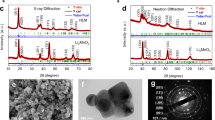
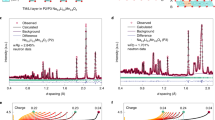
Abstract
The design of materials that efficiently catalyse the electrochemical reaction of molecular oxygen to hydroxide ions is key to the development of electrochemical devices. Here we demonstrate an approach to control the orbital hybridization of 3d and 4d/5d metals to tune the adsorption strength and stabilize the catalytic sites in the platinum-free catalysts Li2Mn1−xRuxO3. We show that in these materials, the stabilization of O 2p holes by changing the M–O covalency (M = 4d/5d metal) can help to mitigate structural instability. Operando X-ray absorption spectroscopy revealed that the Mn and Ru atoms are the active sites for the oxygen reduction reaction (ORR) and exhibit a high ORR activity with noteworthy stability compared with the Pt/C catalyst and outperform NiFe layered double hydroxides and RuO2 in the oxygen evolution reaction. Notably, Li2Mn0.85Ru0.15O3 shows a high power density of 1.2 W cm−2 and current density of 1.2 A cm−2 at 1.9 V in the anion exchange membrane fuel cell and water electrolyser, respectively.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All of the data that support the findings of this study are available in the article. The atomic coordinates of the computational models developed in this study have been deposited at figshare at https://doi.org/10.6084/m9.figshare.25287826.v2 (ref. 70). Source data are provided with this paper.
Change history
26 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41929-024-01166-9
References
Lu, S. F., Pan, J., Huang, A. B., Zhuang, L. & Lu, J. T. Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts. Proc. Natl Acad. Sci. USA 105, 20611–20614 (2008).
Gewirth, A. A. & Thorum, M. S. Electroreduction of dioxygen for fuel-cell applications: materials and challenges. Inorg. Chem. 49, 3557–3566 (2010).
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Firouzjaie, H. A. & Mustain, W. E. Catalytic advantages, challenges, and priorities in alkaline membrane fuel cells. ACS Catal. 10, 225–234 (2020).
Omasta, T. J. et al. Strategies for reducing the PGM loading in high power AEMFC anodes. J. Electrochem. Soc. 165, F710–F717 (2018).
Liang, Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011).
Yin, J. et al. NiO/CoN porous nanowires as efficient bifunctional catalysts for Zn–air batteries. ACS Nano 11, 2275–2283 (2017).
Ng, J. W. D. et al. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat. Energy 1, 16053 (2016).
Cheng, F. et al. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem. Int. Ed. 52, 2474–2477 (2013).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Meng, Y. et al. Structure–property relationship of bifunctional MnO2 nanostructures: highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 136, 11452–11464 (2014).
Zhong, X. et al. Boosting oxygen reduction activity and enhancing stability through structural transformation of layered lithium manganese oxide. Nat. Commun. 12, 3136 (2021).
Douglin, J. C. et al. High-performance ionomerless cathode anion-exchange membrane fuel cells with ultra-low-loading Ag–Pd alloy electrocatalysts. Nat. Energy 8, 1262–1272 (2023).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Zhang, M. et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat. Rev. Mater. 7, 522–540 (2022).
Lu, Z., MacNeil, D. & Dahn, J. Layered Li[NixCo1−2xMnx]O2 cathode materials for lithium-ion batteries. Electrochem. Solid-State Lett. 4, A200 (2001).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Sathiya, M. et al. Electron paramagnetic resonance imaging for real-time monitoring of Li-ion batteries. Nat. Commun. 6, 6276 (2015).
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 3, 546–550 (2011).
Hong, W. T. et al. Probing LaMO3 metal and oxygen partial density of states using X-ray emission, absorption, and photoelectron spectroscopy. J. Phys. Chem. C 119, 2063–2072 (2015).
Zhou, J. et al. Voltage- and time-dependent valence state transition in cobalt oxide catalysts during the oxygen evolution reaction. Nat. Commun. 11, 1984 (2020).
Grimaud, A., Hong, W. T., Shao-Horn, Y. & Tarascon, J.-M. Anionic redox processes for electrochemical devices. Nat. Mater. 15, 121–126 (2016).
