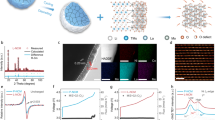
Abstract
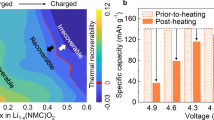
The use of state-of-the-art Ni-rich layered oxides (LiNixCoyMn1−x−yO2, x > 0.5) as the cathode material for lithium-ion batteries can push the energy and power density to a higher level than is currently available1,2. However, volume variation associated with anisotropic lattice strain and stress that is being developed during lithium (de)intercalation induces severe structural instability and electrochemical decay of the cathode materials, which is amplified further when the battery is operating at a high voltage (above 4.5 V), which is essential for unlocking its high energy3,4,5,6. Even after much effort by the research community, an intrinsic strain-retardant method for directly alleviating the continuous accumulation of lattice strain remains elusive. Here, by introducing a coherent perovskite phase into the layered structure functioning as a ‘rivet’, we significantly mitigate the pernicious structural evolutions by a pinning effect. The lattice strain evolution in every single cycle is markedly reduced by nearly 70% when compared with conventional materials, which significantly enhances morphological integrity leading to a notable improvement in battery cyclability. This strain-retardant approach broadens the perspective for lattice engineering to release the strain raised from lithium (de)intercalation and paves the way for the development of high-energy-density cathodes with long durability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Grey, C. P. & Hall, D. S. Prospects for lithium-ion batteries and beyond—a 2030 vision. Nat. Commun. 11, 6279 (2020).
Li, M., Lu, J., Chen, Z. & Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 30, 1800561 (2018).
de Biasi, L. et al. Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion batteries. Adv. Mater. 31, e1900985 (2019).
Zhang, S. S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 24, 247–254 (2020).
Mao, Y. et al. High‐voltage charging‐induced strain, heterogeneity, and micro‐cracks in secondary particles of a nickel‐rich layered cathode material. Adv. Funct. Mater. 29, 1900247 (2019).
Bianchini, M., Roca-Ayats, M., Hartmann, P., Brezesinski, T. & Janek, J. There and back again – the journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. Engl. 58, 10434–10458 (2019).
Heenan, T. M. M. et al. Identifying the origins of microstructural defects such as cracking within Ni‐rich NMC811 cathode particles for lithium‐ion batteries. Adv. Energy Mater. 10, 2002655 (2020).
Xu, C. et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 20, 84–92 (2021).
Ohzuku, T., Ueda, A. & Yamamoto, N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 142, 1431–1435 (1995).
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2020).
Bianchini, M. et al. The interplay between thermodynamics and kinetics in the solid-state synthesis of layered oxides. Nat. Mater. 19, 1088–1095 (2020).
Singer, A. et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging. Nat. Energy 3, 641–647 (2018).
Park, J. et al. Fictitious phase separation in Li layered oxides driven by electro-autocatalysis. Nat. Mater. 20, 991–999 (2021).
Li, M. & Lu, J. Cobalt in lithium-ion batteries. Science 367, 979–980 (2020).
Xu, C., Reeves, P. J., Jacquet, Q. & Grey, C. P. Phase behavior during electrochemical cycling of Ni‐rich cathode materials for Li‐ion batteries. Adv. Energy Mater. 11, 2003404 (2020).
Yoon, M. et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 6, 362–371 (2021).
Marker, K., Xu, C. & Grey, C. P. Operando NMR of NMC811/graphite lithium-ion batteries: structure, dynamics, and lithium metal deposition. J. Am. Chem. Soc. 142, 17447–17456 (2020).
Wang, L. et al. Structural distortion induced by manganese activation in a lithium-rich layered cathode. J. Am. Chem. Soc. 142, 14966–14973 (2020).
Liu, T. et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 6, 277–286 (2021).
Cha, H. et al. Boosting reaction homogeneity in high-energy lithium-ion battery cathode materials. Adv. Mater. 32, e2003040 (2020).
Weigel, T. et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 4, 508–516 (2019).
Xin, F. et al. What is the role of Nb in nickel-rich layered oxide cathodes for lithium-ion batteries? ACS Energy Lett. 6, 1377–1382 (2021).
Dixit, M., Markovsky, B., Aurbach, D. & Major, D. T. Unraveling the effects of Al doping on the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 Using first principles. J. Electrochem. Soc. 164, A6359–A6365 (2017).
Yan, P. et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability ofl lithium-ion batteries. Nat. Energy 3, 600–605 (2018).
Xu, X. et al. Radially oriented single‐crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium‐ion batteries. Adv. Energy Mater. 9, 1803963 (2019).
Ryu, H.-H. et al. Microstrain alleviation in high-energy Ni-rich NCMA cathode for long battery life. ACS Energy Lett. 6, 216–223 (2020).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020).
Langdon, J. & Manthiram, A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 37, 143–160 (2021).
Li, J. et al. Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J. Electrochem. Soc. 164, A1534–A1544 (2017).
