Abstract
The primordial ingredient of cuprate superconductivity is the CuO2 unit cell. Theories usually concentrate on the intra-atom Coulombic interactions dominating the 3d9 and 3d10 configurations of each copper ion. However, if Coulombic interactions also occur between electrons of the 2p6 orbitals of each planar oxygen atom, spontaneous orbital ordering may split their energy levels. This long-predicted intra-unit-cell symmetry breaking should generate an orbitally ordered phase, for which the charge transfer energy ε separating the 2p6 and 3d10 orbitals is distinct for the two oxygen atoms. Here we introduce sublattice-resolved ε(r) imaging to CuO2 studies and discover intra-unit-cell rotational symmetry breaking of ε(r). Spatially, this state is arranged in disordered Ising domains of orthogonally oriented orbital order bounded by dopant ions, and within whose domain walls low-energy electronic quadrupolar two-level systems occur. Overall, these data reveal a Q = 0 orbitally ordered state that splits the oxygen energy levels by ~50 meV, in underdoped CuO2.
Similar content being viewed by others
Main
Unforeseen and unexplained among the quantum matter states of hole-doped CuO2 is an electronic nematic phase1. Analyses of the three-orbital model2,3,4,5,6,7,8,9 for the CuO2 charge transfer insulator have long predicted a candidate mechanism for the nematic state involving energy splitting between the two planar oxygen 2p6 orbitals within each unit cell. Although never observed, such effects should be experimentally identifiable as a difference in charge transfer energy ε(r) separating each oxygen 2p6 orbital from the relevant copper 3d10 orbital configuration. Moreover, if extant, such ordering of the oxygen 2p6 orbitals may be juxtaposed with iron-based high-temperature superconductors where intra-unit-cell rotational symmetry breaking between the iron dzx and dzy orbitals10,11,12 is pivotal13,14.
Soon after the discovery of Fe-based high-temperature superconductivity, the profound significance of ordering in the iron dzx and dzy orbitals was identified10,11,12, along with the subsequent realization that such orbital ordering (lifting energy degeneracy of dzx and dzy orbitals) was a fundamentally important phenomenon13,14. In these materials, such orbital ordering is pivotal for the tetragonal-to-orthorhombic phase transition into an electronic nematic phase1,13. This lifting of dzx:dzy energy degeneracy certainly has profound global effects13,14: sequentially with falling temperature, the orbital ordering renders the electronic structure strongly nematic along with a strongly anisotropic antiferromagnetic state; its spectrum of magnetic fluctuations exhibits equivalently reduced symmetry13; finally, a strongly anisotropic14 and even orbital-selective15 form of high-temperature superconductivity emerges. Hence, intra-unit-cell orbital ordering produces powerful, wide-ranging effects on the electronic structure, quantum magnetism, high-temperature superconductivity and on the global phase diagram of the Fe-based high-temperature superconductive materials13,14,16,17. However, although long anticipated2,3,4,5,6,7,8,9, analogous intra-unit-cell orbital ordering for Cu-based high-temperature superconductive materials has never been observed.
Charge transfer superexchange
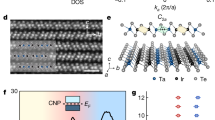
In these materials, planar Cu++ ions are in the 3d9 configuration with a singly occupied \(d_{x^2-y^2}\) orbital, whereas the planar O−− ions have filled 2p6 orbitals. The Cu 3d10 configuration, which is energetically disfavoured by the Coulomb energy U required to doubly occupy each \(d_{x^2-y^2}\) orbital, is separated from the intervening oxygen 2p6 energy levels by the charge transfer energy ε (Fig. 1d). Although at half-filling, the d electrons are fully localized in a charge transfer insulator state, the introduction of holes into the oxygen 2p6 orbitals radically alters the situation, necessitating a three-orbital Hamiltonian18,19 description:
Here α and β label any of the three orbitals, \({t}_{{ij}}^{\alpha \beta }/\hslash\) are transition rates for electrons between orbitals α and β at sites i and j, εα are the orbital energies and \({n}_{i\uparrow }^{d},{n}_{i\downarrow }^{d}\) are the d-orbital occupancies. High-temperature superconductivity is believed to emerge within this model driven by the charge transfer superexchange pairing interactions between the Cu d electrons (Methods and Extended Data Fig. 1). In theory1, however, many other ordered phases could emerge on the hole doping of CuO2, with a planar oxygen orbitally ordered phase2,3,4,5,6,7,8,9 due to additional inter-oxygen repulsion terms \({V}_{{pp}}{\sum }_{{ij}\sigma \sigma {\prime} }{n}_{i\sigma }^{p}{n}_{j\sigma {\prime} }^{p}\) (equation (1) being a prime example).
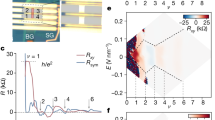
a, Signatures of nematic phase appearing at the cuprate pseudogap (PG) temperature T*(p). Each data point is the average of multiple measurements from each technique (Methods). AF represents the antiferromagnetic state and DSC represents d-wave superconductivity. The error bars are accordingly derived from these estimates. b, Schematic of the relevant orbitals in the CuO2 plane depicting the crucial inter-oxygen-orbital Coulomb interaction Vpp. c, Degeneracy of the Cu \(3d_{x^2-y^2}\) orbital (grey) is lifted by the Coulomb energy U. The px orbital of the oxygen along the x axis of the Cu atom (red) is separated from the upper Cu band by the charge transfer energy εx. At the oxygen site along the y axis from the Cu atom (blue), the py orbital is separated from the upper Cu band by the charge transfer energy εy. d, Schematic of the density of electronic states (top) where the Coulomb energy U and charge transfer energy ε are indicated. Typical measured differential conductance g(V) spectrum (bottom) where the top of the lower band and the bottom of the upper band are visualized, their separation being a direct measure of charge transfer energy ε (ref. 25). CTB represents the charge transfer band, LHB represents the lower Hubbard band and UHB represents the upper Hubbard band.
One enigmatic phase that does emerge is the pseudogap regime. Its essential phenomenology20,21 is that for T < T*(p) and p < p* ≈ 0.19 (Fig. 1a, yellow shading), the Fermi surface becomes partially gapped, thereby diminishing the spectrum of electronic states, magnetic susceptibility, electronic specific heat, c-axis electrical conductivity and spatially averaged density of electronic states. Strikingly, however, in this same region of the phase diagram, there is pervasive evidence for a nematic state in which the electronic structure breaks 90°-rotational (C4) symmetry at wavevector Q = 0, or equivalently within the CuO2 unit cell. Experimental signatures of this nematic state have been widely reported based on multiple techniques (Fig. 1a), and this nematic phenomenology is observed for p < p* in the Bi2Sr2CaCu2O8+x, Bi2Sr2CuO6+x, Bi2–zPbzSr2–yLayCuO6+x, YBa2Cu3O7–x, La2–xBaxCuO4 and HgBa2CuO4+δ material families, thereby strongly indicative of universality (Methods). However, no microscopic mechanism has yet been experimentally established for this CuO2 nematic phase.
Motivation for cuprate orbital ordering
Classically, a nematic metallic state may occur due to the Pomeranchuk instability of the Fermi surface22. But, for cuprates, theoretical analyses using a three-orbital band structure2,3,4,5,6,7,8,9 predict the emergence of low-energy electronic nematicity primarily driven by high-energy orbital ordering. Specifically, when the Coulomb repulsions between electrons on nearest-neighbour O sites within the same unit cell are included in the strong-coupling limit2 and in self-consistent mean-field theory3, an orbitally ordered nematic phase is predicted. On the same microscopic basis, such CuO2 orbital order is also predicted using diagrammatic perturbation theory4, perturbative expansion5, functional renormalization group techniques7, Hartree–Fock-based models8 and slave-boson mean-field theory9. Figure 1b conceptually represents such planar oxygen orbital order, whereas Fig. 1c represents a schematic of its impact on the intra-unit-cell characteristics of charge transfer energy ε. Here the 2p6 orbital of the oxygen atom along the CuO2 x axis (Ox site) is separated from the upper Cu band by the charge transfer energy εx, whereas the notionally equivalent oxygen orbital along the y axis (Oy site) exhibits a different charge transfer energy εy. Our objective is then a direct search for such rotational symmetry breaking at the charge transfer energy scale by the visualization of ε within each CuO2 unit cell.
