
Guidance for Industry. Codevelopment of Two or More New Investigational Drugs for Use in Combination FDA-2010-D-0616 (Food and Drug Administration, 2013).
Drawz, S. M. & Bonomo, R. A. Three decades of beta-lactamase inhibitors. Clin. Microbiol Rev. 23, 160–201 (2010).
Caesar, L. K. & Cech, N. B. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep. 36, 869–888 (2019).
Wang, M. et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 34, 828–837 (2016).
Zhang, J. J., Tang, X. & Moore, B. S. Genetic platforms for heterologous expression of microbial natural products. Nat. Prod. Rep. 36, 1313–1332 (2019).
Diaz, J. E. et al. The transcriptomic response of cells to a drug combination is more than the sum of the responses to the monotherapies. Elife https://doi.org/10.7554/eLife.52707 (2020).
Rokas, A., Wisecaver, J. H. & Lind, A. L. The birth, evolution and death of metabolic gene clusters in fungi. Nat. Rev. Microbiol. 16, 731–744 (2018).
Acknowledgements
We thank S. Tan, M. Lefebvre and S. Pimentel-Elardo for comments on the manuscript. Figures were created with Biorender.com. This work was supported by the Canadian Institutes of Health Research (MID-406688, to J.R.N.).
Author information
Authors and Affiliations
Contributions
K.J.M. and J.R.N. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Gregory Challis, Linda Kinkel, Marnix Medema and Gerry Wright for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meyer, K.J., Nodwell, J.R. Biology and applications of co-produced, synergistic antimicrobials from environmental bacteria. Nat Microbiol 6, 1118–1128 (2021). https://doi.org/10.1038/s41564-021-00952-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-021-00952-6
This article is cited by
-
Going to extremes: progress in exploring new environments for novel antibiotics
npj Antimicrobials and Resistance (2024)