Abstract
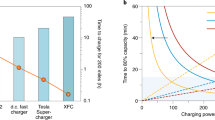
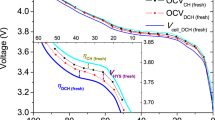
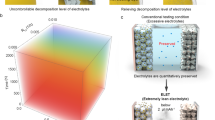
Fast charging of most commercial lithium-ion batteries is limited due to fear of lithium plating on the graphite anode, which is difficult to detect and poses considerable safety risk. Here we demonstrate the power of simple, accessible and high-throughput cycling techniques to quantify irreversible Li plating spanning data from over 200 cells. We first observe the effects of energy density, charge rate, temperature and state of charge on lithium plating, use the results to refine a mature physics-based electrochemical model and provide an interpretable empirical equation for predicting the plating onset state of charge. We then explore the reversibility of lithium plating and its connection to electrolyte design for preventing irreversible Li accumulation. Finally, we design a method to quantify in situ Li plating for commercially relevant graphite|LiNi0.5Mn0.3Co0.2O2 (NMC) cells and compare with results from the experimentally convenient Li|graphite configuration. The hypotheses and abundant data herein were generated primarily with equipment universal to the battery researcher, encouraging further development of innovative testing methods and data processing that enable rapid battery engineering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings in this study are available within the paper and the Supplementary Information. Source data are provided with this paper.
References
Frost & Sullivan. Global Li-ion Battery Materials Growth Opportunities. https://www.marketresearch.com/Frost-Sullivan-v383/Global-Li-ion-Battery-Materials-14856305/ (2021).
Zhang, S. S., Xu, K. & Jow, T. R. Study of the charging process of a LiCoO2-based Li-ion battery. J. Power Sources 160, 1349–1354 (2006).
Tobishima, S. I. & Yamaki, J. I. A consideration of lithium cell safety. J. Power Sources 81–82, 882–886 (1999).
DNV-GL and Arizona Public Service. McMicken Battery Energy Storage System Event Technical Analysis and Recommendations. https://www.aps.com/-/media/APS/APSCOM-PDFs/About/Our-Company/Newsroom/McMickenFinalTechnicalReport.ashx?la=en&hash=50335FB5098D9858BFD276C40FA54FCE (2020).
Arora, P., Doyle, M. & White, R. E. Mathematical modeling of the lithium deposition overcharge reaction in lithium-ion batteries using carbon-based negative electrodes. J. Electrochem. Soc. 146, 3543 (1999).
Tang, M., Albertus, P. & Newman, J. Two-dimensional modeling of lithium deposition during cell charging. J. Electrochem. Soc. 156, A390 (2009).
Yang, X. G., Ge, S., Liu, T., Leng, Y. & Wang, C. Y. A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells. J. Power Sources 395, 251–261 (2018).
Ren, D. et al. Investigation of lithium plating-stripping process in Li-ion batteries at low temperature using an electrochemical model. J. Electrochem. Soc. 165, A2167–A2178 (2018).
Colclasure, A. M. et al. Requirements for enabling extreme fast charging of high energy density Li-ion cells while avoiding lithium plating. J. Electrochem. Soc. 166, A1412–A1424 (2019).
Campbell, I. D., Marzook, M., Marinescu, M. & Offer, G. J. How observable is lithium plating? Differential voltage analysis to identify and quantify lithium plating following fast charging of cold lithium-ion batteries. J. Electrochem. Soc. 166, A725–A739 (2019).
Paul, P. P. et al. A review of existing and emerging methods for lithium detection and characterization in Li-ion and Li-metal batteries. Adv. Energy Mater. 11, 2100372 (2021).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
McShane, E. J. et al. Quantification of inactive lithium and solid–electrolyte interphase species on graphite electrodes after fast charging. ACS Energy Lett. 5, 2045–2051 (2020).
Deng, Z. et al. Towards autonomous high-throughput multiscale modelling of battery interfaces. Energy Environ. Sci. 15, 579–594 (2022).
Kang, K., Meng, Y. S., Bréger, J., Grey, C. P. & Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311, 977–980 (2006).
Qie, L. et al. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 24, 2047–2050 (2012).
Smith, A. J., Burns, J. C., Trussler, S. & Dahn, J. R. Precision measurements of the coulombic efficiency of lithium-ion batteries and of electrode materials for lithium-ion batteries. J. Electrochem. Soc. 157, A196–A202 (2009).
Dahn, J. R., Burns, J. C. & Stevens, D. A. Importance of coulombic efficiency measurements in R&D efforts to obtain long-lived Li-ion batteries. Electrochem. Soc. Interface 25, 75–78 (2016).
