Abstract
Suramin has been a primary early-stage treatment for African trypanosomiasis for nearly 100 yr. Recent studies revealed that trypanosome strains that express the variant surface glycoprotein (VSG) VSGsur possess heightened resistance to suramin. Here, we show that VSGsur binds tightly to suramin but other VSGs do not. By solving high-resolution crystal structures of VSGsur and VSG13, we also demonstrate that these VSGs define a structurally divergent subgroup of the coat proteins. The co-crystal structure of VSGsur with suramin reveals that the chemically symmetric drug binds within a large cavity in the VSG homodimer asymmetrically, primarily through contacts of its central benzene rings. Structure-based, loss-of-contact mutations in VSGsur significantly decrease the affinity to suramin and lead to a loss of the resistance phenotype. Altogether, these data show that the resistance phenotype is dependent on the binding of suramin to VSGsur, establishing that the VSG proteins can possess functionality beyond their role in antigenic variation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Coordinates and structural factors have been uploaded to the RCSB PDB (www.rcsb.org): VSGsur I3C (PDB ID 6Z79), VSGsur WT native (PDB ID 6Z7A), VSGsur + suramin (PDB ID 6Z7B), VSGsur H122A (PDB ID 6Z7C), VSGsur H122A 0.77 mM suramin (PDB ID 6Z7D), VSGsur H122A 7.7 mM (PDB ID 6Z7E), VSG13 NaBr (PDB ID 6Z8G6Z7G) and VSG13 native (PDB ID 6Z8H). Other data supporting the findings of this study are available from the authors upon request. Source data are provided with this paper.
References
Ponte-Sucre, A. An overview of Trypanosoma brucei infections: an intense host–parasite interaction. Front. Microbiol. 7, 2126 (2016).
Keating, J., Yukich, J. O., Sutherland, C. S., Woods, G. & Tediosi, F. Human African trypanosomiasis prevention, treatment and control costs: a systematic review. Acta Trop. 150, 4–13 (2015).
Radwanska, M., Vereecke, N., Deleeuw, V., Pinto, J. & Magez, S. Salivarian trypanosomosis: a review of parasites involved, their global distribution and their interaction with the innate and adaptive mammalian host immune system. Front. Immunol. 9, 2253 (2018).
Matthews, K. R., McCulloch, R. & Morrison, L. J. The within-host dynamics of African trypanosome infections. Philos. Trans. R. Soc. Lond. B 370, 20140288 (2015).
Mugnier, M. R., Stebbins, C. E. & Papavasiliou, F. N. Masters of disguise: antigenic variation and the VSG coat in Trypanosoma brucei. PLoS Pathog. 12, e1005784 (2016).
Cross, G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology 71, 393–417 (1975).
Overath, P. & Engstler, M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol. Microbiol. 53, 735–744 (2004).
Aresta-Branco, F., Erben, E., Papavasiliou, F. N. & Stebbins, C. E. Mechanistic similarities between antigenic variation and antibody diversification during Trypanosoma brucei infection. Trends Parasitol. 35, 302–315 (2019).
Bangs, J. D. Evolution of antigenic variation in African trypanosomes: variant surface glycoprotein expression, structure, and function. BioEssays 40, 1800181 (2018).
Carrington, M. & Higgins, M. K. O-h what a surprise. Nat. Microbiol. 3, 856–857 (2018).
Schnitzer, R. J. & Hawking, F. Experimental Chemotherapy (Elsevier, 2013).
Steverding, D. The development of drugs for treatment of sleeping sickness: a historical review. Parasit. Vectors 3, 15 (2010).
Lindner, A. K. et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: substantial changes for clinical practice. Lancet Infect. Dis. 20, e38–e46 (2020).
Sanderson, L., Khan, A. & Thomas, S. Distribution of suramin, an antitrypanosomal drug, across the blood–brain and blood–cerebrospinal fluid interfaces in wild-type and P-glycoprotein transporter-deficient mice. Antimicrob. Agents Chemother. 51, 3136–3146 (2007).
Gill, B. S. & Malhotra, M. N. Prophylactic activity of suramin complexes in ‘Surra’ (Trypanosoma evansi). Nature 200, 285–286 (1963).
