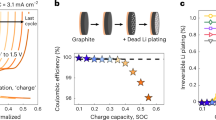
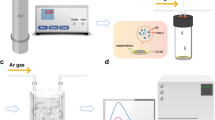
Abstract
Tracking the active lithium (Li) inventory in an electrode shows the true state of a Li battery, akin to a fuel gauge for an engine. However, non-destructive Li inventory tracking is currently unavailable. Here we used the theoretical capacity of a transition metal oxide to convert capacity into a Li inventory analysis. The Li inventory in electrodes was tracked reliably to show how battery formulations and test methods affect performance. Contrary to capacity, Li inventory tracking reveals stoichiometric variations near the electrode–electrolyte interface. Verifiable results rationalized differences in measurements, clarifying and reducing interferences from cell formulations and experimental manipulations. By tracing four variables from formation to end-of-life, we characterize electrode and cell performance with a thermodynamic framework. Accurate rationalization of subtle differences in Li inventory utilization promises precise battery engineering, evaluation, failure analysis and risk mitigation. The method could be applicable from cell design optimization and fabrication to battery management, improving battery performance and reliability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data used in this work are contained in the article and Supplementary Information. The datasets are also available at https://doi.org/10.17605/OSF.IO/2W4K3.
References
Xiao, J. et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Tornheim, A. & O’Hanlon, D. C. What do coulombic efficiency and capacity retention truly measure? A deep dive into cyclable lithium inventory, limitation type, and redox side reactions. J. Electrochem. Soc. 167, 110520 (2020).
Bond, T. M., Burns, J. C., Stevens, D. A., Dahn, H. M. & Dahn, J. R. Improving precision and accuracy in coulombic efficiency measurements of Li-ion batteries. J. Electrochem. Soc. 160, A521–A527 (2013).
Smith, A. J., Burns, J. C., Trussler, S. & Dahn, J. R. Precision measurements of the coulombic efficiency of lithium-ion batteries and of electrode materials for lithium-ion batteries. J. Electrochem. Soc. 157, A196–A202 (2010).
Gyenes, B., Stevens, D. A., Chevrier, V. L. & Dahn, J. R. Understanding anomalous behavior in coulombic efficiency measurements on Li-ion batteries. J. Electrochem. Soc. 162, A278–A283 (2015).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. G. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Li, X. et al. Review on comprehending and enhancing the initial coulombic efficiency of anode materials in lithium-ion/sodium-ion batteries. Nano Energy 77, 105143 (2020).
Li, Z., Huang, J., Liaw, B. Y. & Zhang, J. On state-of-charge determination for lithium-ion batteries. J. Power Sources 348, 281–301 (2017).
Dubarry, M., Vuillaume, N. & Liaw, B. Y. Origins and accommodation of cell variations in Li-ion battery pack modeling. Int. J. Energy Res. 34, 216–231 (2010).
Dubarry, M., Svoboda, V., Hwu, R. & Liaw, B. Y. Capacity loss in rechargeable lithium cells during cycle life testing: the importance of determining state-of-charge. J. Power Sources 174, 1121–1125 (2007).
Dubarry, M. et al. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part I: Initial characterizations. J. Power Sources 196, 10328–10335 (2011).
Wang, F. et al. Does polarization increase lead to capacity fade? J. Electrochem. Soc. 167, 090549 (2020).
Zhang, Y. et al. A quantitative failure analysis on capacity fade in rechargeable lithium metal cells. J. Electrochem. Soc. 167, 090502 (2020).
Zhang, Y. et al. Cell qualification—a performance metric-based approach. J. Phys. Energy 2, 034003 (2020).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Kasnatscheew, J. et al. The truth about the 1st cycle coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 18, 3956–3965 (2016).
Kang, S.-H., Yoon, W.-S., Nam, K.-W., Yang, X.-Q. & Abraham, D. P. Investigating the first-cycle irreversibility of lithium metal oxide cathodes for Li batteries. J. Mater. Sci. 43, 4701–4706 (2008).
Choi, J. & Manthiram, A. Investigation of the irreversible capacity loss in the layered LiNi1/3Co1/3Mn1/3O2 cathodes. Electrochem. Solid State Lett. 8, C102–C105 (2005).
Zhang, S. S., Xu, K. & Jow, T. R. Formation of solid electrolyte interface in lithium nickel mixed oxide electrodes during the first cycling. Electrochem. Solid State Lett. 5, A92–A94 (2002).
Lee, K.-K. & Kim, K.-B. Electrochemical and structural characterization of LiNi1–2yCoyO2 (0 ≤ y ≤ 0.2) positive electrodes during initial cycling. J. Electrochem. Soc. 147, 1709–1717 (2000).
