Abstract
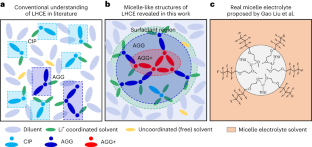
Liquid electrolytes in batteries are typically treated as macroscopically homogeneous ionic transport media despite having a complex chemical composition and atomistic solvation structures, leaving a knowledge gap of the microstructural characteristics. Here, we reveal a unique micelle-like structure in a localized high-concentration electrolyte, in which the solvent acts as a surfactant between an insoluble salt in a diluent. The miscibility of the solvent with the diluent and simultaneous solubility of the salt results in a micelle-like structure with a smeared interface and an increased salt concentration at the centre of the salt–solvent clusters that extends the salt solubility. These intermingling miscibility effects have temperature dependencies, wherein a typical localized high-concentration electrolyte peaks in localized cluster salt concentration near room temperature and is used to form a stable solid–electrolyte interphase on a Li metal anode. These findings serve as a guide to predicting a stable ternary phase diagram and connecting the electrolyte microstructure with electrolyte formulation and formation protocols of solid–electrolyte interphases for enhanced battery cyclability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are included within the paper and its Supplementary Information. Source data are available from the corresponding authors (B.L. and Y.Q.) upon reasonable request.
Code availability
The Python scripts that have been used for MD analyses are available from the corresponding author (Y.Q.) upon request.
References
Wang, Z. et al. Structural regulation chemistry of lithium ion solvation for lithium batteries. EcoMat 4, e12200 (2022).
Cheng, H. et al. Emerging era of electrolyte solvation structure and interfacial model in batteries. ACS Energy Lett. 7, 490–513 (2022).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Wang, J. et al. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 7, 12032 (2016).
Yamada, Y. & Yamada, A. Review—superconcentrated electrolytes for lithium batteries. J. Electrochem. Soc. 162, A2406–A2423 (2015).
Suo, L., Hu, Y. S., Li, H., Armand, M. & Chen, L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
Cao, X., Jia, H., Xu, W. & Zhang, J.-G. Review—localized high-concentration electrolytes for lithium batteries. J. Electrochem. Soc. 168, 010522–010522 (2021).
Cao, X. et al. Monolithic solid-electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Cao, X. et al. Optimization of fluorinated orthoformate based electrolytes for practical high-voltage lithium metal batteries. Energy Storage Mater. 34, 76–84 (2021).
Li, T. et al. Stable anion-derived solid electrolyte interphase in lithium metal batteries. Angew. Chem. Int Ed. 60, 22683–22687 (2021).
Adams, B. D., Zheng, J. M., Ren, X. D., Xu, W. & Zhang, J. G. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Jia, H. P. et al. Controlling ion coordination structure and diffusion kinetics for optimized electrode-electrolyte interphases and high-performance Si anodes. Chem. Mater. 32, 8956–8964 (2020).
Peng, X. D., Lin, Y. K., Wang, Y., Li, Y. J. & Zhao, T. S. A lightweight localized high-concentration ether electrolyte for high-voltage Li-ion and Li-metal batteries. Nano Energy 96, 107102 (2022).
Wang, Y. D. et al. Enhanced sodium metal/electrolyte interface by a localized high-concentration electrolyte for sodium metal batteries: first-principles calculations and experimental studies. ACS Appl. Energy Mater. 4, 7376–7384 (2021).
Wang, N. et al. Stabilized rechargeable aqueous zinc batteries using ethylene glycol as water blocker. ChemSusChem 13, 5556–5564 (2020).
Xue, R. F. et al. Highly reversible zinc metal anodes enabled by a three-dimensional silver host for aqueous batteries. J. Mater. Chem. A 10, 10043–10050 (2022).
Du, X. Q. & Zhang, B. A. Robust solid electrolyte interphases in localized high concentration electrolytes boosting black phosphorus anode for potassium-ion batteries. ACS Nano 15, 16851–16860 (2021).
Qin, L. et al. Pursuing graphite-based K-ion O2 batteries: a lesson from Li-ion batteries. Energy Environ. Sci. 13, 3656–3662 (2020).