Visualization of charge transfer energy variations
In practice, Bi2Sr2CaCu2O8+x samples with hole density p ≈ 0.17 and Tc = 88 K are inserted into a spectroscopic imaging scanning tunnelling microscope and cleaved in a cryogenic ultrahigh vacuum at T = 4.2 K. The technique of imaging charge transfer energies in cuprates has been established by several scanning tunnelling microscopy studies23,24,25 of variations in the dI/dV spectra measured over the energy range of −1.5 < E < 2.0 eV. However, intra-unit-cell resolution imaging of the charge transfer energies has proven to be challenging due to the high tunnelling junction resistance (and thus large tip–sample separation) required for such high-voltage imaging. Here we overcome such challenges and introduce sublattice-resolved26,27,28,29 imaging of ε(r) into CuO2 studies. A typical topographic image, T(r), of the terminal BiO surface from these studies is shown in Fig. 2a. To visualize the intra-unit-cell structure of the charge transfer energy scale, we use a recently developed technique25 that analyses high-voltage tunnelling conductance spectra g(r, E) ≡ dI/dV(r, eV) so as to yield the spatial variations in ε(r). Figure 2b shows an example of a high-voltage g(r, V) spectrum, measured using junction resistance RN ≈ 85 GΩ at a specific site r within the CuO2 unit cell (Fig. 2a, yellow dot). Compared with the spatially averaged \(\overline{g\left(V\right)}\) in the same field of view (FOV), we see that they have different energy separations between the lower band (filled) and upper band (empty) (Fig. 2b, red and blue arrows). Hence, by visualizing g(r, V) in the Vmin (–1.6 V) ≤ V ≤ Vmax (2.0 V) range at these junction resistances, one can find departures in ε(r) from the average, for every unit cell in the FOV. To do so, we first use Fourier filtering to remove the structural supermodulation with wavevector QSM from the measured g(r, V); this is necessary because supermodulation is known to modulate the charge transfer energy25 with an amplitude of approximately 150 meV. We then integrate each measured g(r, V) followed by the evaluation of \({I}_{+}({\bf{r}})={\int }_{0}^{{V}_{\max }}g({\mathbf{r}},V){\mathrm{d}}V\), the integrated density of states giving rise to the current for all positive-energy states and similarly for the negative-energy states, \({I}_{-}({\bf{r}})={\int }_{{V}_{\min }}^{0}g({\bf{r}},V\;){{\rm{d}}V}\), where \(\bar{{I}_{\pm }}\) are the equivalent integrals for the spatially averaged spectrum \(\overline{g\left(V\right)}\). We normalize the current by the junction resistance, which is given by 1/(gmax – gmin), consequently leading to energy variations in the charge transfer band. Both filled and empty states are used in this calculation (Methods and Extended Data Fig. 1). Next, to estimate the variations in the charge transfer energy away from its mean, we use
which averages over a wide range of tunnel conductances g between gmin (0.01 nS) and gmax (0.22 nS). This integration algorithm is demonstrated to provide an improved signal-to-noise ratio (Methods and Extended Data Fig. 1) in measuring the variations in charge transfer energy compared with the previous algorithm25. Finally, the Lawler–Fujita (LF) procedure26 is used to morph the simultaneously measured topograph T′(r) into a perfectly periodic tetragonal image T(r) (Fig. 2a, Methods and Extended Data Fig. 2), in which the geometry of every unit cell is equivalent; the identical transformation carried out on δε′(r) yields an image of charge transfer energy variations δε(r) that are equally unit-cell periodic26,27,28,29. This δε(r) and its power spectral density (PSD) Fourier transform δε(q) become the basis for studies of intra-unit-cell symmetry breaking, where Fig. 2c shows the configuration of δε(r) measured in the FOV shown in Fig. 2a. The distribution of the measured δε values (Fig. 2c) is shown in Fig. 2d and yields a root mean square (r.m.s.) value of δεr.m.s. ≈ 90 meV. As demonstrated below, this distribution is dominated by the spatial arrangements of an intra-unit-cell ordered state.
a, Topograph of the BiO surface of Bi2Sr2CaCu2O8+x (VS = –750 mV:IS =25 pA). The bulk-crystal supermodulation, a quasi-periodic modulation along the (1, 1) direction, is clearly evident. It is at 45° to—and therefore is the mirror plane between—the x and y axes, as always26. Any distinctions between the states of oxygen orbitals along the x and y axes are not influenced by supermodulation for this symmetry reason, as empirically demonstrated in Methodsand Extended Data Fig. 9. Therefore, supermodulation has no discernable influence on the intra-unit-cell symmetry breaking of δε(r). b, High-voltage differential conductance spectra g(r, V) are shown as a solid black curve, whereas the spatially averaged spectrum \(\overline{g\left(V\right)}\) is shown as a dashed curve. The example spectrum is measured at a location (yellow dot) in a. Such high junction resistances of 85 GΩ or large tip–sample distances preclude the effects of the tip–sample electric field on g(V). The separation between the lower and upper bands is clearly visible for the example spectrum (blue arrows) as well as for the average spectrum (red double-headed arrow). Setpoint VS = –600 mV:IS = 7 pA. c, Visualization of charge transfer energy variations δε(r) from a. d, Histogram of charge transfer energy variations δε in c. e, PSD Fourier transform T(q) of the topograph measured simultaneously as c. The QSM peaks (orange arrow) signify the supermodulation. f, Linecuts from q = (0, 0) to (1, 0)2π/a0 and from q = (0, 0) to (0, 1)2π/a0 in T(q). The values at Qx = (1, 0)2π/a0 and Qy = (0, 1)2π/a0 are indistinguishable; thus, the PSD T(q) does not break C4 symmetry at its Bragg peaks. g, PSD Fourier transform δε(q) of charge transfer energy map from c. The QSM peaks are removed in δε(q). h, Linecuts from q = (0, 0) to (1, 0)2π/a0 and from q = (0, 0) to (0, 1)2π/a0 in δε(q) from g. The δε(q) breaks C4 symmetry at its Bragg peaks as the plots of δε(q) are distinct at Qx = (1, 0)2π/a0 and Qy = (0, 1)2π/a0. This is direct evidence of intra-unit-cell rotational symmetry breaking at the charge transfer energy in cuprates.
Intra-unit-cell symmetry breaking of charge transfer energy
Next, we explore the symmetry of intra-unit-cell structure of δε(r) by studying the values of δε(q) measured at the two symmetry-inequivalent Bragg peaks \({{\bf{Q}}}_{{\bf{x}}}^{{\rm{B}}}=2\uppi /{a}_{0}(\mathrm{1,0})\) and \({{\bf{Q}}}_{{\bf{y}}}^{{\rm{B}}}{\boldsymbol{=}}2\uppi /{a}_{0}(\mathrm{0,1})\) of the CuO2 plane26,27. Figure 2e shows the measured PSD Fourier transform of the simultaneous topograph T(q) and Fig. 2f shows these data plotted along the two orthogonal axes. The magnitudes of the two Bragg peaks are indistinguishable (T\(\left({{\bf{Q}}}_{{\bf{x}}}^{{\rm{B}}}\right){\boldsymbol{\approx }}T\left({{\bf{Q}}}_{{\bf{y}}}^{{\rm{B}}}\right)\)) within the error bars, meaning that neither the crystal nor the scanning tunnelling microscope tip breaks the intra-unit-cell rotational symmetry. In contrast, Fig. 2g shows the measured magnitude of δε(q), whereas Fig. 2h shows these data plotted along the two orthogonal axes. Now, the magnitudes of the two Bragg peaks in δε(q) are different (Methods and Extended Data Figs. 3–5 show a repeatable observation), revealing the existence of Q = 0 rotational symmetry breaking of the electronic structure at the charge transfer energy range in Bi2Sr2CaCu2O8+x. To double check, by intra-unit-cell imaging in real space, we also directly demonstrate that this rotational symmetry breaking is a valid empirical property at the charge transfer energy scale of all the unprocessed high-voltage sublattice-resolved dI/dV(r, eV) data (Methods and Extended Data Fig. 6).
