Abstract
The discoveries of penicillin and streptomycin were pivotal for infection control with the knowledge subsequently being used to enable the discovery of many other antibiotics currently used in clinical practice. These valuable compounds are generally derived from mesophilic soil microorganisms, predominantly Streptomyces species. Unfortunately, problems with the replication of results suggested that this discovery strategy was no longer viable, motivating a switch to combinatorial chemistry in conjunction with existing screening programmes to derive new antimicrobials. However, the chemical space occupied by these synthetic products is vastly reduced compared to those of natural products. More recent approaches such as using artificial intelligence to ‘design’ synthetic ligands to dock with molecular targets suggest that chemical synthesis is still a promising option for discovery. It is important to employ diverse discovery strategies to combat the worrying increase in antimicrobial resistance (AMR). Here, we reconsider whether nature can supply innovative solutions to recalcitrant infections. Specifically, we assess progress in identifying novel antibiotic-producing organisms from extreme and unusual environments. Many of these organisms have adapted physiologies which often means they produce different repertoires of bioactive metabolites compared to their mesophilic counterparts, including antibiotics. In addition, we examine insights into the regulation of extremotolerant bacterial physiologies that can be harnessed to increase the production of clinically important antibiotics and stimulate the synthesis of new antibiotics in mesophilic microorganisms. Finally, we comment on the insights provided by combinatorial approaches to the treatment of infectious diseases that might enhance the efficacy of antibiotics and reduce the development of AMR.
Similar content being viewed by others
Introduction
Physicians in the early 20th century employed various chemical agents such as mercury salts and sulpha drugs to treat pathogenic microbial infections, however, many of these were also associated with problems of toxicity or poor efficacy1. A fortuitous breakthrough came in 1928 with the discovery of penicillin by Dr Alexander Fleming, and subsequently streptomycin from a Gram-positive Streptomyces bacterium by Schatz and Waksman in 19432,3. These antibiotics are produced by a filamentous fungus and a Gram-positive Streptomyces bacterium, respectively. Selman Waksman coined the term antibiotic to describe compounds of biological origin that can kill or inhibit the growth of microorganisms4. Although this definition has changed slightly in the intervening period, antibiotics have revolutionised clinical care and gone on to be one of the greatest medical success stories of the 20th century. This progress was significantly aided by governments and a collective of pharmaceutical companies who ensured the mass production and distribution of these life-saving drugs in those early years which were commonly referred to as the beginning of the ‘golden era of antibiotic discovery’1.
Inspired by Waksman’s pioneering research, scientists went on to discover and isolate many antibiotic-producing soil microorganisms. Although these antibiotics now comprise approximately 70% of our current frontline antibiotics, this discovery process trickled to a halt in the 1970s when scientists realised that they were (re)discovering the same compounds over and over again in what was described as a phenomenon of replication5. The pharmaceutical industry tried to compensate for this gap in the discovery pipeline by using combinatorial chemistry, however, even though millions of compounds were tested in these screening programmes, very few progressed to further developmental stages due to problems with toxicity, stability, and overall efficacy6. In comparison, the success rate for the development of drugs from natural products is about 0.6%, which is approximately 100 times greater than that of synthetic compounds7. This is in part due to the realisation that novel synthetic molecules occupy a much smaller chemical space in terms of their underlying chemical structures in comparison to natural products, and has motivated other efforts focused on combinatorial biosynthesis8. Essentially this process offers the prospect of redesigning known antibiotic structures to create new activities based on knowledge of biosynthetic pathways. This approach has been partly hindered by low yields, but it was also acknowledged that as the resulting compounds share a core chemical scaffold with their natural counterparts, resistance would arise quickly. Consequently, with a low return on the investment for pharmaceutical companies associated with these programmes, there has been a scaling down of antibiotic R&D investment by the pharmaceutical industry9, unfortunately coinciding with increasing problems with AMR.
More recently, artificial intelligence has been exploited, in place of traditional screening of chemical libraries, to discover new compounds that inhibit bacterial growth10. This is an encouraging advance, as it underlines the importance of accessing chemical diversity, be it sourced via synthesis or through natural products. Still, the adoption of next-generation genome sequencing with genome mining, combined with a wider exploration of natural habitats and a deeper understanding of the biology of antibiotic-producing microorganisms, suggests that only a small fraction of natural products with potential antibiotic activity have been examined to date. Indeed, mathematical modelling of the potential for antibiotic biosynthesis by the genus Streptomyces has predicted that they could produce up to 100,000 different antibiotics, of which only a small fraction have been discovered to date11. This review focuses on the exploration of extreme and unusual habitats for new antibiotic-producing organisms, with emphasis on actinobacteria and, in particular, the most prolific producers that belong to the genus Streptomyces. Rather than provide an exhaustive view of all antimicrobials sourced from these extreme and unusual environments, we provide an overview and reference key detailed reports where further information can be gleaned.
The biology of antibiotic-producing microorganisms
Two of the main producers of antibiotics, filamentous fungi and actinobacteria, typically share similar life-styles, inhabiting soil and/or marine sediments. They are often non-motile, grow as branched mycelium, adopt a saprophytic lifestyle and many produce reproductive spores. However, much of what we know concerning the biology and synthesis of antibiotics has been derived from a rather limited study of cultures of individual microbial species grown under laboratory conditions rather than examining in situ the physiology of antibiotic synthesis.