Piao, N. et al. Countersolvent electrolytes for lithium-metal batteries. Adv. Energy Mater. 10, 1903568 (2020).
Qian, K., Winans, R. E. & Li, T. Insights into the nanostructure, solvation, and dynamics of liquid electrolytes through small-angle X-ray scattering. Adv. Energy Mater. 11, 2002821 (2021).
Su, C. C. et al. Solvating power series of electrolyte solvents for lithium batteries. Energy Environ. Sci. 12, 1249–1254 (2019).
Cao, X., Zhang, J.-G. & Xu, W. Electrolyte for stable cycling of rechargeable alkali metal and alkali ion batteries. US patent: US20200161706A1 (2019).
McBain, J. W. Mobility of highly-charged micelles. Trans. Faraday Soc. (1913).
McClements, D. J. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8, 1719–1729 (2012).
Zhao, Y. et al. A micelle electrolyte enabled by fluorinated ether additives for polysulfide suppression and Li metal stabilization in Li-S battery. Front. Chem. https://doi.org/10.3389/fchem.2020.00484 (2020).
Ren, F. et al. Solvent–diluent interaction-mediated solvation structure of localized high-concentration electrolytes. ACS Appl. Mater. Interfaces 14, 4211–4219 (2022).
Beltran, S. P., Cao, X., Zhang, J. G. & Balbuena, P. B. Localized high concentration electrolytes for high voltage lithium-metal batteries: correlation between the electrolyte composition and its reductive/oxidative stability. Chem. Mater. 32, 5973–5984 (2020).
Genovese, M. et al. Hot formation for improved low temperature cycling of anode-free lithium metal batteries. J. Electrochem. Soc. 166, A3342–A3347 (2019).
Yoshida, H. & Matsuura, H. Density functional study of the conformations and vibrations of 1,2-dimethoxyethane. J. Phys. Chem. A 102, 2691–2699 (1998).
Cote, J. F. et al. Dielectric constants of acetonitrile, gamma-butyrolactone, propylene carbonate, and 1,2-dimethoxyethane as a function of pressure and temperature. J. Solut. Chem. 25, 1163–1173 (1996).
Pham, T. A., Kweon, K. E., Samanta, A., Lordi, V. & Pask, E. J. Solvation and dynamics of sodium and potassium in ethylene carbonate from ab initio molecular dynamics simulations. J. Phys. Chem. C 121, 21913–21920 (2017).
Kerner, M., Plylahan, N., Scheers, J. & Johansson, P. Thermal stability and decomposition of lithium bis(fluorosulfonyl)imide (LiFSI) salts. RSC Adv. 6, 23327–23334 (2016).
Suo, L., Zheng, F., Hu, Y. S. & Chen, L. FT-Raman spectroscopy study of solvent-in-salt electrolytes. Chin. Phys. B 25, 016101 (2015).
Wang, J. et al. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019).
Sun, B. et al. At the polymer electrolyte interfaces: the role of the polymer host in interphase layer formation in Li-batteries. J. Mater. Chem. A 3, 13994–14000 (2015).
Nagarajan, R. in Structure-Performance Relationships in Surfactants (eds Esumi, K. & Ueno, M.) 1–89 (Marcel Dekker, 2003).
Pal, A. & Chaudhary, S. Ionic liquids effect on critical micelle concentration of SDS: conductivity, fluorescence and NMR studies. Fluid Phase Equilib. 372, 100–104 (2014).
Perez-Rodriguez, M. et al. A comparative study of the determination of the critical micelle concentration by conductivity and dielectric constant measurements. Langmuir 14, 4422–4426 (1998).
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Chen, S. R. et al. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2, 1548–1558 (2018).
Ren, X. D. et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 4, 1877–1892 (2018).
Su, L. S. et al. Uncovering the solvation structure of LiPF6-based localized saturated electrolytes and their effect on LiNiO2-based lithium-metal batteries. Adv. Energy Mater. 12, 2201911 (2022).
Materials Studio 2020 (Dassault Systèmes BIOVIA, 2020).
