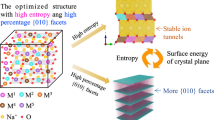
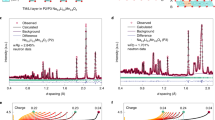
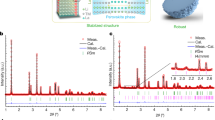
Abstract
Manganese-rich NASICON-type materials have triggered widespread attention for developing advanced polyanionic cathodes, primarily driven by their abundant reserves and promising cycling performance with high operating voltages (~3.8 V for Mn2+/3+/4+, versus Na+/Na). However, the charge/discharge profiles exhibit significant voltage hysteresis, which leads to a limited reversible capacity, thereby preventing their application. Here, we demonstrate that the voltage hysteresis in manganese-rich NASICON-type cathodes (Na3MnTi(PO4)3) is closely related to the intrinsic anti-site defect (IASD), which forms during synthesis and is captured in our characterizations. Combining electrochemical analysis and spectroscopic techniques, we draw a comprehensive picture of sluggish Na+ diffusion behaviours in the IASD-affected structure during cycling, and rationalize the relationship of voltage hysteresis, phase separation and delayed charge compensation. Furthermore, a Mo-doping strategy is developed to decrease the defect concentration, which enhances the initial Coulombic efficiency from 76.2% to 85.9%. Overall, this work sheds light on the voltage hysteresis in NASICON-type cathodes and provides guidelines for designing high-performance polyanionic electrodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files. Source data are provided with this paper.
References
Dunn, B., Kamath, H. & Tarascon, J. M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Rudola, A., Sayers, R., Wright, C. J. & Barker, J. Opportunities for moderate-range electric vehicles using sustainable sodium-ion batteries. Nat. Energy 8, 215–218 (2023).
Jin, T. et al. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 49, 2342–2377 (2020).
Pan, H., Hu, Y.-S. & Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 6, 2338–2360 (2013).
Gao, H., Li, Y., Park, K. & Goodenough, J. B. Sodium extraction from NASICON-structured Na3MnTi(PO4)3 through Mn(III)/Mn(II) and Mn(IV)/Mn(III) redox couples. Chem. Mater. 28, 6553–6559 (2016).
Gao, H. et al. Na3MnZr(PO4)3: a high-voltage cathode for sodium batteries. J. Am. Chem. Soc. 140, 18192–18199 (2018).
Goodenough, J. B., Hong, H. Y. P. & Kafalas, J. A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976).
Deng, Z. et al. Fundamental investigations on the sodium-ion transport properties of mixed polyanion solid-state battery electrolytes. Nat. Commun. 13, 4470 (2022).
Wang, Q. et al. Experimental and theoretical investigation of Na4MnAl(PO4)3 cathode material for sodium-ion batteries. Chem. Eng. J. 425, 130680 (2021).
Zhu, T. et al. Dual carbon decorated Na3MnTi(PO4)3: a high-energy-density cathode material for sodium-ion batteries. Nano Energy 70, 104548 (2020).
Sun, X. et al. Dual carbon decorated Na3MnTi(PO4)3 as an advanced cathode for sodium-ion batteries. Ionics 26, 3919–3927 (2020).
Zhu, T. et al. Realizing three-electron redox reactions in NASICON-structured Na3MnTi(PO4)3 for sodium-ion batteries. Adv. Energy Mater. 9, 2338–2360 (2019).
Liu, R. et al. Counter-intuitive structural instability aroused by transition metal migration in polyanionic sodium ion host. Adv. Energy Mater. 11, 2003256 (2020).
Liu, R. et al. Exploring highly reversible 1.5-electron reactions (V3+/V4+/V5+) in Na3VCr(PO4)3 cathode for sodium-ion batteries. ACS Appl. Mater. Interfaces 9, 43632–43639 (2017).
Zhang, J., Lin, C., Xia, Q., Wang, C. & Zhao, X. S. Improved performance of Na3MnTi(PO4)3 using a non-stoichiometric synthesis strategy. ACS Energy Lett. 6, 2081–2089 (2021).
Gao, H. & Goodenough, J. B. An aqueous symmetric sodium-ion battery with NASICON-structured Na3MnTi(PO4)3. Angew. Chem. Int. Ed. Engl. 55, 12768–12772 (2016).
