Abstract
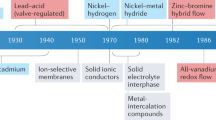
Aqueous batteries have been considered as the most promising alternatives to the dominant lithium-based battery technologies because of their low cost, abundant resources and high safety. The output voltage of aqueous batteries is limited by the narrow stable voltage window of 1.23 V for water, which theoretically impedes further improvement of their energy density. However, the pH-decoupling electrolyte with an acidic catholyte and an alkaline anolyte has been verified to broaden the operating voltage window of the aqueous electrolyte to over 3 V, which goes beyond the voltage limitations of the aqueous batteries, making high-energy aqueous batteries possible. In this Review, we summarize the latest decoupled aqueous batteries based on pH-decoupling electrolytes from the perspective of ion-selective membranes, competitive redox couples and potential battery prototypes. The inherent defects and problems of these decoupled aqueous batteries are systematically analysed, and the critical scientific issues of this battery technology for future applications are discussed.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Pasta, M., Wessells, C. D., Huggins, R. A. & Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 3, 1149 (2012).
Kim, H. et al. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 114, 11788–11827 (2014).
Tripathi, A. M., Su, W. N. & Hwang, B. J. In situ analytical techniques for battery interface analysis. Chem. Soc. Rev. 47, 736–851 (2018).
Chao, D. et al. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci. Adv. 6, eaba4098 (2020).
Sui, Y. & Ji, X. Anticatalytic strategies to suppress water electrolysis in aqueous batteries. Chem. Rev. 121, 6654–6695 (2021).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015). This paper reports that water-in-salt electrolytes significantly widen the operation voltage windows of aqueous electrolytes and enable high-voltage aqueous LIBs.
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Xie, J., Liang, Z. & Lu, Y. C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 19, 1006–1011 (2020). This paper reports that molecular crowding agents are able to break the hydrogen-bond network of water, thereby substantially suppressing the activity of water.
Bi, H. et al. A universal approach to aqueous energy storage via ultralow-cost electrolyte with super-concentrated sugar as hydrogen-bond-regulated solute. Adv. Mater. 32, 2000074 (2020).
Zhong, C. et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat. Energy 5, 440–449 (2020). This paper reports a decoupled Zn–MnO2 battery based on the multiple IEMs, including CEM and AEM.
Wang, F. et al. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 17, 543–549 (2018).
McEldrew, M., Goodwin, Z. A., Kornyshev, A. A. & Bazant, M. Z. Theory of the double layer in water-in-salt electrolytes. J. Phys. Chem. Lett. 9, 5840–5846 (2018).
Wang, X., Chandrabose, R. S., Jian, Z., Xing, Z. & Ji, X. A 1.8 V aqueous supercapacitor with a bipolar assembly of ion-exchange membranes as the separator. J. Electrochem. Soc. 163, 1853–1858 (2016).
Gu, S., Gong, K., Yan, E. Z. & Yan, Y. A multiple ion-exchange membrane design for redox flow batteries. Energy Environ. Sci. 7, 2986–2998 (2014). This paper introduces the multiple IEM designs into flow batteries to improve the working voltages of flow batteries.
Chao, D. et al. Atomic engineering catalyzed MnO2 electrolysis kinetics for a hybrid aqueous battery with high power and energy density. Adv. Mater. 32, 2001894 (2020).
Logan, B. E. & Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 488, 313–319 (2012).
Liu, X. et al. Power generation by reverse electrodialysis in a single-layer nanoporous membrane made from core–rim polycyclic aromatic hydrocarbons. Nat. Nanotechnol. 15, 307–312 (2020).
Yip, N. Y., Brogioli, D., Hamelers, H. V. M. & Nijmeijer, K. Salinity gradients for sustainable energy: primer, progress, and prospects. Environ. Sci. Technol. 50, 12072–12094 (2016).
Yang, M., Chen, R., Shen, Y., Zhao, X. & Shen, X. A high-energy aqueous manganese–metal hydride hybrid battery. Adv. Mater. 32, 2001106 (2020).
Jin, Z., Li, P. & Xiao, D. A hydrogen-evolving hybrid-electrolyte battery with electrochemical/photoelectrochemical charging from water oxidation. ChemSusChem 10, 483–488 (2017).
Ran, J. et al. Ion exchange membranes: new developments and applications. J. Membr. Sci. 522, 267–291 (2017).
Luo, T., Abdu, S. & Wessling, M. Selectivity of ion exchange membranes: a review. J. Membr. Sci. 555, 429–454 (2018).