Ising domains of orbital ordering
This motivates the development of a CuO2 orbital order parameter for the efficient visualization of intra-unit-cell energy-splitting variations. We specifically focus on the two planar oxygen atom sites within each CuO2 unit cell. First, we sample the values of δε(r) surrounding the oxygen atom sites along the x axis from Cu atom Rij and label them \({\delta {\varepsilon }}_{{{\rm{O}}_x}}\left({{\bf{R}}}_{{\bf{ij}}}{\bf{-}}a\hat{{\bf{x}}}/2\right)\): \({\delta {\varepsilon }}_{{{\rm{O}}_x}}\left({{\bf{R}}}_{{\bf{ij}}}{\bf{-}}a\hat{{\bf{x}}}/2\right)\); equivalently, we sample the two nearest planar oxygen atom sites along the y axis from Rij and label them \({\delta {\varepsilon }}_{{{\rm{O}}_y}}\left({{\bf{R}}}_{{\bf{ij}}}{\bf{-}}a\hat{{\bf{y}}}/2\right)\):\({\delta {\varepsilon }}_{{{\rm{O}}_y}}\left({{\bf{R}}}_{{\bf{ij}}}{\bf{-}}a\hat{{\bf{y}}}/2\right)\) (Fig. 3c, inset), where a is the CuO2 lattice constant. Using this approach, a nematic order parameter Nε(r) may be defined26,30 as the difference between the average \({\delta {\varepsilon }}_{{{\rm{O}}_x}}({\bf{r}})\) and \({\delta {\varepsilon }}_{{{\rm{O}}_y}}({\bf{r}})\) inside each unit cell:
a, Image of nematic intra-unit-cell order parameter Nε(Rij) sampled on oxygen sites. Here \({\delta {\varepsilon }}_{{{{\rm{O}}_x}}{{({\rm{O}}_y)}}}\) represent the four oxygen sites of each unit cell (inset in c) from the FOV shown in Fig. 2a. The continuous Nε(r) image is achieved by the Gaussian smoothing of Nε(Rij) with a radius of 3.2 Å (dashed circle). The powerful breaking of intra-unit-cell C4 symmetry is clearly observed in δε(r), whereas the predominant disorder is revealed as Ising domains of opposite sign Nε. b, Schematic of the charge-quadrupole two-level systems within the Ising-domain walls. The top panel shows the schematic of the charge distribution in the four intra-unit-cell oxygen sites consequent to the intra-unit-cell charge transfer symmetry breaking. The bottom panel shows a schematic of two orbitally ordered domains consisting of orthogonally oriented charge quadrupoles, and a domain wall within which the lower-energy barrier to fluctuations in the charge quadrupolar orientation can be exceeded at finite temperatures. The darker circles indicate oxygen sites with higher charge transfer energy ε, whereas lighter circles represent those with lower ε. c, Histogram of Nε(r) in a with an r.m.s. value of 25 meV. This histogram represents a combination of two populations of ordered Ising nematic domains plus all the randomized values of δε(r) within the intervening domain walls. The inset shows the unit cell sampled on four oxygen sites (red along the x axis and blue along the y axis) for calculating the nematic order parameter, with Cu sites marked by yellow circles. d, Histogram Nε from all the non-ordered regions of a. Such regions are identified as the white domain walls in a, where |Nε| < 5 meV. The thermal energy barrier of 5 meV is equivalent to approximately 60 K. e, Location of oxygen dopant ions (black dots) measured simultaneously as Nε(r) (Methods and Extended Data Fig. 7). They occur proximate to the domain walls (yellow contours) where |Nε(r)| < 5 meV. f, Histogram of the shortest dopant ion–domain-wall distance ddopant (pink bars). The histogram of the expected averaged distance drandom between the simulated Poisson random point and its nearest domain-wall point (grey area), uncorrelated with Nε(r) (Methods and Extended Data Fig. 7).
Figure 3a shows our measured Nε(r) from the FOV shown in Fig. 2a, where a continuous image is achieved by the Gaussian smoothing of Nε(Rij) with the radius shown by a small circle in the image. A strong breaking of intra-unit-cell C4 symmetry is now observed in δε(r), whereas the predominant disorder is revealed as Ising domains of opposite sign Nε (a phenomenology that cannot be the result of anisotropy of the scan tip). The relative preponderance of the two orbitally ordered domains is about 2:1 in these p ≈ 0.17 hole-doped samples (Methods and Extended Data Figs. 3–5), indicating that the quenched disorder from dopant ions pins the orbitally ordered domains such that some domains of the opposite sign to the predominant order are stabilized. This intra-unit-cell structure in charge transfer energy implies a redistribution of electric charge that breaks rotational symmetry in the form of a charge quadrupole. But each such charge quadrupole has two possible orientations relative to the local environment, with two different energies (Fig. 3b): it is, thus, an electronic quadrupolar two-level system. Figure 3c shows the histogram of the measured Nε(r) in Fig. 3a, whose r.m.s. value is approximately 25 meV. The histogram of the measured |Nε| < 5 meV within the proposed Ising-domain walls is presented in Fig. 3d, representing a dense reservoir of thermally active quadrupolar two-level systems that should undergo rapid thermal fluctuations at finite temperatures. Such two-level systems occupy an area fraction of approximate 25% in this sample. Another issue is the size of the Ising domains, the largest of which (when averaged over the entire FOV; Methods and Extended Data Figs. 3–5) typically has a size of 30 nm2, equivalent to approximately 200 CuO2 unit cells. In the same FOV of these studies, we can identify the locations of interstitial oxygen dopant ions (Methods and Extended Data Fig. 7). When superimposed on the simultaneously measured Nε(r) images (Fig. 3e), we find a propensity for these ions to lie in the domain walls (Fig. 3f) between the orbitally ordered regions (Methods and Extended Data Fig. 7).
Determination of energy splitting of Ox and Oy orbitals
To illustrate the internal electronic structure of orbitally ordered CuO2 (Fig. 3a), we select all the regions with Nε > +5 meV (domain α) and all the regions with Nε < –5 meV (domain β). For each of these two zones, we first average the topographic image T(r) over all the CuO2 unit cells contained therein (Methods and Extended Data Fig. 8). Figure 4a,b shows that neither the topography nor the scan tip break the intra-unit-cell C4 symmetry. We then present δε(r) unit cell averaged over exactly the same two zones. The results (Fig. 4c,d) vividly demonstrate how strongly the intra-unit-cell rotational symmetry is broken at the charge transfer energy, within the two orthogonal Ising nematic domains. Focusing on the Cu sites (Fig. 4c,d) reveals the breaking of rotational symmetry, whereas the Ox and Oy sites (Fig. 4c,d) exhibit different charge transfer energies with separation on the 50 meV scale (Methods and Extended Data Figs. 3–5 report the repeatable observation). Indeed, this intra-unit-cell splitting of the charge transfer energies can be directly seen from the unprocessed data where the dI/dV spectra are separately identified at the Ox and Oy sites within the same unit cell (Methods and Extended Data Fig. 6). These results are indicative that the 2p6 oxygen orbital at Ox is separated from the upper Cu band by a different energy than that at Oy, or equivalently, the existence of intra-unit-cell orbital order. Many characteristics of CuO2 intra-unit-cell orbital ordering become evident in Figs. 3 and 4: the spatial structure of δε(r) within CuO2 including the specific values at each Cu and inequivalent O site; the energy scale for these orbital-ordering phenomena near 50 meV; the statistical distribution δε(r) due to the short-correlation-length Ising domains; and the population of unit-cell-localized quadrupolar two-level systems whose energy barriers range continuously from zero. Overall, Figs. 3 and 4 show that the Q = 0 splitting between ε(r) at the two oxygen sites within a CuO2 unit cell is near 50 meV for Bi2Sr2CaCu2O8+x samples with a hole density of p ≈ 0.17, but that long-range orbital order is absent resulting in two populations of Ising nematic domains.
a, Unit-cell-averaged structure of T(r) averaged over all the regions shown in Fig. 3a where Nε > +5 meV. b, Unit-cell-averaged structure of T(r) averaged over all the regions shown in Fig. 3a where Nε < –5 meV. In both a and b, C4 symmetry is preserved. c, Unit-cell-averaged structure of δε(r) averaged over all the regions shown in Fig. 3a where Nε > +5 meV. The charge transfer energy strongly breaks C4 rotation symmetry about every Cu site (yellow dots), and consequently, there is an energy splitting of approximately 50 meV between the charge transfer energies at the two crystal-equivalent oxygen sites (indicated by the crosses). Unprocessed high-voltage dI/dV images directly reveal the subtending data (Methods and Extended Data Fig. 6). d, Unit-cell-averaged structure of δε(r) averaged over all the regions shown in Fig. 3a where Nε < –5 meV. Virtually identical phenomena as in c, but rotated by 90°. The difference between c and d is the difference in the internal structure of the CuO2 unit cell in the two distinct Ising domains of orbital order. Unprocessed high-voltage dI/dV images directly reveal the subtending data(Methods and Extended Data Fig. 6).
These data may be compared with earlier X-ray scattering studies of orbital ordering across the family of La2–xBaxCuO4 materials. In these crystals, signatures of intra-unit-cell symmetry breaking should occur as anisotropy in the scattering tensor at planar Cu sites. Here it is the intensity of the (001) scattering peak at the Cu L edge, which directly measures the electronic nematicity of the Cu 3d states within a single CuO2 plane and provides the indications of intra-unit-cell nematicity31. Figure 4c,d also shows that C4 symmetry is broken in the charge transfer electronic structure at the Cu sites (yellow dots), which—if due to Coulomb potentials inside the CuO2 unit cell—also indicates quadrupolar charge distributions surrounding the Cu sites, in agreement with other work31. With respect to other order parameters, we note that the orbitally ordered state at Q = 0 would not have any direct relation to the surface supermodulation or a perfectly harmonic charge density wave (CDW) state at finite QCDW = (2π/4a0, 0);(0, 2π/4a0) because any distinctions between Ox and Oy inside the unit cell are due to the density wave, average to 0 over one period26,27. However, higher harmonics of a CDW can, in principle, produce composite order parameters that break symmetry at Q = 0, as can ‘vestigial’ CDW states in the presence of disorder32. Another important subject is the effect of electron–lattice interactions: although the predominant theoretical motivation for such a state has been based on the repulsive Vpp potential between the oxygen atoms2,3,4,5,6,7,8,9 (Fig. 1b), it is inevitable that such orbital order will also couple to the B1g phonon mode. If this mode softens in Bi2Sr2CaCu2O8+x, the O atom elevated above the CuO2 plane experiences a different potential than its neighbour, which is depressed below it33. One surprising new revelation is the distribution of spatially localized, low-activation-energy two-level systems within the boundaries between orbitally ordered domains. These are the electronic quadrupoles within each unit cell (Fig. 4c,d) whose energy barrier between the two orientations of Nε tends to zero (Fig. 3d), making thermally activated fluctuations conceivable. Ultimately, as to the key issue of the 50 meV scale of energy splitting between Ox:Oy, its precise microscopic cause is an outstanding theoretical challenge apparently requiring realistically parameterized theory for the three-orbital model (equation (3)) within the CuO2 unit cell.