Antibiotics are the products of secondary metabolism whereby microorganisms can repurpose their metabolites from primary metabolism to generate often quite complex organic compounds using a dedicated set of enzymes for each secondary metabolite they produce. The specific enzymes required for antibiotic biosynthesis of any given secondary metabolite are encoded on a set of genes organised in a biosynthetic gene cluster (BGC), permitting coordination of their expression. Studies in Streptomyces indicate that this antibiotic production often coincides with reproductive growth when elements of the mycelium are recycled to fuel the growth of spore-bearing aerial hyphae12. In this context, antibiotics may provide a defence against predation by other competing organisms. However, there is some debate about the ecological role of these compounds and whether sufficient amounts (of antibiotics) are produced in natural environments to function in antibiosis. This may be due to the fact that antibiotics can have many physiological roles including acting as potent signalling molecules at lower concentrations13. Further, each antibiotic-producing organism can simultaneously express several secondary metabolites which may act in a synergistic manner14. Genome sequencing has also revealed that, in addition to the BGCs that are constitutively expressed and whose products can be determined, there are also many BGCs that are ‘cryptic’ or ‘silent’, for which no end-product can be determined15. This may be a consequence of growing pure cultures in lab conditions, which cannot fully recreate the complexities of the natural environment. In addition, it should be noted that genome annotation algorithms such as antiSMASH16, which are used for the identification of BGCs in a genome sequence, are trained in reference to known BGCs. One of the consequences of this is that entirely novel BGCs, with the potential to direct the synthesis of a new class of antibiotic, could theoretically be overlooked.
One increasing area of study, albeit as yet limited in breadth, is investigating how antibiotic-producing microbes behave under in situ conditions—a critical issue with respect to understanding the cues that may trigger antibiotic biosynthesis, particularly by cryptic pathways. For example, terrestrial antibiotic-producing microorganisms such as Streptomyces spp. are frequently associated with the soil rhizosphere and can provide growth advantages to plants in return for physiologically favourable growth conditions17. Indeed, elegant studies have shown how leaf-cutter ants exploit biosynthesis of antifungal compounds using symbiotic actinobacteria to protect their ‘gardens’ of a cellulose-digesting fungus from competing fungi18. Evidently, these types of interactions vary considerably according to the local fauna and flora specific to any given habitat, particularly in the case of extreme environments. Together with the challenges posed by adaptations to physiologically extreme or unusual environments, the specific conditions encountered by extremophilic, extremotolerant or other microorganisms have likely provided an evolutionary pressure to diversify their antibiotic production capacity. Based on this rationale and with the global AMR crisis as a key driver, bioprospecting for new antibiotics sourced from previously untapped extreme and unusual environments is of vital importance.
For those organisms isolated from inhospitable habitats, the types of microorganisms to consider are those associated with long periods of extreme temperatures, such as extremes of heat (thermophiles/thermotolerant) or close to freezing (psychrophiles/psychrotolerant); habitats with extreme pressures, like high plateaus or deep ocean trenches (barophiles/barotolarant); habitats with extreme osmotic pressure, such as salt plains (halophiles/halotolerant); habitats with extreme pH, such as acid (acidophiles/acidotolerant) or alkali (alkaliphiles/alkalitolerant); and habitats with extreme arid conditions (xerophiles/xerotolerant), such as the desert. More than one of these conditions may persist in many habitats, and consequently, the succeeding sections in this review are at best arbitrary. The related scientific literature frequently alludes to antibiotics produced by extremophiles; however, we note that for many examples these isolated organisms are better described as having extremotolerant characteristics.
It should also be noted that in many cases, especially with respect to the streptomycetes, bacterial systematics has depended heavily on 16s rDNA sequence comparisons and, prior to this, chemical taxonomy. Access to next-generation sequencing has now enabled multi-locus sequencing or whole genome comparisons, providing a more reliable means for the speciation of members of the genus, albeit applied so far to only a small proportion of isolated species.
Antibiotics from thermotolerant producers
Desert ecosystems have attracted considerable attention from microbiologists due to the discovery of microorganisms that live in seemingly uninhabitable temperatures which at their peak can reach as high as 56.7 °C (July 10, 1913, Death Valley, California) in the daytime and −3.9 °C in the night-time. Microorganisms are able to survive in such inhospitable conditions by physiological adaptations and/or associations with other organisms. For example, many deserts and drylands have a biological soil crust on top of the sand which is formed primarily by the adhesion of soil particles to extracellular polysaccharides excreted mainly by cyanobacteria19. These biofilm mats also include a complex microbial consortium consisting of fungi, algae and occasionally lichens and are vital for creating and maintaining the fertility of this layer by fixing both carbon and nitrogen19. It is in these environments that researchers have identified many extremophiles20. One important area for these discoveries is the Atacama desert in Chile21. This is a large desert plateau on the Pacific coast of Chile which covers approximately 100,000 km2 and whose core region has been described as too extreme for life22. Researchers have identified at least 50 novel natural products from this source alone, many with antibiotic activity20. These bacteria can also be considered to be xerotolerant and/or halotolerant, that is, organisms that can survive in very arid conditions or those with high salt stress23. Other important sources of antibiotic-producing desert extremophiles have been reported for the Sahara desert in North Africa (9.2 million km²), the Taklamakan Desert in China (337,000 km2) and the Thar desert in India and Pakistan (200,000 km2)23 (Table 1).