Severson, K. A. et al. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy 4, 383–391 (2019).
Attia, P. M. et al. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature 578, 397–402 (2020).
Aykol, M., Herring, P. & Anapolsky, A. Machine learning for continuous innovation in battery technologies. Nat. Rev. Mater. 5, 725–727 (2020).
Konz, Z. M., McShane, E. J. & McCloskey, B. D. Detecting the onset of lithium plating and monitoring fast charging performance with voltage relaxation. ACS Energy Lett. 5, 1750–1757 (2020).
Adam, A., Knobbe, E., Wandt, J. & Kwade, A. Application of the differential charging voltage analysis to determine the onset of lithium-plating during fast charging of lithium-ion cells. J. Power Sources 495, 229794 (2021).
Colclasure, A. M. et al. Electrode scale and electrolyte transport effects on extreme fast charging of lithium-ion cells. Electrochim. Acta 337, 135854 (2020).
Finegan, D. P. et al. Spatial dynamics of lithiation and lithium plating during high-rate operation of graphite electrodes. Energy Environ. Sci. 13, 2570–2584 (2020).
Robertson, D. C. et al. Effect of anode porosity and temperature on the performance and lithium plating during fast-charging of lithium-ion cells. Energy Technol. 9, 2000666 (2021).
Chen, Y. et al. Operando video microscopy of Li plating and re-intercalation on graphite anodes during fast charging. J. Mater. Chem. A 9, 23522–23536 (2021).
Dees, D. W. et al. Apparent increasing lithium diffusion coefficient with applied current in graphite. J. Electrochem. Soc. 167, 120528 (2020).
Uhlmann, C., Illig, J., Ender, M., Schuster, R. & Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 279, 428–438 (2015).
Gao, T. et al. Interplay of lithium intercalation and plating on a single graphite particle. Joule 5, 393–414 (2021).
Adam, A. et al. Development of an innovative workflow to optimize the fast-charge capability of lithium-ion battery cells. J. Power Sources 512, 230469 (2021).
Hobold, G. M. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Gunnarsdóttir, A. B., Amanchukwu, C. V., Menkin, S. & Grey, C. P. Noninvasive in situ NMR study of ‘dead lithium’ formation and lithium corrosion in full-cell lithium metal batteries. J. Am. Chem. Soc. 142, 20814–20827 (2020).
Wandt, J., Jakes, P., Granwehr, J., Eichel, R. A. & Gasteiger, H. A. Quantitative and time-resolved detection of lithium plating on graphite anodes in lithium ion batteries. Mater. Today 21, 231–240 (2018).
Tanim, T. R., Dufek, E. J., Dickerson, C. C. & Wood, S. M. Electrochemical quantification of lithium plating: challenges and considerations. J. Electrochem. Soc. 166, A2689–A2696 (2019).
Martin, C., Genovese, M., Louli, A. J., Weber, R. & Dahn, J. R. Cycling lithium metal on graphite to form hybrid lithium-ion/lithium metal cells. Joule 4, 1296–1310 (2020).
Cai, W. et al. The boundary of lithium plating in graphite electrode for safe lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 60, 13007–13012 (2021).
Mei, W., Jiang, L., Liang, C., Sun, J. & Wang, Q. Understanding of Li-plating on graphite electrode: detection, quantification and mechanism revelation. Energy Storage Mater. 41, 209–221 (2021).
Brown, D. E., McShane, E. J., Konz, Z. M., Knudsen, K. B. & McCloskey, B. D. Detecting onset of lithium plating during fast charging of Li-ion batteries using operando electrochemical impedance spectroscopy. Cell Rep. Phys. Sci. 2, 100589 (2021).
Ho, A. S. et al. 3D detection of lithiation and lithium plating in graphite anodes during fast charging. ACS Nano 15, 10480–10487 (2021).
Duangdangchote, S. et al. Effect of fluoroethylene carbonate on the transport property of electrolytes towards Ni-rich Li-ion batteries with high safety. Chem. Commun. 57, 6732–6735 (2021).
Yan, S. et al. Regulating the growth of lithium dendrite by coating an ultra-thin layer of gold on separator for improving the fast-charging ability of graphite anode. J. Energy Chem. 67, 467–473 (2022).
Shin, H., Park, J., Sastry, A. M. & Lu, W. Effects of fluoroethylene carbonate (FEC) on anode and cathode interfaces at elevated temperatures. J. Electrochem. Soc. 162, A1683–A1692 (2015).