Jian, Z. et al. Atomic structure and kinetics of NASICON NaxV2(PO4)3 cathode for sodium-ion batteries. Adv. Funct. Mater. 24, 4265–4272 (2014).
De Backer, A., Martinez, G. T., Rosenauer, A. & Van Aert, S. Atom counting in HAADF STEM using a statistical model-based approach: methodology, possibilities, and inherent limitations. Ultramicroscopy 134, 23–33 (2013).
Lee, K. et al. STEM image analysis based on deep learning: identification of vacancy defects and polymorphs of MoS2. Nano Lett. 22, 4677–4685 (2022).
Lin, R., Zhang, R., Wang, C., Yang, X. Q. & Xin, H. L. TEMImageNet training library and AtomSegNet deep-learning models for high-precision atom segmentation, localization, denoising, and deblurring of atomic-resolution images. Sci. Rep. 11, 5386 (2021).
Chung, S. Y., Choi, S. Y., Lee, S. & Ikuhara, Y. Distinct configurations of antisite defects in ordered metal phosphates: comparison between LiMnPO4 and LiFePO4. Phys. Rev. Lett. 108, 195501 (2012).
Zhang, Z. et al. Correlated migration invokes higher Na+‐ion conductivity in NaSICON‐type solid electrolytes. Adv. Energy Mater. 9, 1902373 (2019).
Zou, Z. et al. Relationships between Na+ distribution, concerted migration, and diffusion properties in rhombohedral NASICON. Adv. Energy Mater. 10, 2001486 (2020).
Park, S. et al. Crystal structure of Na2V2(PO4)3, an intriguing phase spotted in the Na3V2(PO4)3–Na1V2(PO4)3 system. Chem. Mater. 34, 451–462 (2021).
Zhuo, Z., Hu, J., Duan, Y., Yang, W. & Pan, F. Transition metal redox and Mn disproportional reaction in LiMn0.5Fe0.5PO4 electrodes cycled with aqueous electrolyte. Appl. Phys. Lett. 109, 023901 (2016).
Qiao, R. et al. Direct evidence of gradient Mn(II) evolution at charged states in LiNi0.5Mn1.5O4 electrodes with capacity fading. J. Power Sources 273, 1120–1126 (2015).
Gruenert, W. et al. Analysis of molybdenum(3d) XPS spectra of supported molybdenum catalysts: an alternative approach. J. Phys. Chem. 95, 1323–1328 (2002).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Shi, S. et al. Multi-scale computation methods: their applications in lithium-ion battery research and development. Chin. Phys. B 25, 018212 (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Hart, G. L. W., Nelson, L. J. & Forcade, R. W. Generating derivative structures at a fixed concentration. Comput. Mater. Sci. 59, 101–107 (2012).
Acknowledgements
Y.-S.H. acknowledges support by the National Key Technologies R&D Program (2022YFB3807800), the National Natural Science Foundation of China (NSFC) (52122214) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020006). J.Z. acknowledges support by the Beijing Natural Science Foundation (2222078) and National Natural Science Foundation of China (52072370). The authors wish to thank the support of the BL11W and BL02B02 beamlines of Shanghai Synchrotron Radiation Facility.
Author information
Authors and Affiliations
Contributions
Y.-S.H. and J.Z. designed and supervised the project. Y.L. synthesized, characterized (X-ray diffraction, XAS, TG, SEM, NPD, Raman) and electrochemically tested the samples and analysed the data with X.R. and R.B. X.L. performed the NMR measurements and analysis. Q.Z. performed the STEM measurements and analysis. J.X. and W.Y. performed neutron diffraction measurements and analysis. R.X. designed and performed DFT calculations and analysis. C.Z. performed the TEM and electron energy loss spectroscopy measurements and analysis. Y.L., X.R., and Y.-S.H. wrote the manuscript. All the authors participated in the discussion to improve the paper and made revisions of the whole manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Zdeněk Sofer, Yan Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–33, Tables 1–3, Note 1 and References.
Source data
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Rong, X., Bai, R. et al. Identifying the intrinsic anti-site defect in manganese-rich NASICON-type cathodes. Nat Energy 8, 1088–1096 (2023). https://doi.org/10.1038/s41560-023-01301-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01301-z
This article is cited by
-
Capturing and reducing defects
Nature Energy (2023)