Li, L. et al. In situ/operando capturing unusual Ir6+ facilitating ultrafast electrocatalytic water oxidation. Adv. Funct. Mater. 31, 2104746 (2021).
Lin, C. et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012–1023 (2021).
Liu, H. et al. Insight into the role of metal–oxygen bond and O 2p hole in high-voltage cathode LiNixMn2–xO4. J. Phys. Chem. C 121, 16079–16087 (2017).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Saubanère, M., McCalla, E., Tarascon, J.-M. & Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Mitra, C. et al. Direct observation of electron doping in La0.7Ce0.3MnO3 using X-ray absorption spectroscopy. Phys. Rev. B 67, 092404 (2003).
Vasiliev, A. N. et al. Valence states and metamagnetic phase transition in partially B-site-disordered perovskite EuMn0.5Co0.5O3. Phys. Rev. B 77, 104442 (2008).
Hollmann, N. et al. Local symmetry and magnetic anisotropy in multiferroic MnWO4 and antiferromagnetic CoWO4 studied by soft X-ray absorption spectroscopy. Phys. Rev. B 82, 184429 (2010).
Nemrava, S. et al. Three oxidation states of manganese in the barium hexaferrite BaFe12–xMnxO19. Inorg. Chem. 56, 3861–3866 (2017).
Chen, G. et al. Ultrahigh-performance tungsten-doped perovskites for the oxygen evolution reaction. J. Mater. Chem. A 6, 9854–9859 (2018).
Croft, M. et al. Systematic Mn d-configuration change in the La1−xCaxMnO3 system: a Mn K-edge XAS study. Phys. Rev. B 55, 8726 (1997).
Guan, D. et al. Exceptionally robust face‐sharing motifs enable efficient and durable water oxidation. Adv. Mater. 33, 2103392 (2021).
Gao, T. et al. Synthesis and properties of layered-structured Mn5O8 nanorods. J. Phys. Chem. C 114, 922–928 (2010).
Lü, M., Deng, X. L., Waerenborgh, J. C., Wu, X. J. & Meng, J. Redox chemistry and magnetism of LaSrM0.5Ru0.5O4±δ (M = Co, Ni and Zn) Ruddlesden–Popper phases. Dalton Trans. 41, 11507–11518 (2012).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Calle-Vallejo, F., Martínez, J. I., García-Lastra, J. M., Abad, E. & Koper, M. T. M. Oxygen reduction and evolution at single-metal active sites: comparison between functionalized graphitic materials and protoporphyrins. Surf. Sci. 607, 47–53 (2013).
Rossmeisl, J., Qu, Z.-W., Zhu, H., Kroes, G.-J. & Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Ríos, E. et al. Electrocatalysis of oxygen reduction on CuxMn3−xO4 (1.0 ≤ x ≤ 1.4) spinel particles/polypyrrole composite electrodes. Int. J. Hydrogen Energy 33, 4945–4954 (2008).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 408–413 (2020).
Li, X. et al. Exceptional oxygen evolution reactivities on CaCoO3 and SrCoO3. Sci. Adv. 5, eaav6262 (2019).
Sathiya, M. et al. High performance Li2Ru1−yMnyO3 (0.2 ≤ y ≤ 0.8) cathode materials for rechargeable lithium-ion batteries: their understanding. Chem. Mater. 25, 1121–1131 (2013).
Nilsson, A. et al. The electronic structure effect in heterogeneous catalysis. Catal. Lett. 100, 111–114 (2005).
Wang, J. et al. Redirecting dynamic surface restructuring of a layered transition metal oxide catalyst for superior water oxidation. Nat. Catal. 4, 212–222 (2021).
Wu, T. et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
Grimaud, A. et al. Oxygen evolution activity and stability of Ba6Mn5O16, Sr4Mn2CoO9, and Sr6Co5O15: the influence of transition metal coordination. J. Phys. Chem. C 117, 25926–25932 (2013).
Pao, C.-W. et al. The new X-ray absorption fine-structure beamline with sub-second time resolution at the Taiwan Photon Source. J. Synchrotron Radiat. 28, 930–938 (2021).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Paulus, U., Schmidt, T., Gasteiger, H. & Behm, R. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: a thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 495, 134–145 (2001).
McCrory, C. C., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013).