Nevertheless, important consequences do emerge from these studies. First, when physically realistic models of CuO2 electronic structure are used (for example, equation (1) with parameters relevant to real materials), Coulomb interactions between electrons at the two crystal-equivalent oxygen sites should generate intra-unit-cell rotational symmetry breaking, that is, orbital ordering2,3,4,5,6,7,8,9. By introducing sublattice-resolved ε(r) imaging techniques into CuO2 studies, this state has now been detected (Fig. 4). By visualizing an oxygen-site-specific nematic order parameter Nε(r), we reveal robust domains of C2 symmetry and with typical energy splitting of ~50 meV between the two intra-unit-cell oxygen sites (Fig. 3). Hence, a strong orbitally ordered state occurs in underdoped Bi2Sr2CaCu2O8+x at the charge transfer energy scale. Spatially, the state is arranged in Ising domains that are highly disordered. This disorder appears to be bounded in shape and size by randomly sited oxygen dopant ions. Moreover, within the domain walls, as Nε→0 (Fig. 3d), we find an ensemble of low-energy-barrier electronic quadrupolar two-level systems. Most fundamentally, the microscopic mechanism proposed theoretically2,3,4,5,6,7,8,9 for the cuprate nematic phase, that is, orbital order between oxygen orbitals at the two separate oxygen sites of CuO2, is highly consistent with the observed intra-unit-cell rotational symmetry breaking of ε(r) that splits the energy between the two oxygen atoms by ~50 meV in Bi2Sr2CaCu2O8+x.
Methods
Visualization of spatially modulating charge transfer energy
The spatially resolved charge transfer energy ε(r) may be visualized in cuprates using high-voltage23,34,35 differential tunnel conductance (g(r, V) ≡ dI/dV(r, V)) imaging of Bi2Sr2CaCu2O8+x (ref. 25). Here g(r, V) was visualized in the range of –1.6 ≤ V ≤ 2.0 V and at a very high junction resistance of RN ≈ 85 GΩ (VS = –600 mV:IS = 7 pA), which is necessary to suppress tip-induced electric-field effects. Figure 2b shows a typical example of a g(r, V) spectrum measured at T = 4.2 K (ref. 25). The edges of the filled lower band and the empty upper band can be identified from the appearance of an extremely rapid increase in the density of states. Here the value of charge transfer energy ε(r) is estimated at every location by subtracting these band edges at a constant differential conductance (G ≈ 20 pS) as follows:
Crystal supermodulation is a quasi-periodic lattice modulation along the (1, 1) direction in the crystal structure at wavevector QSM. The resulting ε(r) map for Bi2Sr2CaCu2O8+x shows strong modulations at the same wavevector, with an amplitude of ~0.3 eV, and has a spatial average value of 〈ε(r)〉 ≈ 1.2 eV, which agrees well with the charge transfer energy of Bi2Sr2CaCu2O8+x, as independently measured using a variety of different experimental techniques24,36,37. The analysis of the supermodulation influence on ε(r) and the corresponding supermodulation influence on the electron-pair density np(r) measured by scanned Josephson tunnelling microscopy have revealed25 that the charge transfer energy ε(r) controls the density of condensed electron pairs np(r) as \({\rm{d}}{\bar{n}}_{{{p}}}/{\rm{d}}{\varepsilon }\) ≈ –0.81 ± 0.17 eV–1.
Extended Data Fig. 1 compares the images of charge transfer energy ε(r) derived from the simple algorithm in equation (4) and the more general algorithm for charge transfer energy variation δε(r) used in the main text. The images of ε and δε bear high similarities in real space and reciprocal space. Their linecuts show identical and strong symmetry breaking at the Bragg peaks where the ratio of the intensity of the Bragg peak in the y direction to the Bragg peak in the x direction is \(\frac{{{\bf{Q}}}_{y}}{{{\bf{Q}}}_{x}}=1.9\). Thus, our general algorithm for calculating the charge transfer energy variation δε(r) throughout this paper is consistent with the earlier simple algorithm25 for calculating ε(r). From Extended Data Fig. 1d,e, the δε(r) histogram features a narrower σ and therefore yields a higher signal-to-noise ratio. Hence, we implement our algorithm to measure the charge transfer energy variation δε(r) throughout.
Experimental evidence of Q = 0 rotational symmetry breaking in cuprates
The existence of a nematic state in cuprates has been extensively demonstrated on the basis of multiple experimental techniques (Fig. 1a). Neutron scattering, particularly with polarized neutrons, is extensively used to detect anisotropy at Bragg peaks, indicating intra-unit-cell (IUC) symmetry breaking38,39,40,41,42,43. Angle-resolved photoemission spectroscopy44,45,46 shows that above T*, left- and right-circularly polarized light have the same intensity at the mirror plane, but when cooled below T*, a difference in intensities of the left- and right-circularly polarized light at the mirror plane emerges, which is a signature of Q = 0 rotational symmetry breaking. Torque magnetometry studies47,48 have reported a kink of the in-plane anisotropy of susceptibility at T*, which also relates to rotational symmetry breaking. Optical anisotropy49 measurements show a substantial change in the second harmonic of the optical response near T*, whereas the linear response remains unchanged in the same region. This could be explained if bulk inversion symmetry was broken; alongside this, nematicity is also seen. In polarization-resolved Raman scattering50, the suppression of susceptibility near T* is observed. Elastoresistance measurements51 of in-plane anisotropy that onsets near T* also indicate a nematic state. Resonant ultrasound spectroscopy52 finds a phase transition at T* by noting discontinuities in the frequencies and widths of the vibrational normal modes of a crystal. Scanning tunnelling microscopy experiments53 have detected the intra-unit-cell rotational symmetry breaking in the low-energy density of states, and this nematicity exhibits Ising domains that diminish in size and intensity approaching the pseudogap endpoint. In La1.875Ba0.125CuO4 (refs. 31,54), La1.65Eu0.2Sr0.15CuO4 (refs. 31,55) and La1.6–xNd0.4SrxCuO4 (refs. 31,55,56), resonant soft-X-ray scattering also reveals a nematic phase. This electronic phase transition is explored using resonant X-ray scattering, particularly measuring the [001] Bragg peak. The intensity of the Bragg peak is proportional to the electronic nematic order parameter that is associated with the symmetry breaking at the Cu d state. When the photon energies sensitive to Cu are used, the emerging [001] intensity reveals a nematic phase. Importantly, a nematic phase is reported across numerous cuprate families, including YBa2Cu3O7–x (refs. 38,39,42,43,47,49,52), Bi2Sr2CaCu2O8+x (refs. 44,46,50,51,53), Bi2Sr2CuO6+x (ref. 45), Bi2–zPbzSr2–yLayCuO6+x (ref. 57), HgBa2CuO4+δ (refs. 40,41,48) and La1.875Ba0.125CuO4 (refs. 31,54,56). But its microscopic mechanism was unknown.
Visualization of charge transfer energy variations
The averaged differential conductance g(V) is shown as a dashed curve in Extended Data Fig. 1f,g. The variations in charge transfer energy are determined from the deviation of a point spectrum from the averaged spectrum. The states on the V > 0 side have a positive integral \({I}_{+}\left({\bf{r}}\right)={\int }_{0}^{{V}_{\max }}g(V,{\bf{r}}){{\rm{d}}V}\), where the maximum energy is Vmax = 2 V. The states on the V < 0 side have a negative integral \({I}_{-}\left({\bf{r}}\right)={\int }_{{V}_{\min }}^{0}g(V,{\bf{r}})\) where the minimum energy is Vmin = –1.6 V. The variation in each integral I+(r) and I–(r) from the average values \(\bar{{I}_{+}}\) and \(\bar{{I}_{\pm }}\), respectively, occurs due to the variation in energy separation δε(r) between the lower and upper bands from its average value. To efficiently evaluate the energy splitting between the point spectrum and averaged spectrum, the integral difference is normalized by the difference between the maximum differential conductance gmax and the minimum difference conductance gmin. Here gmax = 0.220 nS is given by the maximum of the FOV-averaged spectrum and gmin = 0.009 nS is the minimum differential conductance of the averaged spectrum.
Hence, we define
There are the two typical cases shown in Extended Data Fig. 1f,g.