Arai, H., Okada, S., Sakurai, Y. & Yamaki, J.-I. Reversibility of LiNiO2 cathode. Solid State Ion. 95, 275–282 (1997).
Xin, F. et al. Li–Nb–O coating/substitution enhances the electrochemical performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) cathode. ACS Appl. Mater. Interfaces 11, 34889–34894 (2019).
Zhou, H., Xin, F., Pei, B. & Whittingham, M. S. What limits the capacity of layered oxide cathodes in lithium batteries? ACS Energy Lett. 4, 1902–1906 (2019).
Märker, K., Reeves, P. J., Xu, C., Griffith, K. J. & Grey, C. P. Evolution of structure and lithium dynamics in LiNi0.8Mn0.1Co0.1O2 (NMC 811) cathodes during electrochemical cycling. Chem. Mater. 31, 2545–2554 (2019).
Weppner, W. & Huggins, R. A. Determination of the kinetic parameters of mixed-conducting electrodes and application to the system Li3Sb. J. Electrochem. Soc. 124, 1569–1578 (1977).
Huggins, R. A. Advanced Batteries: Materials Science Aspects (Springer, 2008); https://doi.org/10.1007/978-0-387-76424-5
Li, Z. et al. Synchrotron operando depth profiling studies of state-of-charge gradients in thick Li(Ni0.8Mn0.1Co0.1)O2 cathode films. Chem. Mater. 32, 6358–6364 (2020).
Mao, Y. et al. High-voltage charging-induced strain, heterogeneity, and micro-cracks in secondary particles of a nickel-rich layered cathode material. Adv. Funct. Mater. 29, 1900247 (2019).
Wu, F. et al. Removing the intrinsic NiO phase and residual lithium for high-performance nickel-rich materials. Energy Mater. Adv. 4, 0007 (2023).
Rahimi Eichi H. & Chow, M.-Y. Modeling and analysis of battery hysteresis effects. In Proc. 2012 IEEE Energy Conversion Congress and Exposition 4479–4486 (IEEE, 2012).
Lim, J. et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 353, 566–571 (2016).
Hess, M., Sasaki, T., Villevieille, C. & Novák, P. Combined operando X-ray diffraction-electrochemical impedance spectroscopy detecting solid solution reactions of LiFePO4 in batteries. Nat. Commun. 6, 8169 (2015).
Ovejas, V. J. & Cuadras, A. Effects of cycling on lithium-ion battery hysteresis and overvoltage. Sci. Rep. 9, 14875 (2019).
Fang, Z. et al. Formation of surface impurities on lithium-nickel-manganese-cobalt oxides in the presence of CO2 and H2O. J. Am. Chem. Soc. 143, 10261–10274 (2021).
Gering, K. L. et al. Investigation of path dependence in commercial lithium-ion cells chosen for plug-in hybrid vehicle duty cycle protocols. J. Power Sources 196, 3395–3403 (2011).
Su, L. et al. Path dependence of lithium ion cells aging under storage conditions. J. Power Sources 315, 35–46 (2016).
Acknowledgements
The work presented in this article comprised three stages of effort and development that should be recognized. The initial concept was developed in a project supported in part under Idaho National Laboratory’s Laboratory Directed Research and Development programme (project no. 19P45-013FP) for a lithium-ion battery reliability and safety diagnostic study. This work was later supported by a project funded by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy in the Advanced Battery Materials Research Program (Battery500 Consortium) to apply the concept to diagnose the performance and degradation of high-energy rechargeable lithium metal batteries, where the bulk of the data was used in this study. The extension of the experimental work for the analysis is supported under the Earth-Abundant Cathode Material Consortium led by Argonne National Laboratory, and the goal is to assist material research with a better diagnostic tool and approach. Idaho National Laboratory is operated by Battelle Energy Alliance under contract no. DE-AC07-05ID14517 for the US Department of Energy.
Author information
Authors and Affiliations
Contributions
B.L. and M.L. conceived the conceptual approach for the analysis. M.L. and Y.Z. conducted the analyses. H.Z., F.X. and M.S.W. conducted the experiments and produced the data for the analyses. B.L. wrote the paper with the help of all authors.
Corresponding author
Ethics declarations
Competing interests
B.L., M.L. and Y.Z. disclosed that Battelle Energy Alliance has filed US patent applications 17/149,046 and 18/185,018. US Patent 11,740,290 has been issued. The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks David Ansean and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Zhang, Y., Zhou, H. et al. Lithium inventory tracking as a non-destructive battery evaluation and monitoring method. Nat Energy (2024). https://doi.org/10.1038/s41560-024-01476-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41560-024-01476-z