Abstract
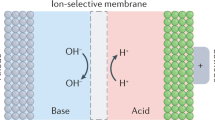
Establishing a pH difference between the two electrolytes (pH decoupling) of an aqueous redox flow battery (ARFB) enables cell voltages exceeding the 1.23 V thermodynamic water-splitting window, but acid–base crossover penalizes efficiency and lifetime. Here we employ mildly acidic and mildly alkaline electrolytes to mitigate crossover, achieving high round-trip energy efficiency with open circuit voltage >1.7 V. We implemented an acid–base regeneration system to periodically restore electrolytes to their initial pH values. The combined system exhibited capacity fade rate <0.07% per day, round-trip energy efficiency >85% and approximately 99% Coulombic efficiency during stable operation for over a week. Cost analysis shows that the tolerance of acid–base crossover could be increased if the pH-decoupling ARFB achieved a higher voltage output and lower resistance. This work demonstrates principles for improving lifespan, rate capability and energy efficiency in high-voltage pH-decoupling ARFBs and pH recovery concepts applicable for pH-decoupling systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets analysed and generated during the current study are included in the paper, its Supplementary Information and uploaded to Figshare at https://doi.org/10.6084/m9.figshare.23816796.
References
Zhang, L., Feng, R., Wang, W. & Yu, G. Emerging chemistries and molecular designs for flow batteries. Nat. Rev. Chem. 6, 524–543 (2022).
Chao, D. & Qiao, S.-Z. Toward high-voltage aqueous batteries: super- or low-concentrated electrolyte? Joule 4, 1846–1851 (2020).
Yao, Y., Lei, J., Shi, Y., Ai, F. & Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 6, 582–588 (2021).
Perry, M. L., Rodby, K. E. & Brushett, F. R. Untapped potential: the need and opportunity for high-voltage aqueous redox flow batteries. ACS Energy Lett. 7, 659–667 (2022).
Walsh, F. C. et al. The development of Zn-Ce hybrid redox flow batteries for energy storage and their continuing challenges. ChemPlusChem 80, 288–311 (2015).
Robb, B. H., Farrell, J. M. & Marshak, M. P. Chelated chromium electrolyte enabling high-voltage aqueous flow batteries. Joule 3, 2503–2512 (2019).
Yan, Z. et al. High-voltage aqueous redox flow batteries enabled by catalyzed water dissociation and acid-base neutralization in bipolar membranes. ACS Cent. Sci. 7, 1028–1035 (2021).
Weng, G.-M., Li, C.-Y. V. & Chan, K.-Y. High-voltage pH differential vanadium-hydrogen flow battery. Mater. Today Energy 10, 126–131 (2018).
Gu, S., Gong, K., Yan, E. Z. & Yan, Y. A multiple ion-exchange membrane design for redox flow batteries. Energy Environ. Sci. 7, 2986–2998 (2014).
Gong, K. et al. A zinc–iron redox-flow battery under $100 per kW h of system capital cost. Energy Environ. Sci. 8, 2941–2945 (2015).
Xie, X., Mushtaq, F., Wang, Q. & Daoud, W. A. The renaissance of the Zn-Ce flow battery: dual-membrane configuration enables unprecedentedly high efficiency. ACS Energy Lett. 7, 3484–3491 (2022).
Beh, E. S. et al. A neutral pH aqueous organic–organometallic redox flow battery with extremely high capacity retention. ACS Energy Lett. 2, 639–644 (2017).
Zhong, C. et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 5, 440–449 (2020).
Xia, Y. et al. A cost-effective alkaline polysulfide-air redox flow battery enabled by a dual-membrane cell architecture. Nat. Commun. 13, 2388 (2022).
Zhu, Y.-h et al. Decoupled aqueous batteries using pH-decoupling electrolytes. Nat. Rev. Chem. 6, 505–517 (2022).
Kwabi, D. G., Ji, Y. & Aziz, M. J. Electrolyte lifetime in aqueous organic redox flow batteries: a critical review. Chem. Rev. 120, 6467–6489 (2020).
Wang, C. et al. Molecular design of fused-ring phenazine derivatives for long-cycling alkaline redox flow batteries. ACS Energy Lett. 5, 411–417 (2020).
Gao, J. et al. A high potential, low capacity fade rate iron complex posolyte for aqueous organic flow batteries. Adv. Energy Mater. 12, 2202444 (2022).
Park, M. et al. A high voltage aqueous zinc–organic hybrid flow battery. Adv. Energy Mater. 9, 1900694 (2019).
Kong, T. et al. Stable operation of aqueous organic redox flow batteries in air atmosphere. Angew. Chem. Int. Ed. 62, e202214819 (2023).
Zhang, F. et al. Decoupled redox catalytic hydrogen production with a robust electrolyte-borne electron and proton carrier. J. Am. Chem. Soc. 143, 223–231 (2021).
Hohenadel, A. et al. Electrochemical characterization of hydrocarbon bipolar membranes with varying junction morphology. ACS Appl. Energy Mater. 2, 6817–6824 (2019).
Metlay, A. S. et al. Three-chamber design for aqueous acid–base redox flow batteries. ACS Energy Lett. 7, 908–913 (2022).