Antibiotics from psychrotolerant producers
Antibiotics have also been discovered in psychrotolerant microorganisms. These organisms are able to survive freezing temperatures and repeated heat-thaw cycles and are generally found in cold environments such as polar regions, cold deserts, glaciers, deep oceans and vast areas of permafrost. Although these microorganisms may be better known for having cryoprotective compounds, they have also produced novel antibiotics24,25,26,27. Many of these discoveries were made in dedicated research facilities such as those in the Arctic region25, the Barents sea27, Svalbard in the Norwegian archipelago24 and the Antarctica28 (Table 2). A good summary of many of these discoveries from Antarctic is provided in a review by Núñez-Montero et al.28.
Antibiotics from aquatic environments
Considerable attention has also been devoted to aquatic environments in the search for new antibiotics29. These ecosystems are easily some of the largest in the world and are the source of many antibiotic-producing organisms including halophiles, barophiles, and thermophiles. A recent review of the progress in this area between 1984 and 2022, indicated that 182 natural products were derived from predominantly filamentous fungi and Streptomyces species growing in these extreme conditions; half of these being novel compounds with antibiotic activity30. We have compiled a list of some of these antibiotics in Table 3. For Actinobacteria, 70% of antimicrobial compounds were sourced from bacteria isolated from marine sediment and 24% were isolated from bacteria associated with marine flora and fauna31. Amongst this later group, algae, sponges, corals and mangroves were the most common environments to isolate antibiotic-producing fungi and actinobacteria32 (Table 4). One feature of antibiotics sourced from the marine environment is that they generally possess a greater abundance of halogen-containing compounds in comparison to those obtained from terrestrial organisms, reflecting the halogenated nature of seawater.
There are also many discoveries of antibiotic-producing organisms originating from cold seeps and hydrothermal vents. Cold seeps typically occur over fissures in the ocean floor in environments that are usually the result of tectonic activity. These areas are typically characterised by the seepage of either methane or other hydrocarbons possibly with the addition of hydrogen sulfide. It is this chemically rich environment that sustains the cold-seep ecosystem which is dominated by chemosynthetic primary producers33. Contrary to their name, these environments are not necessarily colder than the surrounding water, just cold relative to the hydrothermal vents in their vicinity.
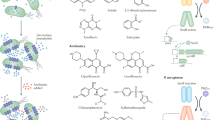
Hydrothermal vents are also caused by fissures on the sea floor but in this case, superheated water is extruded into the surrounding cold water where dissolved minerals such as iron, copper and zinc are precipitated quickly; many of these precipitates form chimneys which can be colonised by chemosynthetic bacteria34. As an example of bioprospecting in such an environment researchers were able to induce the expression of a novel (structurally different) antibiotic produced by Streptomyces sp. WU20 follows a strategy based on metal induction of silent biosynthetic gene clusters combined with metabolomic analytical methods. This streptomycete was isolated from a metal-rich hydrothermal vent off the coast of Kueishantao island, which is situated off the coast of Taiwan35 (Fig. 1).
WU20 isolated from a metal-rich hydrothermal vent near Taiwan. Bioactive metabolite screening of silent gene induction and analytical metabolomics revealed the structure of a new antibiotic (compound 1). Illustrations of the differential HPLC and the structure of the new antibiotic are adapted from Shi et al.35. NMR illustration adapted from “NMR gyrotron” and bioassay illustration adapted from “petri dish antibiotic sensitivity test” both from BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates. Streptomyces WU20 and hydrothermal vent illustrations by Val Romani.
Antibiotics from halophiles
Halophiles or halotolerant microorganisms can grow in a range of salt concentrations, including hypersaline habitats such as the Dead Sea, or the salt flats of the Salar de Atacama36. Describing these halophilic/halotolerant antibiotic producers as a separate entity is confounded to a large extent by many of these organisms being associated either with deserts or with the marine environment. Some of the most notable halophilic microorganisms that produce antibiotics can be found in the domain Archaea. For example, the archaeocins are ribosomally synthesised bacteriocin-like antimicrobial peptides (AMPs) with antagonistic activity against other microorganisms37. Archaeocins fall into two phylogenetic groups: halocins produced by haloarchaea and sulfolobicins produced by the genus Sulfolobus. Two novel antibiotic compounds, kribbellichelin A and B, have been isolated from a halophilic actinobacterial Kribbella species associated with the rhizosphere of Limonium majus which grows in a saline wetland in Spain38.
Antibiotics from unculturable or previously uncultured bacteria
It has been postulated by several scientists that unculturable or uncultivated microorganisms might provide one of the largest sources of new antibiotics, given that more than 99% of an estimated 1011–1012 microbial species remain undiscovered39. One of the solutions to growing unculturable microorganisms may be to conduct the process in situ. This method is exemplified by the iChip40 which is seen as a great step forward because it allows for the in situ cultivation of cells in the environment by allowing diffusion of localised nutrients into a chamber41. There are also other methods that are currently utilised for uncultivated organisms such as microbial traps or double isolation methods39. The use of the iChip led to the discovery of teixobactin, derived from the bacteria Eleftheria terrae, after screening 10,000 isolates. Teixobactin has inhibitory activity against Gram-positive bacteria and mycobacteria but not Gram-negative organisms. Its mode of action is the inhibition of peptidoglycan and teichoic acid synthesis by inhibiting lipids42. Another antibiotic isolated using the iChip is clovibactin. This also blocks cell wall synthesis by targeting multiple peptidoglycan precursors43. Further antibiotic discovery using this method included a macrolide known as amicobactin, which inhibits Mycobacterium tuberculosis, and hypeptin (produced by Lysobacter sp. K5869) which is similar to teixobactin and displays a broad range of activity against Gram-positive bacteria40. Bacteria which are described as “just difficult to isolate” have also been seen as a good source of novel compounds. This is exemplified by the research of Rolf Müller’s group44 resulting in the discovery a new antibiotic, rowithocin, from Sorangium cellulosum which has an uncommon phosphorylated polyketide scaffold45. Applying innovative culturing and isolation techniques for extremophilic/extremotolerant microorganisms offers the potential for the discovery of many new antibiotics.