Kazyak, E., Chen, K. H., Chen, Y., Cho, T. H. & Dasgupta, N. P. Enabling 4C fast charging of lithium-ion batteries by coating graphite with a solid-state electrolyte. Adv. Energy Mater. 12, 2102618 (2022).
Paul, P. P. et al. Quantification of heterogeneous, irreversible lithium plating in extreme fast charging of lithium-ion batteries. Energy Environ. Sci. 14, 4979–4988 (2021).
Christensen, J. & Newman, J. Cyclable lithium and capacity loss in Li-ion cells. J. Electrochem. Soc. 152, A818–A829 (2005).
Gong, C. et al. Revealing the role of fluoride-rich battery electrode interphases by operando transmission electron microscopy. Adv. Energy Mater. 11, 2003118 (2021).
Huang, W. et al. Onboard early detection and mitigation of lithium plating in fast-charging batteries. Nat. Commun. 13, 7091 (2022).
Logan, E. R., Tonita, E. M., Gering, K. L. & Dahn, J. R. A critical evaluation of the advanced electrolyte model. J. Electrochem. Soc. 165, A3350–A3359 (2018).
Usseglio-Viretta, F. L. E. et al. Resolving the discrepancy in tortuosity factor estimation for Li-ion battery electrodes through micro-macro modeling and experiment. J. Electrochem. Soc. 165, A3403–A3426 (2018).
Hindmarsh, A. C. et al. SUNDIALS: suite of nonlinear and differential/algebraic equation solvers. ACM Trans. Math. Softw. 31, 363–396 (2005).
Bloom, I. et al. Differential voltage analyses of high-power, lithium-ion cells 1. Technique and application. J. Power Sources 139, 295–303 (2005).
Acknowledgements
This work was largely supported by the Vehicle Technologies Office of the US Department of Energy under the XCEL Fast Charging Program (eXtreme Fast Charge Cell Evaluation of Lithium ion Batteries, XCEL). Part of this work was authored by the National Renewable Energy Laboratory, operated by the Alliance for Sustainable Energy, LLC, for the US Department of Energy (DOE) under contract DE-AC36-08GO28308. H.K.B., D.E.B. and E.J.M. acknowledge support from the National Science Foundation Graduate Research Fellowship Program under grant DGE 1106400. T.-Y.H. gratefully acknowledges support from both the Ministry of Education in Taiwan and UC Berkeley College of Chemistry through the Taiwan Fellowship Program. The authors thank S. Trask, A. Jansen, A. Dunlop and B. Polzin from the Argonne National Laboratory Cell Analysis, Modeling, and Prototyping (CAMP) facility for providing laminate electrodes used in the study. Z.M.K. thanks J. Heo for assistance with the design of Fig. 3e.
Author information
Authors and Affiliations
Contributions
Z.M.K. conceived ideas, performed experiments, developed methods, wrote analysis code and wrote the manuscript and Supplementary Information. B.M.W. developed Fig. 3 methods and analysis with Z.M.K. and provided continuous project and manuscript feedback. A.V. and A.M.C. performed EChem modelling simulations and wrote the corresponding manuscript/Supplementary Information sections. T.-Y.H. helped Z.M.K. to build the titration syringe attachment. H.K.B. helped with experiment design for Li plating on copper and electrolyte conductivity measurements. M.J.C. and T.-Y.H. provided feedback and assistance with titrations. D.E.B. and E.J.M. provided project feedback, troubleshooting ideas and mentorship. A.M.C. also conceived Fig. 2 experiments with Z.M.K., led EChem model modifications and provided continuous project feedback. B.D.M. was lead project supervisor, conceived ideas and was primary manuscript editor. All authors edited and provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Peer review information
Nature Energy thanks Tao Gao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–38, Tables 1–5 and Notes 1–8.
Source data
Source Data Fig. 1
All data required to reproduce Fig. 1 plots.
Source Data Fig. 2
All data required to reproduce Fig. 2 plots.
Source Data Fig. 3
All data required to reproduce Fig. 3 plots.
Source Data Fig. 4
All data required to reproduce Fig. 4 plots.
Source Data Fig. 5
All data required to reproduce Fig. 5 plots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konz, Z.M., Wirtz, B.M., Verma, A. et al. High-throughput Li plating quantification for fast-charging battery design. Nat Energy 8, 450–461 (2023). https://doi.org/10.1038/s41560-023-01194-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01194-y
This article is cited by
-
Operando Li metal plating diagnostics via MHz band electromagnetics
Nature Communications (2023)