LF symmetrization of charge transfer energy images
To correct picometre distortions due to piezo-electronic drift in scanning, we apply the affine transformations of the LF algorithm26. This is the key for visualizing the intra-unit-cell electronic structure. To obtain this goal, the dataset with atomic resolution and the dataset with high voltage (–1.6 to 2.0 V) must be registered with atomic accuracy. The two datasets are measured in the same FOV. The topographs of the two datasets, namely, T1′(r) in the atomic-resolution dataset (Extended Data Fig. 2a) and T2′(r) in the high-voltage dataset (Extended Data Fig. 2b), are used to register identical atoms in one dataset with those in the other. LF is applied to produce the corrected images of both topographs. Subsequently, both topographs are registered by spatial translations, whose accuracy is evaluated by their cross-correlation image. T1(r) in Extended Data Fig. 2e and T2(r) in Extended Data Fig. 2f are now registered. All the transformation parameters applied to T2′(r) are subsequently applied to the high-voltage differential conductance map g′(r, V) that is simultaneously measured with the topography. The differential conductance map g(r, V) is registered.
Extended Data Fig. 2a,b shows two topographs of a Bi2Sr2CaCu2O8+x surface taken with atomic resolution and high voltage, respectively. They are LF corrected and registered with atomic resolution (Fig. 2e,f). The precision of the image registration is shown in Extended Data Fig. 2f (inset). The maximum of the cross-correlation between T1(r) and T2(r) coincides with the (0, 0) cross-correlation vector (inset of Extended Data Fig. 2f). The offset of the two registered images are within three pixels, meaning that the precision of registration is better than 80 pm everywhere in the whole FOV.
To demonstrate that the evidence of rotational symmetry breaking is present in the unprocessed charge transfer energy variation δε′(r), the unprocessed data of δε′(r) (Extended Data Fig. 2c,d) and processed δε(r) (Extended Data Fig. 2g,h) are compared. δε′(r) is calculated from the unprocessed dataset and δε(r) is calculated from the LF-corrected and registered dataset. The Fourier transform δε′(q) of the unprocessed data shows anisotropy at the Bragg peaks. The processed data δε(q) shows the same anisotropic Bragg peaks and the background noise is much lower in the drift-corrected data than in the unprocessed data. Thus, the LF algorithm does not alter the conclusion that C4 symmetry is broken in δε.
Intra-unit-cell charge transfer energy imaging: repeatability and reliability
Here we first compare the result of multiple experiments to evaluate the repeatability of the phenomena reported. First, the Fourier analysis was repeated on three different FOVs of the same sample (Extended Data Figs. 3–5). The topograph of each FOV is shown in Extended Data Fig. 3a–5a. The T(r) value is an atomically resolved topograph that is registered with the differential conductance map g(r, V). The PSD Fourier transform of the topograph is then shown in Extended Data Figs. 3b–5b. The Bragg peaks Qx and Qy are of similar magnitude in the topograph; therefore, C4 symmetry is preserved, indicating that neither the lattice nor the scanning tip produces IUC symmetry breaking. The red linecut of T(q) is taken from q = (0, 0) to (1, 0)2π/a0 and the blue linecut is taken from q = (0, 0) to (0, 1)2π/a0 (Extended Data Figs. 3c–5c). These linecuts quantitatively and clearly show that Qx ≈ Qy in T(q) of all the FOVs. This confirms that the tip and lattice preserve C4 symmetry.
Extended Data Figs. 3d–5d show the charge transfer energy variations δε(r). All the FOVs show high levels of disorder, and the same energy ranges from –300 to +200 meV. Next, δε(q), the PSD Fourier transform of charge transfer energy variation, is shown in Extended Data Figs. 3e–5e. In all the FOVs, it is clear that the Bragg peaks at (0, 1)2π/a0 are more intense than the peaks at (1, 0)2π/a0, meaning that IUC C4 rotational symmetry is broken. Linecuts of δε(q), taken from q = (0, 0) to the Bragg peaks, show clear anisotropy between the intensities of Bragg peaks in all the FOVs (Extended Data Figs. 3f–5f). Therefore, IUC rotational symmetry is broken in δε. The histograms of δε are asymmetric and the peak of the histogram is shifted from 0 in all the FOVs (Extended Data Figs. 3f–5f, inset), which is consistent with the anisotropy in charge transfer energy variations δε.
Collectively, the anisotropy in Bragg peaks of the charge transfer energy variations δε is repeatable in multiple experiments at the same hole density. The δε values from independent FOVs show similar statistics. The nematicity is not generated by the crystallography or the scanning tip. Therefore, the Fourier analysis of δε appears robust and reliable.
Second, the oxygen-site-specific imaging Nε(r) of the charge transfer are repeated for the three FOVs shown in Extended Data Figs. 3g–5g. This focuses attention on the charge transfer symmetry breaking occurring on the planar oxygen sites. The three FOVs show disordered Ising domains. The relative strength between the two domains is defined as the area ratio Ared/Ablue, which is approximately 2.1 ± 0.2. This is consistent with the relative strength between the two Bragg peaks in the PSD Fourier transform of δε, that is, \(\frac{\delta {\varepsilon }\left({{\bf{Q}}}_{y}\right)}{\delta {\varepsilon }\left({{\bf{Q}}}_{x}\right)}\) ≈ 1.9 ± 0.3 in Extended Data Figs. 3f–5f. This result demonstrates that the relative preponderance of the two orbitally ordered domains is approximately 2:1 in the 17% hole-doped sample. The histograms of Nε(r) in the inset of these figures are asymmetric and the peak of the histogram is shifted from Nε = 0. This observation is consistent with the data in Extended Data Figs. 3g–5g that the preponderance of the Nε(r) > 0 domain is stronger than the Nε(r) < 0 domain. The r.m.s. value of the IUC energy splitting between the Ox and Oy sites ranges from 20 to 30 meV.
The microscopic structure inside the Ising domains is visualized from the unit-cell-averaged δε(r) images of each domain (Extended Data Figs. 3h,i–5h,i). The unit-cell-averaged δεα(r) from the Nε(r) > 5 meV region is indicated in Extended Data Figs. 3h–5h, and the unit-cell-averaged δεβ(r) from the Nε(r) < –5 meV is indicated in Extended Data Figs. 3i–5i. Clearly, the charge transfer energy variations break the IUC rotational symmetry. Because the number of unit cells in the Nε(r) > 5 meV domain is almost twice that in the other domain, the signal-to-noise ratio is higher in unit-cell averaging than that in \(\delta {{\varepsilon }}_{B}^{R}({\bf{r}})\). The unit-cell-averaged images of the topograph T(r) serve (Extended Data Figs. 3h,i–5h,i (insets)) as a reference of δεα(r) and δεβ(r). The tip preserves IUC rotational symmetry and the crystallography of the CuO2 unit cell does not break rotational symmetry.
Third, from the unprocessed data (Extended Data Fig. 6), we directly demonstrate that the rotational symmetry breaking reported here is a basic property of the charge transfer gap. Three representative examples of this from three different but perfectly typical FOVs are presented in Extended Data Fig. 6. These same phenomena are omnipresent throughout the FOVs in the experiment, meaning that orbital ordering is universal.
We show the topography, the orbital order parameter Nε(r) and 20 unprocessed dI/dV point spectra from each FOV. The first column in Extended Data Fig. 6 is the topographs showing the FOVs where the charge transfer measurements are taken. The locations of five CuO2 unit cells consisting of ten different oxygen sites are highlighted in this figure (blue and red dots). The second column in Extended Data Fig. 6 shows the nematic domains Nε(r) in this FOV. The third column in Extended Data Fig. 6 shows domain 1 (red), where the Ox spectra are shifted in the range from almost −50 to −30 meV with respect to the Oy spectra inside the same unit cell. The charge transfer gap on the Ox sites (red) is higher than the gap on the Oy site (blue) in this domain. Domain 2 (blue) shows that the Oy spectra are shifted in the range from almost −50 to −30 meV with respect to the Ox spectra (Extended Data Fig. 6, fourth column), which means that the Oy sites have higher charge transfer energy than the Ox sites in this domain. The two domains are separated by a domain wall. Such domains are observed throughout the unprocessed data, showing the intra-unit-cell symmetry breaking and Ising domains of orbital ordering.
Dopant-oxygen-ion pinning of orbitally ordered domains
To explore the causes for obvious heterogeneity in the orbitally ordered domains, we search for the dopant oxygen ions and their relationship with the domains. Each dopant oxygen ion is identified as a maximum located at –900 meV in the differential conductance spectrum (Extended Data Fig. 7a, inset). A total of ~70 oxygen dopants are found in the differential conductance map g(r, –900 meV) with an image size of 17 × 17 nm2 (Extended Data Fig. 7a). The locations of the oxygen dopant are overlaid onto the simultaneously measured IUC oxygen-specific nematic order parameter Nε(r) (Extended Data Fig. 7d); visually, we see that the oxygen dopants are near the Nε domain walls (yellow contours).
The distance ddopant between each oxygen dopant and their nearest location on the domain walls are calculated and a distribution is created (Extended Data Fig. 7g). To validate that the oxygen dopants are located near the Nε domain walls, the distribution of ddopant is compared with the distance drandom between randomly generated points and the domain walls. The random points are generated from a two-dimensional Poisson-disc sampling function and the random points are separated from each other. The random points have no spatial correlation with the orbitally ordered domains. The expected averaged distance drandom to the domain walls is calculated and compared with ddopant (Extended Data Fig. 7g).