Weng, G.-M., Li, C.-Y. V. & Chan, K.-Y. High voltage vanadium-metal hydride rechargeable semi-flow battery. J. Electrochem. Soc. 160, A1384 (2013).
Darling, R. M. Techno-economic analyses of several redox flow batteries using levelized cost of energy storage. Curr. Opin. Chem. Eng. 37, 100855 (2022).
Kwabi, D. G. et al. Alkaline quinone flow battery with long lifetime at pH 12. Joule 2, 1894–1906 (2018).
Shin, M., Noh, C., Chung, Y. & Kwon, Y. All iron aqueous redox flow batteries using organometallic complexes consisting of iron and 3-[bis (2-hydroxyethyl)amino]-2-hydroxypropanesulfonic acid ligand and ferrocyanide as redox couple. Chem. Eng. J. 398, 125631 (2020).
Shin, M., Noh, C. & Kwon, Y. Stability enhancement for all‐iron aqueous redox flow battery using iron‐3‐[bis(2‐hydroxyethyl)amino]‐2‐hydroxypropanesulfonic acid complex and ferrocyanide as redox couple. Int. J. Energy Res. 46, 6866–6875 (2021).
Jin, S. et al. A water-miscible quinone flow battery with high volumetric capacity and energy density. ACS Energy Lett. 4, 1342–1348 (2019).
Xi, J., Wu, Z., Qiu, X. & Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 166, 531–536 (2007).
Tan, R. et al. Hydrophilic microporous membranes for selective ion separation and flow-battery energy storage. Nat. Mater. 19, 195–202 (2020).
Xia, Y. et al. Polymeric membranes with aligned zeolite nanosheets for sustainable energy storage. Nat. Sustain. 5, 1080–1091 (2022).
Ye, C. et al. Development of efficient aqueous organic redox flow batteries using ion-sieving sulfonated polymer membranes. Nat. Commun. 13, 3184 (2022).
Li, Z. & Lu, Y.-C. Polysulfide-based redox flow batteries with long life and low levelized cost enabled by charge-reinforced ion-selective membranes. Nat. Energy 6, 517–528 (2021).
Lopez-Atalaya, M., Codina, G., Perez, J., Vazquez, J. & Aldaz, A. Optimization studies on a Fe/Cr redox flow battery. J. Power Sources 39, 147–154 (1992).
Dmello, R., Milshtein, J. D., Brushett, F. R. & Smith, K. C. Cost-driven materials selection criteria for redox flow battery electrolytes. J. Power Sources 330, 261–272 (2016).
Darling, R. M., Gallagher, K. G., Kowalski, J. A., Ha, S. & Brushett, F. R. Pathways to low-cost electrochemical energy storage: a comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 7, 3459–3477 (2014).
Zuo, P. et al. Near-frictionless ion transport within triazine framework membranes. Nature 617, 299–305 (2023).
Lin, C. et al. High-voltage asymmetric metal–air batteries based on polymeric single-Zn2+-ion conductor. Matter 4, 1287–1304 (2021).
Acknowledgements
This research was supported by US Department of Energy (DOE) award DE-AC05-76RL01830 through Pacific Northwest National Laboratory (PNNL) subcontract 428977, by Harvard Climate Solution Change Funding, by the Massachusetts Clean Energy Technology Center and by the Harvard School of Engineering and Applied Sciences. A.M.A. acknowledges the Materials Science and Engineering department at King Fahd University of Petroleum and Minerals and the Ministry of Education of Saudi Arabia for doctoral scholarship. L.C.I.F acknowledges financial support from São Paulo Research Foundation (FAPESP) under the grant numbers: 2019/21089-6, 2021/14537-2. T.Y.G. acknowledges funding support from the NSF Graduate Research Fellowship Program. We thank Y. Jing, K. Amini, E. Fell and J. Sosa for helpful discussions. We thank J. MacArthur from Harvard Electronic Instrument Design Lab for developing multi-channel pH sensors. We thank M. P. Marshak from University of Colorado Boulder for providing CrPDTA sample.
Author information
Authors and Affiliations
Contributions
D.X. and A.M.A. designed and conducted cell tests and electrochemical experiments. D.X. developed the concept of mild pH-decoupling ARFB and acid–base recovery. A.M.A. worked on molecule synthesis and characterization. D.X. and J.G. worked on the posolyte. D.X. and T.C. worked on the design of centre chambers. A.M.A., L.C.I.F., T.Y.G. and T.W. worked on crossover characterization. D.X. and Z.Y. worked on the single-membrane pH-decoupling cell. D.X. did the techno-economic calculation. R.Y.L. and R.G.G. supervised the molecular synthesis and characterization. M.J.A. supervised the project. D.X., A.M.A. and M.J.A. drafted the manuscript. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Qilei Song and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–46 and Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xi, D., Alfaraidi, A.M., Gao, J. et al. Mild pH-decoupling aqueous flow battery with practical pH recovery. Nat Energy 9, 479–490 (2024). https://doi.org/10.1038/s41560-024-01474-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-024-01474-1
This article is cited by
-
Decoupled architecture enables pH decoupling
Nature Energy (2024)