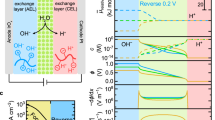
Abstract
Limited understanding exists about the operation of bipolar membranes (BPMs) in forward bias to convert protonic gradients into electrical work, despite their emerging role in many electrochemical devices. In these device contexts, the BPM is typically exposed to complex electrolyte mixtures, but their impact on polarization remains poorly understood. Here we develop a mechanistic model explaining the forward bias polarization behaviour of BPMs in mixed electrolytes with different acidities/basicities. This model invokes that weak acids/bases accumulate in the BPM and impose an ionic blockade that inhibits the recombination of stronger acids/bases, resulting in a substantial neutralization overpotential. We demonstrate the utility of our model for fuel cells and redox flow batteries and introduce two materials design strategies for mitigating this inhibition. Lastly, we apply our findings to enhance the energy efficiency of carbonate management in CO2 electrolysers. This work highlights how non-equilibrium local environments at membrane–membrane interfaces can define the efficiency of protonic-to-electrical energy conversion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the published article and its Supplementary Information. Source data are provided with this paper.
References
Pärnamäe, R. et al. Bipolar membranes: a review on principles, latest developments, and applications. J. Memb. Sci. 617, 118538 (2021).
Giesbrecht, P. K. & Freund, M. S. Recent advances in bipolar membrane design and applications. Chem. Mater. 32, 8060–8090 (2020).
Blommaert, M. A. et al. Insights and challenges for applying bipolar membranes in advanced electrochemical energy systems. ACS Energy Lett. 6, 2539–2548 (2021).
Tufa, R. A. et al. Bipolar membrane and interface materials for electrochemical energy systems. ACS Appl. Energy Mater. 4, 7419–7439 (2021).
Yan, Z. & Mallouk, T. E. Bipolar membranes for ion management in (photo)electrochemical energy conversion. Acc. Mater. Res. 2, 1156–1166 (2021).
Simons, R. & Khanarian, G. Water dissociation in bipolar membranes: experiments and theory. J. Membr. Biol. 38, 11–30 (1978).
Ramírez, P., Rapp, H.-J., Mafé, S. & Bauer, B. Bipolar membranes under forward and reverse bias conditions. Theory vs. experiment. J. Electroanal. Chem. 375, 101–108 (1994).
McDonald, M. B., Ardo, S., Lewis, N. S. & Freund, M. S. Use of bipolar membranes for maintaining steady-state pH gradients in membrane-supported, solar-driven water splitting. ChemSusChem 7, 3021–3027 (2014).
Vargas-Barbosa, N. M., Geise, G. M., Hickner, M. A. & Mallouk, T. E. Assessing the utility of bipolar membranes for use in photoelectrochemical water-splitting cells. ChemSusChem 7, 3017–3020 (2014).
Luo, J. et al. Bipolar membrane-assisted solar water splitting in optimal pH. Adv. Energy Mater. 6, 1600100 (2016).
Mayerhöfer, B. et al. Bipolar membrane electrode assemblies for water electrolysis. ACS Appl. Energy Mater. 3, 9635–9644 (2020).
Oener, S. Z., Foster, M. J. & Boettcher, S. W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020).
Vermaas, D. A. & Smith, W. A. Synergistic electrochemical CO2 reduction and water oxidation with a bipolar membrane. ACS Energy Lett. 1, 1143–1148 (2016).
Li, Y. C. et al. Electrolysis of CO2 to syngas in bipolar membrane-based electrochemical cells. ACS Energy Lett. 1, 1149–1153 (2016).
Salvatore, D. A. et al. Electrolysis of gaseous CO2 to CO in a flow cell with a bipolar membrane. ACS Energy Lett. 3, 149–154 (2018).
Pătru, A., Binninger, T., Pribyl, B. & Schmidt, T. J. Design principles of bipolar electrochemical co-electrolysis cells for efficient reduction of carbon dioxide from gas phase at low temperature. J. Electrochem. Soc. 166, F34–F43 (2019).
Siritanaratkul, B. et al. Zero-gap bipolar membrane electrolyzer for carbon dioxide reduction using acid-tolerant molecular Eelectrocatalysts. J. Am. Chem. Soc. 144, 7551–7556 (2022).
Xie, K. et al. Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products. Nat. Commun. 13, 3609 (2022).
Sullivan, I. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 4, 952–958 (2021).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Ding, Y., Cai, P. & Wen, Z. Electrochemical neutralization energy: from concept to devices. Chem. Soc. Rev. 50, 1495–1511 (2021).
Yan, Z. et al. High-voltage aqueous redox flow batteries enabled by catalyzed water dissociation and acid–base neutralization in bipolar membranes. ACS Cent. Sci. 7, 1028–1035 (2021).
Metlay, A. S. et al. Three-chamber design for aqueous acid–base redox flow batteries. ACS Energy Lett. 7, 908–913 (2022).
Pärnamäe, R. et al. The acid–base flow battery: sustainable energy storage via reversible water dissociation with bipolar membranes. Membranes 10, 409 (2020).
Al-Dhubhani, E., Pärnamäe, R., Post, J. W., Saakes, M. & Tedesco, M. Performance of five commercial bipolar membranes under forward and reverse bias conditions for acid–base flow battery applications. J. Memb. Sci. 7, 119748 (2021).
