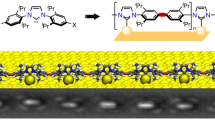
Abstract
Two-dimensional (2D) transition-metal carbides and nitrides (MXenes) combine the electronic and mechanical properties of 2D inorganic crystals with chemically modifiable surfaces, which provides an ideal platform for both fundamental and applied studies of interfaces. Good progress has been achieved in the functionalization of MXenes with small inorganic ligands, but relatively little work has been reported on the covalent bonding of various organic groups to MXene surfaces. Here we synthesize a family of hybrid MXenes (h-MXenes) that incorporate amido- and imido-bonding between organic and inorganic parts by reacting halogen-terminated MXenes with deprotonated organic amines. The resulting hybrid structures unite tailorability of organic molecules with electronic connectivity and other properties of inorganic 2D materials. Describing the structure of h-MXene necessitates the integration of concepts from coordination chemistry, self-assembled monolayers and surface science. The optical properties of h-MXenes reveal coherent coupling between the organic and inorganic constituents. h-MXenes also exhibit superior stability against hydrolysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are provided in the Article and its Supplementary Information and are also available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 1–17 (2017).
VahidMohammadi, A., Rosen, J. & Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 372, eabf1581 (2021).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Ghidiu, M., Lukatskaya, M. R., Zhao, M.-Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014).
Halim, J. et al. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chem. Mater. 26, 2374–2381 (2014).
Kamysbayev, V. et al. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 369, 979–983 (2020).
Li, Y. et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 19, 894–899 (2020).
Li, M. et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 141, 4730–4737 (2019).
Li, M. et al. Halogenated Ti3C2 MXenes with electrochemically active terminals for high-performance zinc ion batteries. ACS Nano 15, 1077–1085 (2021).
Hope, M. A. et al. NMR reveals the surface functionalisation of Ti3C2 MXene. Phys. Chem. Chem. Phys. 18, 5099–5102 (2016).
Khazaei, M. et al. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 23, 2185–2192 (2013).
Liu, Y., Xiao, H. & Goddard, W. A. Schottky-barrier-free contacts with two-dimensional semiconductors by surface-engineered MXenes. J. Am. Chem. Soc. 138, 15853–15856 (2016).
Si, C., Zhou, J. & Sun, Z. Half-metallic ferromagnetism and surface functionalization-induced metal–insulator transition in graphene-like two-dimensional Cr2C crystals. ACS Appl. Mater. Interfaces 7, 17510–17515 (2015).
Griffith, K. J. et al. Bulk and surface chemistry of the niobium MAX and MXene phases from multinuclear solid-state NMR spectroscopy. J. Am. Chem. Soc. 142, 18924–18935 (2020).
Harris, K. J., Bugnet, M., Naguib, M., Barsoum, M. W. & Goward, G. R. Direct measurement of surface termination groups and their connectivity in the 2D MXene V2CTx using NMR spectroscopy. J. Phys. Chem. C 119, 13713–13720 (2015).
Kobayashi, T. et al. Nature of terminating hydroxyl groups and intercalating water in Ti3C2Tx MXenes: a study by 1H solid-state NMR and DFT calculations. J. Phys. Chem. C 124, 13649–13655 (2020).
Beaumier, E. P., Billow, B. S., Singh, A. K., Biros, S. M. & Odom, A. L. A complex with nitrogen single, double, and triple bonds to the same chromium atom: synthesis, structure, and reactivity. Chem. Sci. 7, 2532–2536 (2016).
Paluch, P., Pawlak, T., Amoureux, J.-P. & Potrzebowski, M. J. Simple and accurate determination of X–H distances under ultra-fast MAS NMR. J. Magn. Reson. 233, 56–63 (2013).
Hillhouse, G. L. & Bercaw, J. E. Reactions of water and ammonia with bis(pentamethylcyclopentadienyl) complexes of zirconium and hafnium. J. Am. Chem. Soc. 106, 5472–5478 (1984).
Green, M. L. H. A new approach to the formal classification of covalent compounds of the elements. J. Organomet. Chem. 500, 127–148 (1995).
Anderson, N. C., Hendricks, M. P., Choi, J. J. & Owen, J. S. Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: spectroscopic observation of facile metal-carboxylate displacement and binding. J. Am. Chem. Soc. 135, 18536–18548 (2013).
Ghidiu, M. & Barsoum, M. W. The {110} reflection in X-ray diffraction of MXene films: misinterpretation and measurement via non-standard orientation. J. Am. Ceram. Soc. 100, 5395–5399 (2017).
Bain, C. D. et al. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 111, 321–335 (1989).
Dubois, L. H. & Nuzzo, R. G. Synthesis, structure, and properties of model organic surfaces. Annu. Rev. Phys. Chem. 43, 437–463 (1992).
Badia, A., Cuccia, L., Demers, L., Morin, F. & Lennox, R. B. Structure and dynamics in alkanethiolate monolayers self-assembled on gold nanoparticles: a DSC, FT-IR, and deuterium NMR study. J. Am. Chem. Soc. 119, 2682–2692 (1997).