Li, L. & Manthiram, A. Long-life, high-voltage acidic Zn–air batteries. Adv. Energy Mater. 6, 1502054 (2016). This paper reports a decoupled Zn–air battery based on a NASICON-type Li-ion solid electrolyte.
Chen, L., Guo, Z., Xia, Y. & Wang, Y. High-voltage aqueous battery approaching 3 V using an acidic–alkaline double electrolyte. Chem. Commun. 49, 2204–2206 (2013).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Zhao, Q., Stalin, S., Zhao, C. Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020).
Liu, C., Chi, X., Han, Q. & Liu, Y. A high energy density aqueous battery achieved by dual dissolution/deposition reactions separated in acid-alkaline electrolyte. Adv. Energy Mater. 10, 1903589 (2020).
Yu, F. et al. Aqueous alkaline–acid hybrid electrolyte for zinc-bromine battery with 3V voltage window. Energy Storage Mater. 19, 56–61 (2019). This paper reports a decoupled Zn–Br battery based on a bipolar membrane.
Ding, Y., Cai, P. & Wen, Z. Electrochemical neutralization energy: from concept to devices. Chem. Soc. Rev. 50, 1495–1511 (2021).
Epsztein, R., Shaulsky, E., Qin, M. & Elimelech, M. Activation behavior for ion permeation in ion-exchange membranes: role of ion dehydration in selective transport. J. Membr. Sci. 580, 316–326 (2019).
Feng, J. D. et al. Single-layer MoS2 nanopores as nanopower generators. Nature 536, 197–200 (2016).
Kordesh, K. & Weissenbacher, M. Rechargeable alkaline manganese dioxide/zinc batteries. J. Power Sources 51, 61–78 (1994).
Ovshinsky, S. R., Fetcenko, M. A. & Ross, J. A nickel metal hydride battery for electric vehicles. Science 260, 176–181 (1993).
Putois, F. Market for nickel-cadmium batteries. J. Power Sources 57, 67–70 (1995).
Ji, H. et al. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 5, 3317 (2014).
Fleischmann, S. et al. Pseudocapacitance: from fundamental understanding to high power energy storage materials. Chem. Rev. 120, 6738–6782 (2020).
Wang, X., Xie, Y., Tang, K., Wang, C. & Yan, C. Redox chemistry of molybdenum trioxide for ultrafast hydrogen-ion storage. Angew. Chem. Int. Ed. 57, 11569–11573 (2018).
Hong, J. J. et al. A dual plating battery with the iodine/[ZnIx(OH2)4−x]2−x cathode. Angew. Chem. Int. Ed. 58, 15910–15915 (2019).
Choi, C. et al. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 5, 5–19 (2020).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Sun, Y., Liu, N. & Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 1, 16071 (2016).
Wang, Y. et al. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 155, 706–726 (2019).
Cai, Z. et al. Chemically resistant Cu–Zn/Zn composite anode for long cycling aqueous batteries. Energy Storage Mater. 27, 205–211 (2020).
Lee, J. H. et al. High-energy efficiency membraneless flowless Zn–Br battery: utilizing the electrochemical–chemical growth of polybromides. Adv. Mater. 31, 1904524 (2019).
Liu, T., Wei, X., Nie, Z., Sprenkle, V. & Wang, W. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte. Adv. Energy Mater. 6, 1501449 (2016).
Lin, K. et al. Alkaline quinone flow battery. Science 349, 1529–1532 (2015).
Tian, H. et al. High power rechargeable magnesium/iodine battery chemistry. Nat. Commun. 8, 14083 (2017).
Gurieff, N., Timchenko, V. & Menictas, C. Variable porous electrode compression for redox flow battery systems. Batteries 4, 53 (2018).
Peng, Z., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li-O2 battery. Science 337, 563–566 (2012).
Qiao, Y. et al. Li-CO2 electrochemistry: a new strategy for CO2 fixation and energy storage. Joule 1, 359–370 (2017).
Ma, J. L., Bao, D., Shi, M. M., Yan, J. M. & Zhang, X. B. Reversible nitrogen fixation based on a rechargeable lithium-nitrogen battery for energy storage. Chem 2, 525–532 (2017).
Huang, Z. F. et al. Design of efficient bifunctional oxygen reduction/evolution electrocatalyst: recent advances and perspectives. Adv. Energy Mater. 7, 1700544 (2017).