Antibiotics from traditional and historic medicines
There is growing evidence that the therapeutic basis of some traditional and historic medicines is based on a relationship with antibiotic-producing organisms46,47,48. For example, traces of tetracycline have been found in human skeletal remains of ancient Sudanese Nubia dating back to 350–550 CE47,48. This can only be explained if these ancient people were exposed to tetracycline-containing materials in their diets, consistent with a low rate of infectious disease documented in the population48. In other research, a milky white exudate covering the rock surfaces of some caves which were referred to in ancient texts as ‘moonmilk’ was used to heal multiple ailments49. This moonmilk contains an abundance of Streptomyces that have antibacterial activity against a wide range of bacteria and fungi49,50. Researchers have isolated Streptomyces sp. HZP-2216E from a traditional Chinese medicine that incorporates the sea lettuce Ulva pertusa which is associated with a bacteria that produces a unique indolizinium alkaloid, streptopertusacin A and two previously undescribed bafilomycins with antibiotic activity46.
Another common theme in the discovery of inhibitory compounds from traditional and historic medicines is their association with particular soils; however, these tend to have unique characteristics that differentiate them from other soil types such as higher pH or the presence of specific minerals51,52,53. These soils have been documented in Jordan where a red soil known to contain actinobacteria that produce actinomycin is traditionally used to treat skin infections51, in northern British Columbia in Canada, where generations of indigenous people have used Kisameet clay (glacial clay) that has potent activity against many important clinical pathogens53 and in Northern Ireland, where researchers isolated Streptomyces sp. myrophorea, from an ancient Irish folk cure which was based on soil from a post-glacial alkaline grassland soil which has in-vitro antibiotic activity against clinically important ESKAPE pathogens52. Indeed subsequent investigations of a similar soil from the immediate vicinity identified many more Streptomyces with antibacterial and antifungal activity54.
These shared associations of traditional and historic medicines with antibiotic-producing bacteria52,53 open the door to the possibility that many more discoveries could be made using a more systematic approach. However, this discussion would be incomplete, without at least crediting the pioneering work in this field by Geoffrey Cordell even though his focus was anticancer medicines from plants. In his research, Dr Cordell observed that the screening of historic and traditional medicines yielded a far greater positive rate than the random screening of plants55.
Combinatorial approaches to antimicrobial therapy
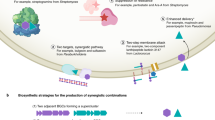
One increasingly relevant factor missing from discussions of traditional and historic medicines is that most of these are rarely purified compounds but instead a combination of ingredients56,57. In many instances, researchers are also uncertain which particular ingredients are strictly necessary to produce maximum efficacy56. So why is this relevant to treating pathogens resistant to conventional therapies or even delaying the onset of AMR? The principal antibiotic producers such as Streptomyces spp. typically produce a cocktail of secondary metabolites that can exhibit complementary antimicrobial activity. For instance, many antibiotic-producing organisms produce biosurfactants that can have synergy with antibiotics. One good example is the combination of sophorolipids (biosurfactant) with tetracycline, which increases the inhibitory activity against Staphylococcus aureus by 25%. Further trials with Gram-negative bacteria (E. coli) demonstrated that sophorolipids combined with cefalor increased inhibitory activity by 48% more than cefalor alone58. In some cases, the biosurfactants have weak antibacterial activity on their own, for instance, lipopeptides from Streptomyces rochei have antagonistic properties against Staphylococcus aureus and Pseudomonas aeruginosa59. The role of co-produced antibiotics has been reviewed in some detail by Meyer and Nodwell14 who noted that biosynthetic genes directing synthesis of complementary compounds are often located adjacent to each other on BGC superclusters. Of course, combining antibiotics is not a new clinical practice and has been used for many years in the treatment of tuberculosis for fear of resistance arising60. However as noted earlier, there are other compounds with no apparent antibiotic activity on their own that can have synergistic effects with antibiotics61. This is demonstrated in the case of a reducing agent, alkylresorcinol which in combination with antibiotics such as gentamicin, polymyxin, ampicillin and vancomycin inhibit various pathogenic bacteria62. Perhaps a more well-known example of these synergies is siderophores which are iron scavenging compounds commonly produced by actinomycetes. These can act as useful adjuvants to antibiotics. For example, desferrioxamine, produced by Streptomyces pilosus has synergistic activity with gentamicin, chloramphenicol, cefalothin, cefotiam and cefsulodin against pathogenic bacteria63. Siderophores are also found naturally in combination with antibiotics in the form of sideromycins64,65. These include albomycins, which are combination of a thioribosyl nucleoside linked to a ferrichrome-type siderophore and have antibacterial activity against Gram-positive and Gram-negative bacteria66 and ferrimycins which are iron-containing siderophores with activity against Gram-positive bacteria65,67.
Even secondary metabolites as seemingly innocuous as pigments can sometimes have some synergistic activity. This is demonstrated in research on a green pigment produced by the marine bacteria Streptomyces tunisiensis W4 which has a synergistic inhibitory activity when combined with cefuroxime and ciprofloxacin68. In addition, some pigments also have antibiotic activity on their own such as undecylprodigiosin, a red pigment produced by Streptomyces sp. JAR6 which has been noted for its antibiotic activity against Salmonella sp., Proteus mirabilis, Shigella sp. and Enterococcus sp,69.