WHO Model Lists of Essential Medicines (WHO, accessed 12 December 2020); https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06
Stein, C. A. Suramin: a novel antineoplastic agent with multiple potential mechanisms of action. Cancer Res. 53, 2239–2248 (1993).
Wiedemar, N. et al. Beyond immune escape: a variant surface glycoprotein causes suramin resistance in Trypanosoma brucei: suramin resistance in T. brucei. Mol. Microbiol. 107, 57–67 (2018).
Babokhov, P., Sanyaolu, A. O., Oyibo, W. A., Fagbenro-Beyioku, A. F. & Iriemenam, N. C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 107, 242–252 (2013).
Thomas, J. A. et al. Insights into antitrypanosomal drug mode-of-action from cytology-based profiling. PLoS Negl. Trop. Dis. 12, e0006980 (2018).
Alsford, S. et al. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature 482, 232–236 (2012).
Vansterkenburg, E. L. et al. The uptake of the trypanocidal drug suramin in combination with low-density lipoproteins by Trypanosoma brucei and its possible mode of action. Acta Trop. 54, 237–250 (1993).
Wiedemar, N. et al. Expression of a specific variant surface glycoprotein has a major impact on suramin sensitivity and endocytosis in Trypanosoma brucei. FASEB BioAdvances 1, 595–608 (2019).
Bartossek, T. et al. Structural basis for the shielding function of the dynamic trypanosome variant surface glycoprotein coat. Nat. Microbiol. 2, 1523–1532 (2017).
Pinger, J. et al. African trypanosomes evade immune clearance by O-glycosylation of the VSG surface coat. Nat. Microbiol. 3, 932–938 (2018).
Freymann, D. et al. 2.9 A resolution structure of the N-terminal domain of a variant surface glycoprotein from Trypanosoma brucei. J. Mol. Biol. 216, 141–160 (1990).
Metcalf, P., Blum, M., Freymann, D., Turner, M. & Wiley, D. C. Two variant surface glycoproteins of Trypanosoma brucei of different sequence classes have similar 6 Å resolution X-ray structures. Nature 325, 84–86 (1987).
Hartel, A. J. et al. N-glycosylation enables high lateral mobility of GPI-anchored proteins at a molecular crowding threshold. Nat. Commun. 7, 12870 (2016).
Zoll, S. et al. The structure of serum resistance-associated protein and its implications for human African trypanosomiasis. Nat. Microbiol. 3, 295–301 (2018).
Higgins, M. K. et al. Structure of the trypanosome haptoglobin–hemoglobin receptor and implications for nutrient uptake and innate immunity. Proc. Natl Acad. Sci. USA 110, 1905–1910 (2013).
Engstler, M. et al. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 117, 1105–1115 (2004).
Zoltner, M. et al. Suramin exposure alters cellular metabolism and mitochondrial energy production in African trypanosomes. J. Biol. Chem. 295, 8331–8347 (2020).
Warren, G. Transport through the Golgi in Trypanosoma brucei. Histochem. Cell Biol. 140, 235–238 (2013).
Manna, P. T., Boehm, C., Leung, K. F., Natesan, S. K. & Field, M. C. Life and times: synthesis, trafficking, and evolution of VSG. Trends Parasitol. 30, 251–258 (2014).
Rotureau, B., Subota, I. & Bastin, P. Molecular bases of cytoskeleton plasticity during the Trypanosoma brucei parasite cycle. Cell. Microbiol 13, 705–716 (2011).
Figueiredo, L. M., Janzen, C. J. & Cross, G. A. M. A histone methyltransferase modulates antigenic variation in African trypanosomes. PLoS Biol. 6, e161 (2008).
Alsford, S. & Horn, D. Single-locus targeting constructs for reliable regulated RNAi and transgene expression in Trypanosoma brucei. Mol. Biochem. Parasitol. 161, 76–79 (2008).
Schumann Burkard, G., Jutzi, P. & Roditi, I. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol. Biochem. Parasitol. 175, 91–94 (2011).
Hirumi, H. & Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75, 985–989 (1989).
Cross, G. A. Release and purification of Trypanosoma brucei variant surface glycoprotein. J. Cell. Biochem. 24, 79–90 (1984).