Hostetler, M. J., Stokes, J. J. & Murray, R. W. Infrared spectroscopy of three-dimensional self-assembled monolayers: N-alkanethiolate monolayers on gold cluster compounds. Langmuir 12, 3604–3612 (1996).
Luk’yanchuk, B. et al. The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater. 9, 707–715 (2010).
Miroshnichenko, A. E., Flach, S. & Kivshar, Y. S. Fano resonances in nanoscale structures. Rev. Mod. Phys. 82, 2257 (2010).
El-Demellawi, J. K., Lopatin, S., Yin, J., Mohammed, O. F. & Alshareef, H. N. Tunable multipolar surface plasmons in 2D Ti3C2Tx MXene flakes. ACS Nano 12, 8485–8493 (2018).
Agrawal, A. et al. Resonant coupling between molecular vibrations and localized surface plasmon resonance of faceted metal oxide nanocrystals. Nano Lett. 17, 2611–2620 (2017).
Israelachvili, J. N. Intermolecular and Surface Forces (Academic Press, 2011).
Pang, Z., Zhang, J., Cao, W., Kong, X. & Peng, X. Partitioning surface ligands on nanocrystals for maximal solubility. Nat. Commun. 10, 2454 (2019).
Zhang, J. et al. Freezing titanium carbide aqueous dispersions for ultra-long-term storage. ACS Appl. Mater. Interfaces 12, 34032–34040 (2020).
Zhang, C. J. et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes). Chem. Mater. 29, 4848–4856 (2017).
Natu, V. et al. Edge capping of 2D‐MXene sheets with polyanionic salts to mitigate oxidation in aqueous colloidal suspensions. Angew. Chem. 131, 12785–12790 (2019).
Mathis, T. S. et al. Modified MAX phase synthesis for environmentally stable and highly conductive Ti3C2 MXene. ACS Nano 15, 6420–6429 (2021).
Wang, X., Wang, Z. & Qiu, J. Stabilizing MXene by hydration chemistry in aqueous solution. Angew. Chem. Int. Ed. 60, 26587–26591 (2021).
Avgustinik, A. I., Drozdetskaya, G. V. & Ordan’yan, S. S. Reaction of titanium carbide with water. Powder Metall. Met. Ceram. 6, 470–473 (1967).
Vasilos, T. & Kingery, W. Note of properties of aqueous suspensions of TiC and TiN. J. Phys. Chem. 58, 486–488 (1954).
Limonov, M. F., Rybin, M. V., Poddubny, A. N. & Kivshar, Y. S. Fano resonances in photonics. Nat. Photonics 11, 543–554 (2017).
Wallwork, S. C. Introduction to the Calculation of Structure Factors. (University College Cardiff Press, 1980).
Acknowledgements
We thank J. Anderson, H. You, W. Cho, H. Wu, J. Ondry, J. Xie, N. Jiang and A. Ramaanathan for help and valuable discussions. We are also grateful to A. Nelson for a critical reading and editing of the manuscript. h-MXene synthesis and surface functionalization studies were supported by the National Science Foundation under award number DMR-2004880 and Advanced Materials for Energy-Water Systems (AMEWS) Center, an Energy Frontier Research Center funded by the US Department of Energy (DOE), Office of Science, BES. Spectroscopic studies were supported by the Department of Defense Air Force Office of Scientific Research under grant number FA9550-22-1-0283. Several concepts discussed here have been formulated during the preparation of NSF Center for Chemical Innovation (CCI) Proposal for NSF Center for MXenes Synthesis, Tunability and Reactivity (M-STAR). This work made use of the shared facilities at the University of Chicago Materials Research Science and Engineering Center, supported by National Science Foundation under award number DMR-2011854. Parts of this work were carried out at the Soft Matter Characterization Facility of the University of Chicago. Computational studies by M.L. and D.J. were supported by the Fluid Interface Reactions, Structures, and Transport (FIRST) Center, an Energy Frontier Research Center (EFRC) funded by the US DOE, Office of Science, Office of Basic Energy Sciences. F.L. and R.F.K. at University of Illinois Chicago (UIC) were supported by a grant from the National Science Foundation (NSF-DMR 1831406). Acquisition of the UIC JEOL ARM200CF was supported by an MRI-R2 grant from the National Science Foundation (DMR-0959470). The Gatan Continuum GIF acquisition at UIC was supported by an MRI grant from the National Science Foundation (DMR-1626065). The work used resources of the Center for Nanoscale Materials, a US DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract number DE-AC02-06CH11357. Solid-state NMR spectroscopy (B.A. and A.J.R.) was supported by the US DOE, Office of Science, Basic Energy Sciences, Materials Science and Engineering Division. The Ames National Laboratory is operated for the US DOE by Iowa State University under contract number DE-AC02-07CH11358.