Xu, W., Lu, Z., Sun, X., Jiang, L. & Duan, X. Superwetting electrodes for gas-involving electrocatalysis. Acc. Chem. Res. 51, 1590–1598 (2018).
Maja, M., Orecchia, C., Strano, M., Tosco, P. & Vanni, M. Effect of structure of the electrical performance of gas diffusion electrodes for metal air batteries. Electrochim. Acta 46, 423–432 (2000).
Lee, W. K., Ho, C. H., Van Zee, J. W. & Murthy, M. The effects of compression and gas diffusion layers on the performance of a PEM fuel cell. J. Power Sources 84, 45–51 (1999).
Pan, J. et al. Advanced architectures and relatives of air electrodes in Zn–air batteries. Adv. Sci. 5, 1700691 (2018).
Sun, W. et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science 371, 46–51 (2021).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Yuan, L. X. et al. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 4, 269–284 (2011).
Chao, D. & Qiao, S. Z. Toward high-voltage aqueous batteries: super- or low-concentrated electrolyte? Joule 4, 1846–1851 (2020).
Shen, Y. & Kordesch, K. The mechanism of capacity fade of rechargeable alkaline manganese dioxide zinc cells. J. Power Sources 87, 162–166 (2000).
Winter, M. & Brodd, R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104, 4245–4270 (2004).
Donne, S. W., Lawrance, G. A. & Swinkels, D. A. Redox processes at the manganese dioxide electrode: I. constant-current intermittent discharge. J. Electrochem. Soc. 144, 2949–2953 (1997).
Gibson, A. J. et al. Dynamic electrodeposition of manganese dioxide: temporal variation in the electrodeposition mechanism. J. Electrochem. Soc. 163, H305 (2016).
Singh, A. et al. Towards a high MnO2 loading and gravimetric capacity from proton-coupled Mn4+/Mn2+ reactions using a 3D free-standing conducting scaffold. J. Mater. Chem. A 9, 1500–1506 (2021).
Xu, Y. et al. High-voltage rechargeable alkali–acid Zn–PbO2 hybrid battery. Angew. Chem. Int. Ed. 59, 23593–23597 (2020).
Noack, J., Roznyatovskaya, N., Herr, T. & Fischer, P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 54, 9776–9809 (2015).
Khor, A. et al. Review of zinc-based hybrid flow batteries: from fundamentals to applications. Mater. Today Energy 8, 80–108 (2018).
Chang, Z. et al. Rechargeable Li//Br battery: a promising platform for post lithium ion batteries. J. Mater. Chem. A 2, 19444–19450 (2014).
Bai, P., Viswanathan, V. & Bazant, M. Z. A dual-mode rechargeable lithium–bromine/oxygen fuel cell. J. Mater. Chem. A 3, 14165–14172 (2015).
Bray, W. C. The hydrolysis of iodine and bromine. J. Am. Chem. Soc. 32, 932–938 (1910).
Jones, G. & Baeckström, S. The standard potential of the bromine electrode. J. Am. Chem. Soc. 56, 1524–1528 (1934).
Fischer, J. & Bingle, J. The vapor pressure of bromine from 24 to 116°. J. Am. Chem. Soc. 77, 6511–6512 (1955).
Küttinger, M., Loichet Torres, P. A., Meyer, E., Fischer, P. & Tübke, J. Systematic study of quaternary ammonium cations for bromine sequestering application in high energy density electrolytes for hydrogen bromine redox flow batteries. Molecules 26, 2721 (2021).
Hoobin, P. M., Cathro, K. J. & Niere, J. O. Stability of zinc/bromine battery electrolytes. J. Appl. Electrochem. 19, 943–945 (1989).
Kautek, W. In situ investigations of bromine-storing complex formation in a zinc-flow battery at gold electrodes. J. Electrochem. Soc. 146, 3211–3216 (1999).
Eustace, D. J. Bromine complexation in zinc-bromine circulating batteries. J. Electrochem. Soc. 127, 528–532 (1980).
Li, Y. et al. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 4, 1805 (2013).
Li, Y. & Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 43, 5257–5275 (2014).
Fu, J. et al. Electrically rechargeable zinc–air batteries: progress, challenges, and perspectives. Adv. Mater. 29, 1604685 (2017).
Lee, D. U., Choi, J. Y., Feng, K., Park, H. W. & Chen, Z. Advanced extremely durable 3D bifunctional air electrodes for rechargeable zinc-air batteries. Adv. Energy Mater. 4, 1301389 (2014).
Zhang, J., Zhao, Z., Xia, Z. & Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 10, 444–452 (2015).