Rypniewski, W. R., Holden, H. M. & Rayment, I. Structural consequences of reductive methylation of lysine residues in hen egg white lysozyme: an X-ray analysis at 1.8-A resolution. Biochemistry 32, 9851–9858 (1993).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
Beck, T., Krasauskas, A., Gruene, T. & Sheldrick, G. M. A magic triangle for experimental phasing of macromolecules. Acta Crystallogr. D 64, 1179–1182 (2008).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. D 62, 859–866 (2006).
Langer, G., Cohen, S. X., Lamzin, V. S. & Perrakis, A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat. Protoc. 3, 1171–1179 (2008).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of coot. Acta Crystallogr. D 66, 486–501 (2010).
Aline, R. et al. (TAA)n within sequences flanking several intrachromosomal variant surface glycoprotein genes in Trypanosoma brucei. Nucleic Acids Res. 13, 3161–3177 (1985).
Cross, G. A. M., Kim, H.-S. & Wickstead, B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol. Biochem. Parasitol. 195, 59–73 (2014).
Eisenberg, D., Schwarz, E., Komaromy, M. & Wall, R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179, 125–142 (1984).
McNicholas, S., Potterton, E., Wilson, K. S. & Noble, M. E. M. Presenting your structures: the CCP4mg molecular-graphics software. Acta Crystallogr. D 67, 386–394 (2011).
The PyMOL Molecular Graphics System, v.1.8 (Schrödinger, LLC, 2015).
de Beer, T. A. P., Berka, K., Thornton, J. M. & Laskowski, R. A. PDBsum additions. Nucleic Acids Res. 42, D292–D296 (2014).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Acknowledgements
We acknowledge time at the European Synchrotron Radiation Facility (ESRF, beamline ID29, proposal MX1975, G. Santoni and colleagues), the Diamond Light Source (DLS, beamline i03, proposal number mx18989-1, N. Paterson and colleagues), the Helmholtz-Zentrum Berlin (BESSY, beamlines 14.1 and 14.2, proposal numbers MX-191-00036 and MX-192-00114, staff member M. Weiss and colleagues, especially C. Feiler for help with processing and solving the I3C SAD dataset) and the Paul Scherrer Institut, Villingen, Switzerland (SLS, beamline X06DA, proposal numbers 20182345, 20191097 and 20191895, V. Olieric and colleagues). We thank L. Figueiredo for supplying us with the VSG13-expressing T. brucei strain, and M. Cal, R. Rocchetti, S. Keller and M. Kaiser for help with drug sensitivity testing. N.W., S.H. and P.M. were supported by the Swiss National Science Foundation (grant no. 310030_185163). The work was supported by funds and resources from the German Cancer Research Center (DKFZ).
Author information
Authors and Affiliations
Contributions
H.H. cloned VSGsur and M.v.S. produced VSGsur mutants. S.H., N.W. and P.M. produced high-resistance VSGsur mutants and associated assays. M.v.S. and J.Z. purified all VSGs. M.v.S. crystallized VSG13. M.v.S. and J.Z. crystallized wild-type and mutant VSGsur proteins. C.E.S. phased and produced the first model of VSG13 and A.H. built and refined the structures of VSG13 native and the sodium bromide soak. J.Z. solved all crystal structures of VSGsur. P.D.J. worked with J.Z. on the refinement of VSGsur bound to suramin. M.v.S. performed the suramin growth and toxicity assays. K.P. conducted ITC experiments. J.V. conducted endocytosis assays.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Raghuvir Arni, Markus Engstler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
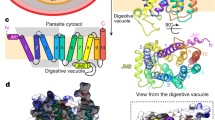
Extended Data Fig. 1 Comparison of the VSG NTDs.
a, A single monomer of the VSGsur NTD is shown as a ribbon diagram. The β-sheet subdomain that forms the top lobe is colored in orange and the rest of the structure in light blue. The N-linked glycan is shown as red space-filling spheres, the cysteine disulfide atoms as space-filling cyan spheres. b, Structural alignment of monomers of VSGsur (orange) and VSG13 (grey) with corresponding glycans shown in space filling representation and colored the same as the protein to which they are linked, while disulfides are shown in cyan and green for VSGsur and VSG13, respectively. The alignment produces an overall root-mean-square deviation of 1Å for the conserved portions of the structure (calculated over 220 Cα positions that primarily encompass the three-helix bundle and elements of the bottom lobe). c, Comparison of the structures of VSGsur, VSG13, and VSG2. The N-linked glycans are displayed as red space-filling atoms and the disulfide bonds are shown in cyan. Approximate dimensions of the molecules are noted, as well as the directions toward the external environment and toward the C-terminal domain (CTD) and plasma membrane of the trypanosome.