The distribution of ddopant is different from drandom with regard to two aspects. Although the ddopant distribution has a sharp peak at 1.6 Å, the drandom distribution has a blunt plateau. The deviation in the distance distribution clearly indicates that the oxygen dopants are located near the domain walls of the orbitally ordered domains, providing statistical evidence that they are pinning the nematic domains.
We repeated the above statistical analysis for three independent FOVs (Extended Data Fig. 7a–c). The data in Extended Data Fig. 7f are also presented in Fig. 3e. The sum of the three histograms (Extended Data Fig. 7g–i) is presented in Fig. 3f. A total of 237 oxygen dopants are studied in the total histogram.
Visualizing intra-unit-cell charge transfer order parameter
The unit cells inside one Ising domain are averaged to increase the signal-to-noise ratio of the microstructure of the IUC symmetry breaking order58. First, each CuO2 unit cell is identified in the topography T(r) (Extended Data Fig. 8a, grey grid). Each unit cell has a size of 10 × 10 pixels2 and is labelled as I(a, b, n). The image series I(a, b, n) is categorized into two datasets, namely, I1(a, b, n) and I2(a, b, n). Here I1 is inside a positive domain with Nε(r) > 5 meV and I2 is inside a negative domain with Nε(r) < –5 meV (Extended Data Fig. 8b). The images I1,2(a, b, n) have lateral dimensions x and y and a counting index n. T(r) has already been atomically registered and the lattice displacements have been corrected using the LF algorithm, making each lattice square and periodic. The image set of I1,2(a, b, n) comprises n images of I1,2(a, b). The images are independently cropped from T(r), where each image has a size of 11 × 11 pixels2 (5.9 × 5.9 Å2) and corresponds to one CuO2 unit cell rotated by 45°. The single CuO2 unit cell is defined by the location of Bi/Cu atoms (Extended Data Fig. 8).
Orbitally ordered domains are unaffected by supermodulation
To further illustrate the lack of effect that supermodulation has on Q = 0 orbital ordering, we have compared the topographs and orbitally ordered domains obtained with and without supermodulation from three independent FOVs (Extended Data Fig. 9). The first column in Extended Data Fig. 9 shows the topographs where the supermodulation is removed via Fourier filtering at the QSM peak. Their orbitally ordered domains are presented in the third column (Extended Data Fig. 9). The second column in Extended Data Fig. 9 presents the topographs where supermodulation is retained in the analysis. Although the atomic structure is strongly modulated by supermodulation, the orbitally ordered domains in the fourth column (Extended Data Fig. 9) remain virtually the same as the orbitally ordered domains where the QSM peak is filtered, as shown in the third column.
Data availability
The data shown in the main figures are available via Zenodo at https://doi.org/10.5281/zenodo.10510971. Source data are provided with this paper.
Code availability
The code is available via Zenodo at https://doi.org/10.5281/zenodo.10515736.
References
Fradkin, E., Kivelson, S. A. & Tranquada, J. M. Colloquium: theory of intertwined orders in high temperature superconductors. Rev. Mod. Phys. 87, 457–482 (2015).
Kivelson, S. A., Fradkin, E. & Geballe, T. H. Quasi-one-dimensional dynamics and nematic phases in the two-dimensional Emery model. Phys. Rev. B 69, 144505 (2004).
Fischer, M. H. & Kim, E. A. Mean-field analysis of intra-unit-cell order in the Emery model of the CuO2 plane. Phys. Rev. B 84, 144502 (2011).
Bulut, S., Atkinson, W. A. & Kampf, A. P. Spatially modulated electronic nematicity in the three-band model of cuprate superconductors. Phys. Rev. B 88, 155132 (2013).
Fischer, M. H., Wu, S., Lawler, M., Paramekanti, A. & Kim, E. A. Nematic and spin-charge orders driven by hole-doping a charge-transfer insulator. New J. Phys. 16, 093057 (2014).
Maier, T. A. & Scalapino, D. J. Pairing interaction near a nematic quantum critical point of a three-band CuO2 model. Phys. Rev. B 90, 174510 (2014).
Tsuchiizu, M., Kawaguchi, K., Yamakawa, Y. & Kontani, H. Multistage electronic nematic transitions in cuprate superconductors: a functional-renormalization-group analysis. Phys. Rev. B 97, 165131 (2018).
Chiciak, A., Vitali, E., Shi, H. & Zhang, S. Magnetic orders in the hole-doped three-band Hubbard model: spin spirals, nematicity, and ferromagnetic domain walls. Phys. Rev. B 97, 235127 (2018).
Yamase, H. Theoretical insights into electronic nematic order, bond-charge orders, and plasmons in cuprate superconductors. J. Phys. Soc. Jpn 90, 111011 (2021).
Krüger, F., Kumar, S., Zaanen, J. & Van Den Brink, J. Spin-orbital frustrations and anomalous metallic state in iron-pnictide superconductors. Phys. Rev. B 79, 054504 (2009).
Lv, W., Wu, J. & Phillips, P. Orbital ordering induces structural phase transition and the resistivity anomaly in iron pnictides. Phys. Rev. B 80, 224506 (2009).
Lee, C. C., Yin, W. G. & Ku, W. Ferro-orbital order and strong magnetic anisotropy in the parent compounds of iron-pnictide superconductors. Phys. Rev. Lett. 103, 267001 (2009).
Fernandes, R. M., Chubukov, A. V. & Schmalian, J. What drives nematic order in iron-based superconductors? Nat. Phys. 10, 97–104 (2014).
Hirschfeld, P. J., Korshunov, M. M. & Mazin, I. I. Gap symmetry and structure of Fe-based superconductors. Rep. Prog. Phys. 74, 124508 (2011).
Sprau, P. O. et al. Discovery of orbital-selective Cooper pairing in FeSe. Science 357, 75–80 (2017).
Yi, M., Zhang, Y., Shen, Z. X. & Lu, D. Role of the orbital degree of freedom in iron-based superconductors. npj Quantum Mater. 2, 57 (2017).
Fernandes, R. M. et al. Iron pnictides and chalcogenides: a new paradigm for superconductivity. Nature 601, 35–44 (2022).
Emery, V. J. Theory of high-Tc superconductivity in oxides. Phys. Rev. Lett. 58, 2794–2797 (1987).
Littlewood, P. B., Varma, C. M. & Abrahams, E. Pairing instabilities of the extended Hubbard model for Cu-O based superconductors. Phys. Rev. Lett. 63, 2602–2605 (1989).
Tallon, J. L. & Loram, J. W. The doping dependence of T*—what is the real high-Tc phase diagram? Physica C 349, 53–68 (2001).
Norman, M. R., Pines, D. & Kallin, C. The pseudogap: friend or foe of high Tc? Adv. Phys. 54, 715–733 (2005).
Fradkin, E., Kivelson, S. A., Lawler, M. J., Eisenstein, J. P. & Mackenzie, A. P. Nematic Fermi fluids in condensed matter physics. Annu. Rev. Condens. Matter Phys. 1, 153–178 (2010).
Cai, P. et al. Visualizing the evolution from the Mott insulator to a charge-ordered insulator in lightly doped cuprates. Nat. Phys. 12, 1047–1051 (2016).
Ruan, W. et al. Relationship between the parent charge transfer gap and maximum transition temperature in cuprates. Sci. Bull. 61, 1826–1832 (2016).
Mahony, S. M. O. et al. On the electron pairing mechanism of copper-oxide high temperature superconductivity. Proc. Natl Acad. Sci. USA 119, e2207449119 (2022).
Lawler, M. J. et al. Intra-unit-cell electronic nematicity of the high-Tc copper-oxide pseudogap states. Nature 466, 347–351 (2010).
Fujita, K. et al. Direct phase-sensitive identification of a d-form factor density wave in underdoped cuprates. Proc. Natl Acad. Sci. USA 111, E3026–E3032 (2014).
Hamidian, M. H. et al. Atomic-scale electronic structure of the cuprate d-symmetry form factor density wave state. Nat. Phys. 12, 150–156 (2016).
Mesaros, A. et al. Topological defects coupling smectic modulations to intra-unit-cell nematicity in cuprates. Science 333, 426–430 (2011).
Mukhopadhyay, S., Sharma, R., Koo, C., Edkins, S. D. & Hamidian, M. H. Evidence for a vestigial nematic state in the cuprate pseudogap phase. Proc. Natl Acad. Sci. USA 116, 13249–13254 (2019).
Achkar, A. J. et al. Nematicity in stripe-ordered cuprates probed via resonant X-ray scattering. Science 351, 576–578 (2016).
Nie, L., Tarjus, G. & Kivelson, S. A. Quenched disorder and vestigial nematicity in the pseudogap regime of the cuprates. Proc. Natl Acad. Sci. USA 111, 7980–7985 (2014).
Banerjee, S., Atkinson, W. A. & Kampf, A. P. Emergent charge order from correlated electron-phonon physics in cuprates. Commun. Phys. 3, 161 (2020).
McElroy, K. et al. Atomic-scale sources and mechanism of nanoscale electronic disorder in Bi2Sr2CaCu2O8+δ. Science 309, 1048–1052 (2005).