Burley, J. C. et al. Magnetism and structural chemistry of the n = 1 Ruddlesden–Popper phase La4LiMnO8 and La3SrLiMnO8. J. Am. Chem. Soc. 124, 620–628 (2002).
Hong, Y.-S. et al. Hierarchical defect engineering for LiCoO2 through low-solubility trace element doping. Chem 6, 2759–2769 (2020).
Hebert, A. & McCalla, E. The role of metal substitutions in the development of Li batteries, Part I: cathodes. Mater Adv 2, 3474–3518 (2021).
Yoon, W.-S., Chung, K. Y., McBreen, J. & Yang, X.-Q. A comparative study on structural changes of LiCo1/3Ni1/3Mn1/3O2 and LiNi0.8Co0.15Al0.05O2 during first charge using in situ XRD. Electrochem. Commun. 8, 1257–1262 (2006).
Grenier, A. et al. Reaction heterogeneity in LiNi0.8Co0.15Al0.05O2 induced by surface layer. Chem. Mater. 29, 7345–7352 (2017).
Lee, W., Lee, D., Kim, Y., Choi, W. & Yoon, W.-S. Enhancing the structural durability of Ni-rich layered materials by post-process: washing and heat-treatment. J. Mater. Chem. A 8, 10206–10216 (2020).
Williamson, G. K. & Hall, W. H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1, 22–31 (1953).
Muhammed Shafi, P. & Chandra Bose, A. Impact of crystalline defects and size on X-Ray line broadening: a phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 5, 057137 (2015).
Uchimura, T. & Yamada, I. A robust thermal-energy-storage property associated with electronic phase transitions for quadruple perovskite oxides. Chem Commun (Camb) 56, 5500–5503 (2020).
Hu, J. et al. Fundamental linkage between structure, electrochemical properties, and chemical compositions of LiNi1-x-yMnxCoyO2 cathode materials. ACS Appl. Mater. Interfaces 13, 2622–2629 (2021).
Chae, M. S. et al. Vacancy‐driven high rate capabilities in calcium‐doped Na0.4MnO2 cathodes for aqueous sodium‐ion batteries. Adv. Energy Mater. 10, 2002077 (2020).
Lee, W. et al. Advances in the cathode materials for lithium rechargeable batteries. Angew. Chem. Int. Ed. 59, 2578–2605 (2020).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Cryst. 46, 544–549 (2013).
Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192, 55–69 (1993).
Wang, L. et al. Reaction inhomogeneity coupling with metal rearrangement triggers electrochemical degradation in lithium-rich layered cathode. Nat. Commun. 12, 5370 (2021).
Juhás, P., Davis, T., Farrow, C. L. & Billinge, S. J. L. PDFgetX3: a rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Cryst. 46, 560–566 (2013).
Juhas, P., Farrow, C. L., Yang, X., Knox, K. R. & Billinge, S. J. Complex modeling: a strategy and software program for combining multiple information sources to solve ill posed structure and nanostructure. Inverse Problems. Acta Cryst. 71, 562–568 (2015).
Acknowledgements
The work at Argonne National Laboratory was supported by the US Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. Argonne National Laboratory is operated for the US DOE Office of Science by UChicago Argonne, LLC, under contract no. DE-AC02-06CH11357. This research used the 9-BM, 11-BM, 11-ID-C and 32-ID-C beamlines at APS, a US DOE Office of Science User Facility operated for the US DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This work was also supported by Clean Vehicles, US–China Clean Energy Research Centre (CERC-CVC2) under US DOE EERE Vehicle Technologies Office. J.L. acknowledges financial support from the start-up research funding of Zhejiang University. We also thank Y. Ren, L. Yin, V. D. Andrade and K. Shelly for their support of the synchrotron-based experiments.
Author information
Authors and Affiliations
Contributions
L.W. and J.L. conceived the ideas and designed the experiments. L.W., T.L. and T.W. carried out the synchrotron-based experiments and analysed the data. L.W. and T.L. prepared the materials and conducted the electrochemical measurements. L.W., T.W. and J.L. wrote the manuscript. J.L. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviews for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–34, Tables 1–7 and references.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Liu, T., Wu, T. et al. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 611, 61–67 (2022). https://doi.org/10.1038/s41586-022-05238-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05238-3
This article is cited by
-
Efficient direct repairing of lithium- and manganese-rich cathodes by concentrated solar radiation
Nature Communications (2024)
-
One-step calcination synthesis of interface-coherent crystallized and surface-passivated LiNi0.5Mn1.5O4 for high-voltage lithium-ion battery
Nano Research (2024)
-
Suppressing strain propagation in ultrahigh-Ni cathodes during fast charging via epitaxial entropy-assisted coating
Nature Energy (2024)
-
Defective oxygen inert phase stabilized high-voltage nickel-rich cathode for high-energy lithium-ion batteries
Nature Communications (2023)
-
Sulfide-Based All-Solid-State Lithium–Sulfur Batteries: Challenges and Perspectives
Nano-Micro Letters (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.