Many of these synergies could potentially enhance the therapeutic activity of antibiotics allowing for a reduced dosage and potentially reducing the development of AMR61 (Fig. 2).
Antibiotic-producing organisms produce a wide array of secondary metabolites. In modern reductionist approaches, the principle antibiotic component is purified and used to treat infections. In combinatorial approaches, various other secondary metabolites may be combined with the principle antibiotic to create a synergistic action.
Exploiting insights learned through isolation and identification of extreme or unusual antibiotic-producing organisms
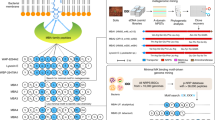
It is generally expected that extremophilic or extremotolerant microorganisms display substantial differences in physiology compared to their mesophilic counterparts. Analysis of these physiological differences could also play a role in elucidating new methods for triggering antibiotic synthesis. Although this is a largely unresearched area, one recent study is encouraging in this context. Streptomyces violaceusniger strain SPC6, a halotolerant strain with an optimal growth temperature of 37 oC, was isolated from the Linze desert in China70. Remarkably this streptomycete completes its life cycle very rapidly, sporulating within 2 days, in contrast to the 4 or 5 days required for typical mesophilic streptomycetes that grow optimally at 28 oC. Sequencing of this SPC6 genome revealed a novel tRNA gene encoding tRNA-Asp-AUC71. Its cognate GAT codon is over-represented in both pleiotropic and pathway-specific transcriptional activators of antibiotic biosynthesis in streptomycetes and translation of this codon in mesophiles is normally dependent on inefficient wobble base-pairing by the conserved tRNA-Asp-GUC. Expression of the new tRNA in mesophilic producers of commercial antibiotics resulted in precocious overproduction of these antibiotics. Also of note is that the new tRNA activated the expression of a cryptic BGC in S. coelicolor, resulting in the production of the antibiotic coelimycin (Fig. 3)71. While it is too early to predict whether this can be a generic tool for activating silent BGCs, it is nonetheless a key advance in unlocking the biosynthetic potential of the actinobacteria and could help in the discovery of new antibiotics in the future.
The new tRNA-Asp-AUC is responsible for the efficient translation of GAU codons in S. violaceusniger SPC6. In the absence of queuosine tRNA anticodon modification, the new tRNA circumvents inefficient wobble base-pairing during translation. When the tRNA is transfected into model mesophilic species S. coelicolor, it greatly enhances synthesis of 4 different antibiotics including the product of a so-called cryptic pathway71. Illustrations from Biorender.com (2023) include NMR, adapted from “NMR gyrotron”, bioassay, adapted from “petri dish antibiotic sensitivity test”, and genome sequence, adapted from “modification of group icon, “DNA transfection (step 4)”. Retrieved from https://app.biorender.com/biorender-templates. Other illustrations of S. violaceuniger and the Linze Desert were by Val Romani.
Conclusions
Bioprospecting for new antibiotic-producing microorganisms in extreme and unusual environments has been a very fruitful exercise, underlined by the novelty of many of the resulting new antimicrobials discovered. One criticism often levelled at this type of exploration is that it has in most cases yielded new activities based on known chemical scaffolds, rather than new classes of compounds. However, these new antibiotics often have potent activities against pathogens resistant to current frontline antibiotics, such as MRSA. An implication is that evolution has finessed new antibiotic activities based on a limited set of optimal chemical scaffolds and begs the question of whether we are misguided in the belief that a solution to AMR depends on the discovery of novel antibiotic classes. Taking forward any of the new antibiotics discovered from producers isolated from extreme environments for clinical trials would require a large investment from the pharmaceutical industry. However, one likely barrier to this investment is the possibility of a repeat of the cycle of emerging resistance that would devalue any new drug. But the success of antibiotic-producing microorganisms in their natural environments suggests that we can learn more from these producers themselves and formulate combinations of antibiotics or antibiotics with adjuvants that could help to increase antibiotic efficacy and minimise resistance. It is now almost a century since Fleming’s discovery of penicillin revolutionised medical practise. Since then, the traditional reductionist approach has been one of isolating an antibiotic-producing organism, growing this in a large-scale monoculture, and purifying the active component. This manner of production and the subsequent prescription of a single antibiotic may be easier in the short-term for health-care regulators to evaluate, but we can now see the long-term (albeit less than 100 years) cost of this approach in terms of lives lost to untreatable infectious diseases due to AMR. Quite possibly, solutions are already at hand, including the contribution of new antibiotics discovered from producers isolated from extreme environments, but we need to be more judicious in how we apply these advances for the benefit of mankind in the future. The key to this is to identify synergies between antibiotic compounds and thereby derive formulations that mitigate against the development of AMR, safeguarding these valuable medicines for the future.
Data availability
All data generated or analysed during this study are included in this published article.
References
Aminov, R. I. A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol. 1, 134 (2010).
Fleming, A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 10, 226–236 (1929).
Schatz, A., Bugie, E. & Waksman, S. Streptomycin: a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55, 66–69 (1944).
Waksman, S. A., Schatz, A. & Reynolds, D. M. Production of antibiotic substances by Actinomycetes. Ann. N. Y. Acad. Sci. 1213, 112–124 (2010).