Extended Data Fig. 2 Growth curves of VSGsur and VSG mutants.
Strains expressing VSGsur and VSGsur mutants were grown with and without suramin (incubation of 0.7µM suramin for 24 h, Methods). The cell densities were determined by cell counting using a Neubauer hemocytometer. This procedure was repeated for 3-4 days in a row. Analysis was performed with GraphPad Prism, using a nonlinear regression model for curve fitting (Exponential growth with log). For statistical comparisons, we tested whether the slopes and intercepts of the best fit curves were significantly different. For VSGsur and VSGsur + 0.7µM suramin, the differences between the slopes is not significant (P value = 0.7720). For H122A and N130A (without suramin) compared to VSGsur the differences are significant (P<.0113 and P<.0001, respectively). Two independent experiments with different cultures were made for each data point.
Extended Data Fig. 3 Sample ITC results.
ITC data for suramin binding to each VSG protein. The upper panels contain the baseline corrected raw data, and the lower panel contains the peak-integrated, concentration normalized data for the heat of reaction vs. molar ratio of suramin per VSG protein. a, VSGSur was measured 3 times, independently: 300µM suramin was titrated into 46µM VSGsur, the curve fitted with a single binding site model to calculate a Kd of 234+/- 28 nM and N of 0.49 +/- 0.03 b, VSGSur H122A was measured 3 times, independently: 450µM suramin was titrated into 51.1µM VSGSur H122A. A Kd could not be fit to the data, although it is clear that the mutation negatively affected the binding affinity. c, VSGSur N130A was measured 2 times, independently: 300µM suramin was titrated into 40µM VSGSur N130A. No binding was detected. d, VSG2 was measured 2 times, independently: 200µM Suramin was titrated into 20µM VSG2 protein. No binding was detected. e, VSG3 was measured 2 times, independently: 300µM suramin was titrated into 53.1µM VSG3. No binding was detected. f, VSG13 was measured 2 times, independently: 200µM Suramin was titrated into 20µM VSG132 protein. No binding was detected.
Extended Data Fig. 4 Two-fold axis of dimer symmetry for VSGsur-suramin complex.
a, The top drawing illustrates the chemical structure of suramin with several of its functional groups denoted, whereas the bottom renders the drug as found in the protein structure with a transparent molecular surface shown. Oxygen atoms are shown in red, nitrogen in blue, and carbon gray. b, Ribbon diagram of VSGsur (one monomer blue and the other gold) looking down the two-fold axis of symmrtry for the dimer. Suramin is shown as a ball-and-stick chemical representation as in (a). In the center of the rotational axis for the dimer, one of the suramin benzene rings is visible.
Extended Data Fig. 5 H122 “open” and “closed” conformations.
a, Two H122 conformations in the native crystals structure of VSGsur with corresponding electron density (b) Closed conformation electron density of H122 (c) Closed conformation surface of VSGsur (d) Position of H122 and the I3C group used in phasing the crystal structure with corresponding electron density.
Extended Data Fig. 6 Creation of VSGsur and VSGsur mutant expressing strains.
The same construct was used multiple times to generate different VSG-expressing cell lines. The top schematic shows the endogenous sub-telomeric expression site in 2T1 cells, which express VSG2 (pink) with its endogenous UTRs (dark green). The vector used to integrate new VSGs was adapted from Pinger et al.2, wherein the homology directed integration of a novel VSG ORF (black) flanked by the UTRs of VSG2 is mediated by 3’ telomere seeds (Tel-S) and 5’ homology to the upstream co-transposed region (CTR – teal). Transfected cells are identified by screening for the integration of a hygromycin selection cassette (red) that is expressed via read-through transcription driven by the subtelomeric promoter.
Extended Data Fig. 7 Panels of WT and H122A with suramin soaks.