Geng, D. et al. From lithium-oxygen to lithium-air batteries: challenges and opportunities. Adv. Energy Mater. 6, 1502164 (2016).
Lin, C. et al. High-voltage asymmetric metal–air batteries based on polymeric single-Zn2+-ion conductor. Matter 4, 1287–1304 (2021). This paper designs a polymeric single-Zn2+-ion conductor, making high-voltage decoupled metal–air batteries possible.
Zhang, L. & Lin, Z. Higher-voltage asymmetric-electrolyte metal-air batteries. Joule 5, 1325–1327 (2021).
Matter, P. H. & Ozkan, U. S. Non-metal catalysts for dioxygen reduction in an acidic electrolyte. Catal. Lett. 109, 115–123 (2006).
Li, L. & Manthiram, A. Decoupled bifunctional air electrodes for high-performance hybrid lithium-air batteries. Nano Energy 9, 94–100 (2014).
Yu, X. & Manthiram, A. A voltage-enhanced, low-cost aqueous iron–air battery enabled with a mediator-ion solid electrolyte. ACS Energy Lett. 2, 1050–1055 (2017).
Wen, H. et al. Ultrahigh voltage and energy density aluminum-air battery based on aqueous alkaline-acid hybrid electrolyte. Int. J. Energy Res. 44, 10652–10661 (2020).
Xu, K. Diffusionless charge transfer. Nat. Energy 4, 93–94 (2019).
Xie, F. et al. Mechanism for zincophilic sites on zinc-metal anode hosts in aqueous batteries. Adv. Energy Mater. 11, 2003419 (2021).
Yuan, L. et al. Regulation methods for the Zn/electrolyte interphase and the effectiveness evaluation in aqueous Zn-ion batteries. Energy Environ. Sci. 14, 5669–5689 (2021).
Zhao, S. et al. All-in-one and bipolar-membrane-free acid-alkaline hydrogel electrolytes for flexible high-voltage Zn-air batteries. Chem. Eng. J. 430, 132718 (2022).
Gong, K. et al. A zinc–iron redox-flow battery under $100 per kW h of system capital cost. Energy Environ. Sci. 8, 2941–2945 (2015).
Li, H., Weng, G., Li, C. Y. V. & Chan, K. Y. Three electrolyte high voltage acid–alkaline hybrid rechargeable battery. Electrochim. Acta 56, 9420–9425 (2011).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant nos. 21725103, 51522101, 51471075, 51631004 and 51401084), National Key R&D Program of China (grant no. 2019YFA0705700) and the China Postdoctoral Science Foundation (grant no. 2020M681035).
Author information
Authors and Affiliations
Contributions
X.Z. and Y.Z. contributed to the writing and editing of all sections of the manuscript. All authors contributed to the research and discussion of data, figure preparation and proofreading of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks W. Hu, S. Qiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Ion-selective membrane
-
(ISM). A semipermeable membrane that has selective transmissibility to specific ions in solution.
- Permselectivity
-
A term to define the preferential permeation of certain ionic species through ion-exchange membranes.
- Donnan equilibrium
-
Also known as the Gibbs–Donnan effect, the behaviour of charged particles near a semipermeable membrane, which sometimes fail to distribute evenly across the two sides of the membrane.
- Bipolar membranes
-
(BPMs). A special class of ion-exchange membranes constituted by a cation-exchange and an anion-exchange layer, which allows the generation of protons and hydroxide ions via a water dissociation mechanism.
- Proton-exchange membrane
-
(PEM). A kind of semipermeable membrane generally made from ionomers and designed to conduct protons.
- Grotthuss mechanism
-
Also known as proton-hopping mechanism, a proton transport mode in the hydrogen-bond network of water molecules or other hydrogen-bonded liquids, which is completed via the cooperative breaking and formation of hydrogen bonds and O–H covalent bonds.
Rights and permissions
About this article
Cite this article
Zhu, Yh., Cui, Yf., Xie, Zl. et al. Decoupled aqueous batteries using pH-decoupling electrolytes. Nat Rev Chem 6, 505–517 (2022). https://doi.org/10.1038/s41570-022-00397-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-022-00397-3
This article is cited by
-
Alkaline-based aqueous sodium-ion batteries for large-scale energy storage
Nature Communications (2024)
-
Mild pH-decoupling aqueous flow battery with practical pH recovery
Nature Energy (2024)
-
Asymmetric Electrolytes Design for Aqueous Multivalent Metal Ion Batteries
Nano-Micro Letters (2024)