Cox, G. et al. A common platform for antibiotic dereplication and adjuvant discovery. Cell Chem. Biol. 24, 98–109 (2017).
Newman, D. J. Old and modern antibiotic structures with potential for today’s infections. ADMET DMPK 10, 131–146 (2022).
Bérdy, J. Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. (Tokyo) 65, 385–395 (2012).
Kirst, H. A. Developing new antibacterials through natural product research. Expert Opin. Drug Discov. 8, 479–493 (2013).
Bartlett, J. G., Gilbert, D. N. & Spellberg, B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 56, 1445–1450 (2013).
Stokes, J. M. et al. A deep learning approach to antibiotic discovery. Cell 180, 688–702.e13 (2020).
Watve, M. G., Tickoo, R., Jog, M. M. & Bhole, B. D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 176, 386–390 (2001).
Ait, B. E. et al. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 80, 1–43 (2015).
Linares, J. F., Gustafsson, I., Baquero, F. & Martinez, J. L. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 103, 19484–19489 (2006).
Meyer, K. J. & Nodwell, J. R. Biology and applications of co-produced, synergistic antimicrobials from environmental bacteria. Nat. Microbiol. 6, 1118–1128 (2021).
Hoskisson P. A. & Seipke R. F. Cryptic or silent? The known unknowns, unknown knowns, and unknown unknowns of secondary metabolism. mBio 11, https://doi.org/10.1128/mbio.02642-20 (2020).
Blin, K. et al. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 51, W46–W50 (2023).
Viaene, T., Langendries, S., Beirinckx, S., Maes, M. & Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 92, fiw119 (2016).
Batey, S. F. D., Greco, C., Hutchings, M. I. & Wilkinson, B. Chemical warfare between fungus-growing ants and their pathogens. Mech. Biol. Energy 59, 172–181 (2020).
Xu, H.-F. et al. Reading and surviving the harsh conditions in desert biological soil crust: the cyanobacterial viewpoint. FEMS Microbiol. Rev. 45, fuab036 (2021).
Xie, F. & Pathom-Aree, W. Actinobacteria from desert: diversity and biotechnological applications. Front. Microbiol. 12, 765531 (2021).
Schulze-Makuch, D. et al. Transitory microbial habitat in the hyperarid Atacama Desert. Proc. Natl. Acad. Sci. USA 115, 2670–2675 (2018).
Rateb, M. E., Ebel, R. & Jaspars, M. Natural product diversity of actinobacteria in the Atacama Desert. Antonie Van Leeuwenhoek 111, 1467–1477 (2018).
Mohammadipanah, F. & Wink, J. Actinobacteria from arid and desert habitats: diversity and biological activity. Front. Microbiol. 6, 1541 (2015).
Ivanova, V. et al. Structural elucidation of a bioactive metabolites produced by streptomyces avidinii sb9 strain, isolated from permafrost soil in spitsbergen, arctic. Biotechnol. Biotechnol. Equip. 24, 2092–2095 (2010).
Moon, K. et al. New benzoxazine secondary metabolites from an arctic actinomycete. Mar. Drugs 12, 2526–2538 (2014).
Wu, B. et al. Lindgomycin, an UNusual Antibiotic Polyketide from A Marine Fungus of the Lindgomycetaceae. Mar. Drugs 13, 4617–4632 (2015).
Corral, P. et al. Identification of a sorbicillinoid-producing aspergillus strain with antimicrobial activity against Staphylococcus aureus: a new polyextremophilic marine fungus from barents sea. Mar. Biotechnol. N. Y. N 20, 502–511 (2018).
Núñez-Montero, K. & Barrientos, L. Advances in Antarctic research for antimicrobial discovery: a comprehensive narrative review of bacteria from antarctic environments as potential sources of novel antibiotic compounds against human pathogens and microorganisms of industrial importance. Antibiot. Basel Switz 7, 90 (2018).
Tortorella, E. et al. Antibiotics from deep-sea microorganisms: current discoveries and perspectives. Mar. Drugs 16, 355 (2018).
Cong, M. et al. Deep-sea natural products from extreme environments: cold seeps and hydrothermal Vents. Mar. Drugs 20, 404 (2022).
Wang, C., Lu, Y. & Cao, S. Antimicrobial compounds from marine actinomycetes. Arch. Pharm. Res. 43, 677–704 (2020).
Chen, J., Xu, L., Zhou, Y. & Han, B. Natural products from actinomycetes associated with marine organisms. Mar. Drugs 19, 629 (2021).
Han, Y. et al. A comprehensive genomic catalog from global cold seeps. Sci. Data 10, 596 (2023).
Wang, L. et al. Metagenomic insights into the functions of microbial communities in sulfur-rich sediment of a shallow-water hydrothermal vent off Kueishan Island. Front. Microbiol. 13, 992034 (2022).
Shi, Y. et al. Stress-driven discovery of a cryptic antibiotic produced by Streptomyces sp. WU20 from Kueishantao hydrothermal vent with an integrated metabolomics strategy. Appl. Microbiol. Biotechnol. 101, 1395–1408 (2017).
Rothschild, L. J. & Mancinelli, R. L. Life in extreme environments. Nature 409, 1092–1101 (2001).
O’Connor, E. M. & Shand, R. F. Halocins and sulfolobicins: the emerging story of archaeal protein and peptide antibiotics. J. Ind. Microbiol. Biotechnol. 28, 23–31 (2002).
Virués-Segovia, J. R. et al. Kribbellichelins A and B, two new antibiotics from Kribbella sp. CA-293567 with activity against several human pathogens. Molecules 27, 6355 (2022).