Kohsaka, Y. et al. Visualization of the emergence of the pseudogap state and the evolution to superconductivity in a lightly hole-doped Mott insulator. Nat. Phys. 8, 534–538 (2012).
Yang, S. et al. Revealing the Coulomb interaction strength in a cuprate superconductor. Phys. Rev. B 96, 245112 (2017).
Itoh, T., Fueki, K., Tanaka, Y. & Lhara, H. Optical conductivity spectra and electronic structure of Bi2Sr2(Y1–xCax)Cu2Oy system. J. Phys. Chem. Solids 60, 41–51 (1999).
Fauqué, B. et al. Magnetic order in the pseudogap phase of high-Tc superconductors. Phys. Rev. Lett. 96, 197001 (2006).
Mook, H. A., Sidis, Y., Fauqué, B., Balédent, V. & Bourges, P. Observation of magnetic order in a superconducting YBa2Cu3O6.6 single crystal using polarized neutron scattering. Phys. Rev. B 78, 020506 (2008).
Li, Y. et al. Unusual magnetic order in the pseudogap region of the superconductor HgBa2CuO4+δ. Nature 455, 372–375 (2008).
Li, Y. et al. Magnetic order in the pseudogap phase of HgBa2CuO4+δ studied by spin-polarized neutron diffraction. Phys. Rev. B 84, 224508 (2011).
Mangin-Thro, L., Sidis, Y., Wildes, A. & Bourges, P. Intra-unit-cell magnetic correlations near optimal doping in YBa2Cu3O6.85. Nat. Commun. 6, 7705 (2015).
Mangin-Thro, L., Li, Y., Sidis, Y. & Bourges, P. a-b anisotropy of the intra-unit-cell magnetic order in YBa2Cu3O6.6. Phys. Rev. Lett. 118, 097003 (2017).
Kaminski, A. et al. Spontaneous breaking of time-reversal symmetry in the pseudogap state of a high-Tc superconductor. Nature 416, 610–613 (2002).
He, R. H. et al. From a single-band metal to a high-temperature superconductor via two thermal phase transitions. Science 331, 1579–1583 (2011).
Nakata, S. et al. Nematicity in a cuprate superconductor revealed by angle-resolved photoemission spectroscopy under uniaxial strain. npj Quantum Mater. 6, 86 (2021).
Sato, Y. et al. Thermodynamic evidence for a nematic phase transition at the onset of the pseudogap in YBa2Cu3Oy. Nat. Phys. 13, 1074–1078 (2017).
Murayama, H. et al. Diagonal nematicity in the pseudogap phase of HgBa2CuO4+δ. Nat. Commun. 10, 3282 (2019).
Zhao, L. et al. A global inversion-symmetry-broken phase inside the pseudogap region of YBa2Cu3Oy. Nat. Phys. 13, 250–254 (2017).
Auvray, N. et al. Nematic fluctuations in the cuprate superconductor Bi2Sr2CaCu2O8+δ. Nat. Commun. 10, 5209 (2019).
Ishida, K. et al. Divergent nematic susceptibility near the pseudogap critical point in a cuprate superconductor. J. Phys. Soc. Jpn 89, 064707 (2020).
Shekhter, A. et al. Bounding the pseudogap with a line of phase transitions in YBa2Cu3O6+δ. Nature 498, 75–77 (2013).
Fujita, K. et al. Simultaneous transitions in cuprate momentum-space topology and electronic symmetry breaking. Science 344, 612–616 (2014).
Achkar, A. J. et al. Orbital symmetry of charge-density-wave order in La1.875 Ba0.125CuO4 and YBa2Cu3O6.67. Nat. Mater. 15, 616–620 (2016).
Gupta, N. K. et al. Vanishing nematic order beyond the pseudogap phase in overdoped cuprate superconductors. Proc. Natl Acad. Sci. USA 118, e2106881118 (2021).
Achkar, A. J. et al. Resonant X-ray scattering measurements of a spatial modulation of the Cu 3d and O 2p energies in stripe-ordered cuprate superconductors. Phys. Rev. Lett. 110, 017001 (2013).
Song, C.-L. et al. Critical nematic correlations throughout the doping range in Bi2−zPbzSr2−yLayCuO6+x. Nat. Commun. 14, 2622 (2023).
Jones, L., Wang, S., Hu, X., ur Rahman, S. & Castell, M. R. Maximising the resolving power of the scanning tunneling microscope. Adv. Struct. Chem. Imaging 4, 7 (2018).
Acknowledgements
We acknowledge and thank E. A. Kim, S. A. Kivelson, M. J. Lawler, P. Bourges, D. Bounoua, V. Madhavan and P. Z. Mai for helpful discussions. K.F. and J.C.S.D. acknowledge support from the Moore Foundation’s EPiQS Initiative through Grant GBMF9457. S.W. and J.C.S.D. acknowledge support from the European Research Council (ERC) under award DLV-788932. J.C.S.D. acknowledges support from the Royal Society under award R64897. N.K., J.C.S.D. and S.M.O. acknowledge support from the Science Foundation Ireland under award SFI 17/RP/5445. S.W. acknowledges support from the John Fell Fund at the University of Oxford under project 0010827. K.F. acknowledges support from the Office of Basic Energy Sciences, Materials Sciences and Engineering Division, US Department of Energy, under contract no. DE-SC0012704. H.E. acknowledges support from JSPS KAKENHI (no. JP19H05823). P.D.J. acknowledges support by QuantEmX grant GBMF9616 from ICAM/Moore Foundation and by a Visiting Fellowship at Wadham College, Oxford, UK. This research programme was also advanced through support from the National Science Foundation under grant no. NSF PHY-1748958 at the Kavli Institute for Theoretical Physics of University of California, Santa Barbara. S.W. and J.C.S.D. thank KITP for its hospitality.
Author information
Authors and Affiliations
Contributions
S.W., N.K. and J.C.S.D. conceived the project. H.E. and S.U. synthesized and characterized the samples. K.F. and J.C.S.D. carried out the experimental measurements. S.W., N.K., K.F. and S.M.O. developed and carried out the comprehensive analysis. P.D.J., J.C.S.D. and S.M.O. supervised the research and wrote the paper with key contributions from S.W. and N.K. The manuscript reflects the contributions and ideas of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Berthold Jäck, Luca de’ Medici and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Validity of the algorithm to measure the charge transfer energy variations δε(r).
(a) Image of the charge transfer energy \({\varepsilon }\left({\boldsymbol{r}}\right)\) calculated from Eq. 4. The supermodulation (~150 meV) wavevector \({{\boldsymbol{Q}}}_{{\rm{SM}}}\) is masked in (a) such that the intra-unit-cell features (~50 meV) are revealed. (b) Fourier transform of \({\varepsilon }\left({\boldsymbol{r}}\right)\). (c). Linecut from (0,0) to the Bragg peaks in \({\varepsilon }\left({\boldsymbol{r}}\right)\), where the ratio of the Bragg peaks intensity \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.9\) indicates IUC symmetry breaking in charge transfer energy \({\varepsilon }\left({\boldsymbol{r}}\right)\). (d) Statistical distribution of \({\varepsilon }\left({\boldsymbol{r}}\right)\). (e) Statistical distribution of \(\delta {\varepsilon }\left({\boldsymbol{r}}\right)\). The narrower distribution in \(\delta {\varepsilon }\left({\boldsymbol{r}}\right)\) demonstrates an improvement in signal-to-noise ratio over the \({\varepsilon }\left({\boldsymbol{r}}\right)\) algorithm. The histogram of \(\delta {\varepsilon }\left({\boldsymbol{r}}\right)\) has a narrower σ than the histogram of \({\varepsilon }\left({\boldsymbol{r}}\right)\), demonstrating the signal-to-noise ratio is higher in the algorithm used here to measure the variations in charge transfer energy \(\delta {\varepsilon }\left({\boldsymbol{r}}\right)\). Thus this \(\delta {\varepsilon }\left({\boldsymbol{r}}\right)\) algorithm with higher SNR was used throughout this paper. (f-g) Typical examples of the splitting of the charge transfer energy. (f) shows a case when the energy variation is positive, \(\delta {\varepsilon } > 0\). (g) shows a case when the energy variation is negative, \(\delta {\varepsilon } < 0\).
Extended Data Fig. 2 Comparison between unprocessed data and Lawler-Fujita corrected data of Bi2Sr2CaCu2O8+x.