Bui, J. C., Digdaya, I., Xiang, C., Bell, A. T. & Weber, A. Z. Understanding multi-ion transport mechanisms in bipolar membranes. ACS Appl. Mater. Interfaces 12, 52509–52526 (2020).
Mitchell, J. B., Chen, L., Langworthy, K., Fabrizio, K. & Boettcher, S. W. Catalytic proton–hydroxide recombination for forward-bias bipolar membranes. ACS Energy Lett. 7, 3967–3973 (2022).
Sokirko, A. V., Ramírez, P., Manzanares, J. A. & Mafés, S. Modeling of forward and reverse bias conditions in bipolar membranes. Ber. Bunsen. Ges. Phys. Chem. 97, 1040–1048 (1993).
Ziv, N., Mustain, W. E. & Dekel, D. R. The effect of ambient carbon dioxide on anion‐exchange membrane fuel cells. ChemSusChem 11, 1136–1150 (2018).
Lee, M.-Y. et al. Current achievements and the future direction of electrochemical CO2 reduction: a short review. Crit. Rev. Environ. Sci. Technol. 50, 769–815 (2020).
Dinh, H. Q., Toh, W. L., Chu, A. T. & Surendranath, Y. Neutralization short-circuiting with weak electrolytes erodes the efficiency of bipolar membranes. ACS Appl. Mater. Interfaces 15, 4001–4010 (2023).
Vermaas, D. A., Wiegman, S., Nagaki, T. & Smith, W. A. Ion transport mechanisms in bipolar membranes for (photo)electrochemical water splitting. Sustain. Energy Fuels 2, 2006–2015 (2018).
Haynes, W. M., Lide, D. R. & Bruno, T. J. CRC Handbook of Chemistry and Physics 97th edn (CRC Press, 2016).
Grew, K. N., McClure, J. P., Chu, D., Kohl, P. A. & Ahlfield, J. M. Understanding transport at the acid–alkaline interface of bipolar membranes. J. Electrochem. Soc. 163, F1572–F1587 (2016).
Ünlü, M., Zhou, J. & Kohl, P. A. Hybrid anion and proton exchange membrane fuel cells. J. Phys. Chem. C. 113, 11416–11423 (2009).
Daud, S. S., Norrdin, M. A., Jaafar, J. & Sudirman, R. The effect of material on bipolar membrane fuel cell performance: a review. IOP Conf. Ser. Mater. Sci. Eng. 736, 032003 (2020).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications 2nd edn (John Wiley & Sons: New York, 2001).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Zhang, Y.-J., Sethuraman, V., Michalsky, R. & Peterson, A. A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 4, 3742–3748 (2014).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, E4585–E4593 (2016).
Ooka, H., Figueiredo, M. C. & Koper, M. T. M. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir 33, 9307–9313 (2017).
Goyal, A., Marcandalli, G., Mints, V. A. & Koper, M. T. M. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. J. Am. Chem. Soc. 142, 4154–4161 (2020).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Xie, K. et al. Eliminating the need for anodic gas separation in CO2 electroreduction systems via liquid-to-liquid anodic upgrading. Nat. Commun. 13, 3070 (2022).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
O’Brien, C. P. et al. 2 Conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Xu, Y. et al. A microchanneled solid electrolyte for carbon-efficient CO2 electrolysis. Joule 6, 1333–1343 (2022).
‘Timothy’ Kim, J. Y. et al. Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor. Nat. Catal. 5, 288–299 (2022).
Ozden, A. et al. Carbon-efficient carbon dioxide electrolysers. Nat. Sustain. 5, 563–573 (2022).
Qiao, Y. et al. Engineering the local microenvironment over Bi nanosheets for highly selective electrocatalytic conversion of CO2 to HCOOH in strong acid. ACS Catal. 12, 2357–2364 (2022).
Iddya, A. et al. A reverse-selective ion exchange membrane for the selective transport of phosphates via an outer-sphere complexation–diffusion pathway. Nat. Nanotechnol. 17, 1222–1228 (2022).
Chen, Y. et al. High-performance bipolar membrane development for improved water dissociation. ACS Appl. Polym. Mater. 2, 4559–4569 (2020).
Acknowledgements
We thank the entire Surendranath Lab for the enriching discussions and their support. This work was supported by the Department of Energy (DOE) under award number DE-SC0021634, which also supported A.T.C. and E.R.S. This work made use of the MRSEC Shared Experimental Facilities at MIT, which is supported by the National Science Foundation under award number DMR-1419807. W.L.T. is supported by a National Science Scholarship awarded by the Agency for Science, Technology and Research (A*STAR), Singapore. H.Q.D. is supported by Undergraduate Research Opportunities Program (UROP) awards from the MIT UROP office and the MIT Energy Initiative. E.R.S. is also supported in part by a Postdoctoral Fellowship awarded by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Contributions
W.L.T., H.Q.D. and Y.S. conceptualized the project. W.L.T., H.Q.D., A.T.C. and E.R.S. conducted experiments. W.L.T., H.Q.D. and Y.S. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Sebastian Oener, Peter Pintauro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26, Tables 1–3 and Notes 1–8.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toh, W.L., Dinh, H.Q., Chu, A.T. et al. The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes. Nat Energy 8, 1405–1416 (2023). https://doi.org/10.1038/s41560-023-01404-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01404-7
This article is cited by
-
Multi-scale physics of bipolar membranes in electrochemical processes
Nature Chemical Engineering (2024)
-
Ions block up the junction
Nature Energy (2023)