Molecular surface of one monomer in the VSGsur dimer shown colored by electrostatic potential (white is neutral, blue is positive/basic, and red is negative/acidic in charge). Electron density in the suramin binding site is shown in green.
Extended Data Fig. 8 Resistance enhancing mutations mapped to VSGsur.
Ribbon diagrams of VSGsur/suramin co-crystal structure in two orientations. Mutations discovered in “supersur” VSGsur mutants with heightened resistance to suramin are shown as green space filling atoms. Suramin and the N-linked glycan of VSGsur are depicted as space filling atoms in purple and crimson respectively.
Extended Data Fig. 9 Replacement of VSGsur with "supermutant" VSGsur at the active expression site of T. b. rhodesiense STIB900_sur1.
a, Construct for gene replacement; mutations in the VSGsur coding sequence (dark red) are shown in light red, arrows indicate primer binding sites. BSD, blasticidin resistance gene; αβ tub, αβ tubulin splice site. (b) 50% inhibitory concentrations of the transfected clones and the parent (sur1) as measured with Alamar Blue assays. The clones sur1_tr1 and sur1_tr2 still expressed VSGsur, while the clones sur1_tr3 and sur1_tr4 expressed the mutant version. The scatter plots represent independent drug assays, each carried out in duplicate. Error bars represent mean ± standard deviation. Small letters indicate significance groups as determined by one-way ANOVA (Df = 4, F-value = 31.02, p-value = 3.6×10−9), followed by Tukey’s multiple pairwise comparisons test (p-values <0.001) as calculated with R 3.6.2. n = 6 for STIB900_sur1, sur1_tr1, sur1_tr2, and sur1_tr4; n = 5 for sur1_tr3.
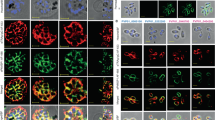
Extended Data Fig. 10 Endocytosis rates by different VSGs relative to VSG2.
Alexa 488-dextran (a), bodipy-LDL (b), and Alexa 488-transferrin (c) endocytosis by T. b. brucei 2T1 cells expressing a variety of different VSG genes. Due to technical limitations, each cell line could not be analyzed simultaneously within each experiment for each ligand. Therefore, VSG2 was used as a control in all experiments, allowing the calculation of each cell line’s relative uptake rate of each ligand as compared to the uptake rate of each ligand observed by VSG2 expressing cells within each separate experiment. Each of these graphs therefore represent the combined experimental results from 2 separate experiments. Cell lines with a relative fluorescence intensity below 1 at a given time point have less efficient endocytic rates compared to VSG2 expressing cells, and vice versa. All graphs share the same Y axis.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Discussion, Methods and Tables 1–4.
Source data
Source Data Fig. 2
The first table gives the cell densities of Trypanosoma strains expressing different VSGs, measured 24 h after addition of various concentrations of suramin. The starting density was 100 (×1,000) cells per ml. (Cells were grown in 24-well tissue culture plates. Cell numbers were determined with a Neubauer haemocytometer.) In the second table, the values are normalized to the cell growth without suramin. (No suramin is 100%.)
Source Data Extended Data Fig. 9
The Excel file contains the data for Extended Data Fig. 9. It is organized in five worksheets, one for each T. b. rhodesiense line: the parental STIB900_sur1 and the four transfected descendants thereof (sur1_tr1 to sur1_tr4). Each line was tested against the drugs melarsoprol (rows 2 to 17) and suramin (rows 19 to 34). Drug concentration in nanomolar is in column A; percentage of growth as compared with untreated controls, tested in six replicates, is in columns B to G. The resulting dose–response curves are shown on the right side. The calculated pharmacodynamic parameters are in rows 14 to 17 for melarsoprol and in rows 31 to 34 for suramin. The IC50 values as plotted in Supplementary Fig. 11 are labelled in green (row 32). Note that melarsoprol was used as an internal reference; the data are not included in Supplementary Fig. 11.
Rights and permissions
About this article
Cite this article
Zeelen, J., van Straaten, M., Verdi, J. et al. Structure of trypanosome coat protein VSGsur and function in suramin resistance. Nat Microbiol 6, 392–400 (2021). https://doi.org/10.1038/s41564-020-00844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-00844-1