(a & b) Show two unprocessed low and high voltage topographs in the same field of view. The low-voltage topograph T'1(r) (a) has atomic resolution and it allows Bi atoms to be identified (RN - 30 GΩ, VS = −750 mV). The high voltage topograph T'2(r) in (b) was acquired simultaneously with the electronic structure \(g({\boldsymbol{r}},V)\) (RN - 85 GΩ, VS = 600 mV). The insets show the broad Bragg peaks of its topo. (c) is an unprocessed map of the charge transfer energy \(\delta {\varepsilon}^{\prime} \left({\boldsymbol{r}}\right)\) and (d) is the corresponding PSD Fourier transform. \(\delta {\varepsilon}^{\prime} \left({\boldsymbol{q}}\right)\) shows anisotropy between its Bragg peaks, which are marked by dashed circles, with \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=\) 2.0. (e & f) show the same topographs after Lawler-Fujita correction. Insets show sharp Bragg peaks indicating that piezo drift has been corrected. Inset: Zoom-in image (center) of the cross-correlation between \({T}_{1}\left({\boldsymbol{r}}\right)\) and \({T}_{2}\left({\boldsymbol{r}}\right)\). The maximum (annotated by the cross) has a width of 3 pixels. The registration precision is better than 80 pm (equivalent to 1.5 pixels). (g) is \(\delta {\varepsilon }\left({\boldsymbol{r}}\right)\) after Lawler-Fujita correction for piezo-drift and (h) the corresponding PSD Fourier transform. The Bragg peaks are single pixel and IUC symmetry breaking remains present at the Bragg peaks with \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.9.\) The disorder at \({\boldsymbol{q}}=\) 0 has been noticeably reduced.
Extended Data Fig. 3 Fourier analysis of charge-transfer energy variations map δε(r) in another FOV.
(a) Second FOV of Bi2Sr2CaCu2O8+x surface topography, T(r). (b) T(q), the Fourier transform PSD of (a). (c) Linecuts of \(T\left({\boldsymbol{q}}\right)\) in Qx (red) and Qy (blue) directions show the ratio of the transverse averaged intensity at the Bragg peaks is \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.3\). (d) Second FOV of charge transfer energy variations, δε(r). (e) δε(q), the Fourier transform PSD of (d). (f) Linecuts of \(\delta {\varepsilon }({\boldsymbol{q}})\) show \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=2.3\) and \({\delta {\varepsilon }}_{{RMS}}=95\) meV. Thus the charge transfer energy variations break rotational symmetry. (g) Nematicity analysis of the same FOV in (a). Image of oxygen-site-specific nematic order parameter \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\). The area ratio \({A}_{{red}}/{A}_{{blue}}\) is 1.8. The largest domain size is ~29 nm2. Inset: histogram of \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\) whose RMS is ~28 meV. (h-i) Unit cell averaged structure of charge transfer energy variations \(\delta {\varepsilon }({\boldsymbol{r}})\) from two Ising domains. Inset: unit cell averaged structure of \(T\left({\boldsymbol{r}}\right)\) of each Ising domain. The yellow circles represent the location of the Cu sites.
Extended Data Fig. 4 Fourier analysis of charge transfer energy variations map δε(r) in another FOV.
(a) Third FOV of Bi2Sr2CaCu2O8+x surface topography, T(r). (b) T(q), the Fourier transform PSD of (a). (c) Linecuts of \(T({\boldsymbol{q}})\) show the ratio of the transverse averaged intensity at the Bragg peaks is \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.3\). (d) Third FOV of charge transfer energy variations, δε(r). (e) δε(q), the Fourier transform PSD of (d). (f) Linecuts of \(\delta {\varepsilon }({\boldsymbol{q}})\) show the intensity ratio of \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.7\) and \({\delta {\varepsilon }}_{{RMS}}\) \({\rm{is}}\) \(\sim 129\) meV. Thus the charge transfer energy variations break rotational symmetry. (g) Nematicity analysis of the same FOV in (a). Image of oxygen-site-specific nematic order parameter \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\). The area ratio \({A}_{{red}}/{A}_{{blue}}\) is 2.3. The largest domain size is ~33 nm2. Inset: histogram of \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\) whose RMS is ~34 meV. (h-i) Unit cell averaged structure of charge transfer energy variations \(\delta {\varepsilon }({\boldsymbol{r}})\) from two Ising domains. Inset: unit cell averaged structure of \(T\left({\boldsymbol{r}}\right)\) from each Ising domain. The yellow circles represent the location of the Cu sites.
Extended Data Fig. 5 Fourier analysis of charge-transfer energy variations map δε(r) in another FOV.
(a) Fourth FOV of Bi2Sr2CaCu2O8+x surface topography, T(r). (b) T(q), the Fourier transform PSD of (a). (c) Linecuts of \(T({\boldsymbol{q}})\) show the ratio of the transverse averaged intensity at the Bragg peaks is \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.3\). (d) Fourth FOV of charge transfer energy variations, δε(r). (e) δε(q), the Fourier transform PSD of (d). (f) \(\delta {\varepsilon }({\boldsymbol{q}})\) shows \(\frac{{{\boldsymbol{Q}}}_{y}}{{{\boldsymbol{Q}}}_{x}}=1.8\) and \({\delta {\varepsilon }}_{{RMS}}\approx 105\) meV. Thus the charge transfer energy variations break rotational symmetry. (g) Nematicity analysis of the same FOV in (a). Image of oxygen-site-specific nematic order parameter \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\). The area ratio \({A}_{{red}}/{A}_{{blue}}\) is 2.2. The largest domain size is ~23 nm2. Inset: histogram of \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\) whose RMS is ~26 meV. (h-i) Unit cell averaged structure of charge transfer energy variations \(\delta {\varepsilon }({\boldsymbol{r}})\). Inset: unit cell averaged structure of \(T\left({\boldsymbol{r}}\right)\) from the corresponding Ising domain. The yellow circles represent the location of the Cu sites.
Extended Data Fig. 6 Intra-unit-cell splitting of unprocessed differential conductance spectra.
(a, e, i) First column: Topograph of the FOV where the spectra are measured. (b, f, j) Second column: Image of nematic intra-unit-cell order parameter \({N}_{\varepsilon }\left({\boldsymbol{r}}\right)\) sampled on oxygen sites. Two Ising domains are labelled. (c, g, k) Third column: 10 dI/dV point spectra sampled on the oxygen sites from Domain 1 in (b). Clearly these point spectra show the Ox spectra are shifted by -50 meV ~ -30 meV with respect to their intra-unit-cell Oy spectra. The locations of the Ox and Oy sites are shown as circles in (a-b). (d, h, l) Fourth column: 10 point spectra sampled on the oxygen sites from Domain 2 reveal that the Oy spectra are shifted by -50 meV ~ -30 meV with respect to the Ox spectra from the same unit cell. The locations of the unit cell and oxygen sites are shown in circles in (a-b). The dI/dV splitting to opposite directions in the two different domains, proves charge transfer energy splitting occurs in unprocessed differential conductance spectra.
Extended Data Fig. 7 Statistical validation of dopant oxygen ion pinning of orbital ordered domains.
(a–c) Three independent differential conductance maps \(g\left({\boldsymbol{r}},-900{\rm{meV}}\right)\) where the locations of the oxygen dopants are identified as black circles. Inset of (a) shows a typical dI/dV spectrum of an oxygen dopant. (d–f) Oxygen-specific order parameter \({N}_{{\varepsilon }}\left({\boldsymbol{r}}\right)\) with overlaying oxygen dopants as black circles. The \({N}_{{\varepsilon }}\left({\boldsymbol{r}}\right)\) are measured simultaneously as \(g\)(r, -900 meV) in (a–c), where the domain walls are highlighted. (g–i) The \({d}_{{\rm{dopant}}}\) histogram (pink bar) is the distance from each dopant to the nearest location on the domain walls. The \({d}_{{\rm{random}}}\) histogram (grey curve) is the expectations of the distance between simulated random points and its nearest point in the domain walls.
Extended Data Fig. 8 Unit-cell averaging (UCA) analysis.
(a) Identification of the Cu site in the topograph \(T\left({\boldsymbol{r}}\right)\). Each unit cell is defined by a Cu site (black circles) in the center and four Cu sites at the corners. The averaged image of all the unit cells is a UCA image presented in the inset. (b) The CuO2 unit cells are categorized into two zones of Ising domains from the parameter \({N}_{{\varepsilon }}\left({\boldsymbol{r}}\right)\) map. The UCA images of each Ising domain is subsequently calculated.
Extended Data Fig. 9 Supermodulation does not affect orbital ordering domains.
(a, e, i) show the topographs where the supermodulation is filtered. (b, f, j) present the topographs where the supermodulation is kept. (c, g, k) show the orbital ordering domains calculated from the topographs in the first column where the supermodulation is removed. (d, h, l) show the orbital ordering domains calculated from the topographs in the second column where the supermodulation remains. The orbital ordering domains from the third (without supermodulation) and fourth columns (with supermodulation) are virtually identical. The supermodulation has virtually no effect on the orbital-order parameter used in this paper.
Source data
Source Data Fig. 1
Analysed data used to plot Fig. 1a.
Source Data Fig. 2
Analysed data used to plot Fig. 2a–h.
Source Data Fig. 3
Analysed data used to plot Fig. 3a,c–f.
Source Data Fig. 4
Analysed data used to plot in Fig. 4a–d.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Kennedy, N., Fujita, K. et al. Discovery of orbital ordering in Bi2Sr2CaCu2O8+x. Nat. Mater. 23, 492–498 (2024). https://doi.org/10.1038/s41563-024-01817-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-024-01817-z