Lewis, K., Epstein, S., D’Onofrio, A. & Ling, L. L. Uncultured microorganisms as a source of secondary metabolites. J. Antibiot. (Tokyo) 63, 468–476 (2010).
Baranova, A. A., Alferova, V. A., Korshun, V. A. & Tyurin, A. P. Modern trends in natural antibiotic discovery. Life 13, 1073 (2023).
Nichols, D. et al. Use of Ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl. Environ. Microbiol. 76, 2445–2450 (2010).
Piddock, L. J. V. Teixobactin, the first of a new class of antibiotics discovered by iChip technology? J. Antimicrob. Chemother. 70, 2679–2680 (2015).
Shukla, R. et al. An antibiotic from an uncultured bacterium binds to an immutable target. Cell 186, 4059–4073.e27 (2023).
Hegemann, J. D., Birkelbach, J., Walesch, S. & Müller, R. Current developments in antibiotic discovery. EMBO Rep. 24, e56184 (2023).
Hoffmann, T. et al. Correlating chemical diversity with taxonomic distance for discovery of natural products in myxobacteria. Nat. Commun. 9, 803 (2018).
Zheng, S. et al. Synthesis and fungicidal activity of tryptophan analogues—the unexpected calycanthaceous alkaloid derivatives. Nat. Prod. Res. 31, 1142–1149 (2017).
Bassett, E. J., Keith, M. S., Armelagos, G. J., Martin, D. L. & Villanueva, A. R. Tetracycline-labeled human bone from ancient Sudanese Nubia (A.D. 350). Science 209, 1532–1534 (1980).
Nelson, M. L. & Levy, S. B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 1241, 17–32 (2011).
Maciejewska, M. et al. A phenotypic and genotypic analysis of the antimicrobial potential of cultivable streptomyces isolated from cave moonmilk deposits. Front. Microbiol. 7, 1455 (2016).
Rangseekaew, P. & Pathom-aree, W. Cave actinobacteria as producers of bioactive metabolites. Front. Microbiol. 10, 387 (2019).
Falkinham, J. O. 3rd et al. Proliferation of antibiotic-producing bacteria and concomitant antibiotic production as the basis for the antibiotic activity of Jordan’s red soils. Appl. Environ. Microbiol. 75, 2735–2741 (2009).
Terra, L. et al. A novel alkaliphilic Streptomyces inhibits ESKAPE pathogens. Front. Microbiol. 9, 2458 (2018).
Behroozian, S., Svensson, S. L. & Davies, J. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. mBio 7, e01842–15 (2016).
Quinn, G. A. et al. Streptomyces Isolates from the Soil of an Ancient Irish Cure Site, Capable of Inhibiting Multi-Resistant Bacteria and Yeasts. Appl. Sci. 11, 4923 (2021).
Cordell, et al. In: Anticancer Drug Discovery and Development: Natural Products and New Molecular Models. Developments in Oncology. (Springer, 1994)
Harrison, F. et al. A 1000-year-old antimicrobial remedy with antistaphylococcal activity. mBio 6, e01129–15 (2015).
Harrison, F., Blower, A., de Wolf, C. & Connelly, E. Sweet and sour synergy: exploring the antibacterial and antibiofilm activity of acetic acid and vinegar combined with medical-grade honeys. Microbiology 169, 001351 (2023).
Joshi-Navare, K. & Prabhune, A. A Biosurfactant-Sophorolipid Acts in Synergy with Antibiotics to Enhance Their Efficiency. BioMed Res. Int. 2013, 512495 (2013).
Al-Healy, N. H. & Al-Sammak, E. Gh. effects of Streptomyces rochi biosurfactants on pathogenic Staphylococcus aureus and Pseudomonas aeruginosa. Al-Mukhtar J. Sci. 37, 261–273 (2022).
Rabahi, M. F., Silva Júnior, J. L. R., da, Ferreira, A. C. G., Tannus-Silva, D. G. S. & Conde, M. B. Tuberculosis treatment. J. Bras. Pneumol. 43, 472–486 (2017).
Mattingly, A. E. et al. Screening an established natural product library identifies secondary metabolites that potentiate conventional antibiotics. ACS Infect. Dis. 6, 2629–2640 (2020).
Nikolaev, Y. A. et al. The use of 4-hexylresorcinol as antibiotic adjuvant. PLoS ONE 15, e0239147 (2020).
van Asbeck, B. S., Marcelis, J. H., van Kats, J. H., Jaarsma, E. Y. & Verhoef, J. Synergy between the iron chelator deferoxamine and the antimicrobial agents gentamicin, chloramphenicol, cefalothin, cefotiam and cefsulodin. Eur. J. Clin. Microbiol 2, 432–438 (1983).
Terra, L., Dyson, P., Ratcliffe, N., Castro, H. C. & Vicente, A. C. P. Biotechnological potential of Streptomyces siderophores as new antibiotics. Curr. Med. Chem. https://doi.org/10.2174/0929867327666200510235512 (2020).
Nüesch, J. & Knüsel, F. in Mech. Action (eds. Gottlieb, D. & Shaw, P. D.) 499–541 (Springer Berlin Heidelberg, 1967).
Wang, M., Zhang, Y., Lv, L., Kong, D. & Niu, G. Biosynthesis and chemical synthesis of albomycin nucleoside antibiotics. Antibiotics 11, 438 (2022).
Sackmann, W. et al. a new iron-containing antibiotic. Antibiot. Chemother. Northfield Ill 12, 34–45 (1962).