Forslund, R. P. et al. Exceptional electrocatalytic oxygen evolution via tunable charge transfer interactions in La0.5Sr1.5Ni1−xFexO4±δ Ruddlesden–Popper oxides. Nat. Commun. 9, 3150 (2018).
Peng, H. et al. Alkaline polymer electrolyte fuel cells stably working at 80 °C. J. Power Sources 390, 165–167 (2018).
Huai, L., Chen, Z. & Li, J. Degradation mechanism of cimethyl carbonate (DMC) dissociation on the LiCoO2 cathode surface: a first-principles study. ACS Appl. Mater. Interfaces 9, 36377–36384 (2017).
He, Q. et al. Accelerating CO2 electroreduction to CO over Pd single-atom catalyst. Adv. Funct. Mater. 30, 2000407 (2020).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 45, 13244 (1992).
Jain, A. et al. A high-throughput infrastructure for density functional theory calculations. Comput. Mater. Sci. 50, 2295–2310 (2011).
Jain, A. et al. Commentary: the Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Device Studio Version 2021A (Hongzhiwei Technology, 2021).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Hansen, H. A., Rossmeisl, J. & Nørskov, J. K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 10, 3722–3730 (2008).
Zhong, X. et al. Atomistic configurations of Li2Mn1−xRuxO3 (x = 0, 0.1, 0.15, 0.2) models. figshare https://doi.org/10.6084/m9.figshare.25287826.v2 (2024).
Wang, Y. et al. Pt–Ru catalyzed hydrogen oxidation in alkaline media: oxophilic effect or electronic effect? Energy Environ. Sci. 8, 177–181 (2015).
Yang, Y. et al. High-loading composition-tolerant Co–Mn spinel oxides with performance beyond 1 W/cm2 in alkaline polymer electrolyte fuel cells. ACS Energy Lett. 4, 1251–1257 (2019).
Ponce-González, J. et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 9, 3724–3735 (2016).
Maurya, S., Fujimoto, C. H., Hibbs, M. R., Narvaez Villarrubia, C. & Kim, Y. S. Toward improved alkaline membrane fuel cell performance using quaternized aryl-ether free polyaromatics. Chem. Mater. 30, 2188–2192 (2018).
Fan, J. et al. Cationic polyelectrolytes, stable in 10 M KOHaq at 100 °C. ACS Macro Lett. 6, 1089–1093 (2017).
US Geological Survey. Platinum-Group Metals in March 2022. Mineral Industry Surveys (2022).
US Geological Survey. Manganese in April 2022. Mineral Industry Surveys (2022).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (22179098, to J.M.). M.Y. acknowledges support from the National Natural Science Foundation of China (52302302), the Fundamental Research Funds for the Central Universities, the National Key R&D Program of China (2022YFE0208000) and HZWTECH for providing computational facilities. Z.H. acknowledges support from the Max Planck-POSTECH-Hsinchu Center for Complex Phase Materials. P.S., T.K. and S.S. acknowledge the funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through project number 403371556 GZ: INST 131/789-1 FUGG and the German–French Project ‘capital ReAlCharge’ (STR 596/13-1) and the German Federal Ministry of Education and Research (BMBF) through the HyThroughGen project within the technology platform ‘H2Giga’ (grant no. 03HY108D). P.S. and M.K. acknowledge the financial support by BMBF under grant numbers 03SF0613D ‘AEMready’, 03HY130B ‘AEM-Direkt’ and 03HY302Q ‘H2Mare’.
Author information
Authors and Affiliations
Contributions
J.M. conceived the project and designed the experiments. X.Z., T.K., M.K., S.S., K.G.R., C.G., L.Z., W.H.K., M.A., M.S., N.A.-V., J.-M.C., S.-C.H., C.-W.P., Y.-C.C., Y.H., Z.H., P.S. and J.M. carried out the experimental work and data analysis. L.S. and M.Y. performed the theoretical calculations. All of the authors discussed the results and commented on the paper. X.Z., Z.H., P.S. and J.M. wrote the paper with contributions from all of the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Peng Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–45 and Tables 1–11.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, X., Sui, L., Yang, M. et al. Stabilization of layered lithium-rich manganese oxide for anion exchange membrane fuel cells and water electrolysers. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01136-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01136-1