Akkermans, R. L. C., Spenley, N. A. & Robertson, S. COMPASS III: automated fitting workflows and extension to ionic liquids. Mol. Simula. 47, 540–551 (2021).
von Cresce, A. & Xu, K. Preferential solvation of Li+ directs formation of interphase on graphitic anode. Electrochem. Solid St. 14, A154–A156 (2011).
Borodin, O. & Smith, G. D. Quantum chemistry and molecular dynamics simulation study of dimethyl carbonate: ethylene carbonate electrolytes doped with LiPF6. J. Phys. Chem. B 113, 1763–1776 (2009).
Borodin, O. et al. Competitive lithium solvation of linear and cyclic carbonates from quantum chemistry. Phys. Chem. Chem. Phys. 18, 164–175 (2016).
Wu, Q. S., McDowell, M. T. & Qi, Y. Effect of the electric double layer (EDL) in multicomponent electrolyte reduction and solid electrolyte interphase (SEI) formation in lithium batteries. J. Am. Chem. Soc. 145, 2473–2484 (2023).
Liu, H. et al. Ultrahigh coulombic efficiency electrolyte enables Li || SPAN batteries with superior cycling performance. Mater. Today 42, 17–28 (2021).
Park, C. et al. Molecular simulations of electrolyte structure and dynamics in lithium–sulfur battery solvents. J. Power Sources 373, 70–78 (2018).
Nose, S. A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Toxvaerd, S. & Dyre, J. C. Role of the first coordination shell in determining the equilibrium structure and dynamics of simple liquids. J. Chem. Phys. 135, 134501–134501 (2011).
Gaussian 09, revision D.01 (Gaussian, Inc., 2016).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on the generalized Born approximation with asymmetric descreening. J. Chem. Theory Comput. 5, 2447–2464 (2009).
Acknowledgements
B.L. on behalf of the authors from National Laboratories and Y.Q. on behalf of the authors from Brown University thank the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy through the Advanced Battery Materials Research Program (Battery500 Consortium) and NASA (grant no. 80NSSC21M0107), respectively, for the financial support. Idaho National Laboratory (INL) is operated by Battelle Energy Alliance under contract no. DE-AC07-05ID14517 for the US Department of Energy. Pacific Northwest National Laboratory (PNNL) is operated by Battelle under contract no. DE-AC05-76RLO1830 for the US Department of Energy. The authors from Boise State University thank the Micron School of Materials Science and Engineering of this university for the additional financial support. We acknowledge the Atomic Films Laboratory at Boise State University for the use of the PHI-5600 XPS system. This research also used resources of the Center for Functional Nanomaterials and the SMI beamline (12-ID) of the National Synchrotron Light Source II, both supported by the US Department of Energy, Office of Science facilities at Brookhaven National Laboratory (BNL) under contract no. DE-SC0012704. We thank E. Graugnard, J. D. Hues and J. Soares for support with XPS, N. Bulloss for support with FESEM and P. H. Davis for support with Raman, as well as S. Tan from BNL for electrolyte sample preparation.
Author information
Authors and Affiliations
Contributions
B.L. and Y.Q. conceived the original idea and designed the experiments. Q.W. and Y.Q. conducted all MD simulations and DFT calculations, as well as computational analyses. C.M.E. and B.L. collected and processed the Raman and FESEM data. C.M.E. and N.G. prepared and cycled the coin cells. X.C. prepared electrolytes and cycled the Coulombic efficiency cells. H.Z. and C.M.E. collected and processed the XPS results. Y.Z., B.L., Y.Q., E.H., X.-Q.Y. and J.L. collected and processed the SAXS-WAXS results. C.M.E., Q.W., Y.Q. and B.L. wrote the manuscript. All authors contributed to the discussions and revisions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Gao Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28 and Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Efaw, C.M., Wu, Q., Gao, N. et al. Localized high-concentration electrolytes get more localized through micelle-like structures. Nat. Mater. 22, 1531–1539 (2023). https://doi.org/10.1038/s41563-023-01700-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01700-3