Ibrahim, W. M. et al. Exploring the antimicrobial, antiviral, antioxidant, and antitumor potentials of marine Streptomyces tunisiensis W4MT573222 pigment isolated from Abu-Qir sediments, Egypt. Microb. Cell Factories 22, 94 (2023).
Abraham, J. & Chauhan, R. Profiling of red pigment produced by Streptomyces sp. JAR6 and its bioactivity. 3 Biotech 8, 22 (2017).
Chen X et al. Genome sequence of Streptomyces violaceusniger Strain SPC6, a halotolerant Streptomycete that exhibits rapid growth and development. Genome Announc. 1, https://doi.org/10.1128/genomea.00494-13 (2013).
Chen, X. et al. A new bacterial tRNA enhances antibiotic production in Streptomyces by circumventing inefficient wobble base-pairing. Nucleic Acids Res. 50, 7084–7096 (2022).
Zhang, L. et al. Characterization of anti-BCG benz[α]anthraquinones and new siderophores from a Xinjiang desert-isolated rare actinomycete Nocardia sp. XJ31. Appl. Microbiol. Biotechnol. 104, 8267–8278 (2020).
Merrouche, R. et al. A new dithiolopyrrolone antibiotic triggered by a long fermentation of Saccharothrix algeriensis NRRL B-24137 in sorbic acid-amended medium. Lett. Appl. Microbiol. 69, 294–301 (2019).
Abdelkader, M. S. A. et al. Asenjonamides A–C, antibacterial metabolites isolated from Streptomyces asenjonii strain KNN 42.f from an extreme-hyper arid Atacama Desert soil. J. Antibiot. (Tokyo) 71, 425–431 (2018).
Rateb, M. E. et al. Diverse metabolic profiles of a Streptomyces strain isolated from a hyper-arid environment. J. Nat. Prod. 74, 1965–1971 (2011).
Rateb, M. E. et al. Chaxamycins A–D, bioactive ansamycins from a hyper-arid desert Streptomyces sp. J. Nat. Prod. 74, 1491–1499 (2011).
Nachtigall, J. et al. Atacamycins A–C, 22-membered antitumor macrolactones produced by Streptomyces sp. C38. J. Antibiot. (Tokyo) 64, 775–780 (2011).
Schulz, D. et al. Abenquines A–D: aminoquinone derivatives produced by Streptomyces sp. strain DB634. J. Antibiot. (Tokyo) 64, 763–768 (2011).
Kavitha, A., Prabhakar, P., Vijayalakshmi, M. & Venkateswarlu, Y. Purification and biological evaluation of the metabolites produced by Streptomyces sp. TK-VL_333. Res. Microbiol. 161, 335–345 (2010).
Masand, M. et al. Biosynthetic potential of bioactive streptomycetes isolated from arid region of the Thar Desert, Rajasthan (India). Front. Microbiol. 9, 687 (2018).
Chi, L.-P., Li, X.-M., Wan, Y.-P., Li, X. & Wang, B.-G. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J. Nat. Prod. 83, 3652–3660 (2020).
Jin, E., Li, H., Liu, Z., Xiao, F. & Li, W. Antibiotic dixiamycins from a cold-seep-derived Streptomyces olivaceus. J. Nat. Prod. 84, 2606–2611 (2021).
Lü, F., Li, X., Chi, L., Meng, L. & Wang, B. A new acyclic peroxide from Aspergillus nidulans SD-531, a fungus obtained from deep-sea sediment of cold spring in the South China Sea. J. Oceanol. Limnol 38, 1225–1232 (2020).
Zhou, X. et al. Marthiapeptide A, an anti-infective and cytotoxic polythiazole cyclopeptide from a 60 L scale fermentation of the deep sea-derived Marinactinospora thermotolerans SCSIO 00652. J. Nat. Prod. 75, 2251–2255 (2012).
Song, Y. et al. Cyclic hexapeptides from the deep south china sea-derived Streptomyces scopuliridis SCSIO ZJ46 active against pathogenic Gram-positive bacteria. J. Nat. Prod. 77, 1937–1941 (2014).
Pérez-Bonilla, M. et al. Phocoenamicins B and C, new antibacterial spirotetronates isolated from a marine micromonospora sp. Mar. Drugs 16, 95 (2018).
Choi, E. J., Kwon, H. C., Ham, J. & Yang, H. O. 6-Hydroxymethyl-1-phenazine-carboxamide and 1,6-phenazinedimethanol from a marine bacterium, Brevibacterium sp. KMD 003, associated with marine purple vase sponge. J. Antibiot. (Tokyo) 62, 621–624 (2009).
Vicente, J. et al. Monacyclinones, new angucyclinone metabolites isolated from Streptomyces sp. M7_15 associated with the Puerto Rican sponge Scopalina ruetzleri. Mar. Drugs 13, 4682–4700 (2015).
Tawfike, A. et al. New bioactive metabolites from the elicited marine sponge-derived bacterium Actinokineospora spheciospongiae sp. nov. AMB Express 9, 12 (2019).
Cheng, C. et al. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 57, 2786–2789 (2016).
Author information
Authors and Affiliations
Contributions
G.A.Q. and P.J.D. contributed equally to preparing this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quinn, G.A., Dyson, P.J. Going to extremes: progress in exploring new environments for novel antibiotics. npj Antimicrob Resist 2, 8 (2024). https://doi.org/10.1038/s44259-024-00025-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44259-024-00025-8