Author information
Authors and Affiliations
Contributions
C.Z. and D.W. performed and designed the experiments and analysed data. F.L. and R.F.K. performed high-resolution STEM studies and image analysis. B.A. and A.J.R. performed solid-state NMR studies and analysis. H.H. participated in the early stages of the project. Z.Z. contributed to the X-ray diffraction data analysis and AFM studies. M.L. and D.J. performed computational studies. A.S.F. performed XPS measurements. D.V.T. conceived and designed experiments, analysed data and supervised the project. C.Z. and D.V.T. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application has been filed on this technology.
Peer review
Peer review information
Nature Chemistry thanks Maria Lukatskaya, Varun Natu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Surface exchange reaction of Ti3C2 MXenes with amines and thermal degradation analysis of h-MXenes.
a, X-ray fluorescence (XRF) spectral and elemental analysis for the Ti3C2Br2 and Ti3C2(bua)2/3 MXenes. XRF spectra were normalized according to Ti Kα1. Based on XRF analysis, Br was completely removed after the surface modification with deprotonated butylamine. b, Powder XRD patterns of products after treatment of Ti3C2Br2 with butylamine and NaH at 120 °C for 2 days, as well as the products of control experiments. We observed that Ti3C2Br2 barely reacted with NaH in an inert solvent at 120 °C. When treated only with butylamine at 120 °C, Ti3C2Br2 is likely to be partially converted or intercalated, as a new peak at 5.4° has emerged, but the majority of Ti3C2Br2 is still intact. When butylamine was deprotonated by NaH and then reacted with Ti3C2Br2, the (0 0 0 2) peak shifted to 4.65° while the \((1 1 \overline{2} 0)\) peak shifted to 60.9°. The absence of diffraction peaks from Ti3C2Br2 suggests the reaction was complete, which was confirmed by XRF analysis. c, Powder XRD patterns of Ti3C2Br2 before and after treatment with triethylamine and NaH at 120 °C for 2 days: The XRD pattern is unchanged, which indicates that no reaction occurred and suggests that deprotonation of amines is crucial to promoting the exchange reaction on MXene surfaces. d, TGA characterization of Ti3C2(pra)2/3 MXene. The sample was heated from room temperature (25 °C) to 650 °C at a rate of 2 °C min−1 under nitrogen. e, SEM-EDS images of pristine Ti3C2(pra)2/3 and that after heating up from room temperature (25 °C) to designated temperature at a rate of 20 °C min−1 and hold for 1 hour under nitrogen flow. XRD and Raman characterizations of the annealed h-MXenes are shown in Supplementary Fig. 1.
Extended Data Fig. 2 Surface exchange reaction of Br- or Cl- terminated MXenes with amines.
a, Powder XRD patterns of Ti3C2(bua)2/3 prepared by using different halogen terminated MXenes and deprotonating agents. Identical products were obtained using different combinations of starting halogen-terminated MXenes and deprotonating agents. b, Powder XRD patterns from diamine-modified h-MXenes and their Le Bail fits. c, Powder XRD patterns from aromatic amine- and 2-methoxyethylamine- modified h-MXenes and their Le Bail fits. d, Powder XRD patterns from different types of Cl-terminated MXenes treated with butylamine and n-BuLi. PXRD patterns suggest that the approach of surface modification can also be applied to other MXenes, for example, Ti2C and Nb2C MXenes.
Extended Data Fig. 3 DFT modeling of h-MXenes.
a, Representative snapshots of propyl groups bond to surface Ti atoms in amido and imido motif, respectively. b. Representative snapshots of propyl or octyl amido/imido groups bond to surface Ti atoms with tilt angle calculated to be 34° and 35°, respectively.
Extended Data Fig. 4 c lattice constant-dependent (0 0 0 l) XRD peak intensity.
a, Side view of the unit cells of Ti3C2 MXene with z coordinates of Ti atoms indicated. b, Relationship between the intensity ratio for (0 0 0 2)/(0 0 0 l) XRD peaks and the c lattice constant. When the c lattice constant varies, the ratio of the peak intensities for the (0 0 0 2) reflection to those of the (0 0 0 l) peaks can change by several orders of magnitude. To quantify these changes, the theoretical peak intensities for each Bragg reflection were calculated using the equation: \({I}_{{hkl}}={({\sum }_{j}^{N}{f}_{j}\cos {\varphi }_{j})}^{2}+{({\sum }_{j}^{N}{f}_{j}{isin}{\varphi }_{j})}^{2}\), where I is the relative integrated intensity, f is the atomic form factor, and φn \(=2\pi (\)hxn \(+k\)yn \(+l\)zn\()\). Here, the equation was simplified by ignoring light atoms with small atomic numbers Z, including N (Z = 7), C (Z = 6), and H (Z = 1), considering their sufficiently lower f ~ Z2 compared to Ti (Z = 22). The imaginary part \({\sum }_{j}^{N}{f}_{j}i\sin {\varphi }_{j}\) is eliminated when a collection of atoms has a center of symmetry41. For (0 0 0 l) lamella peaks, \({\varphi }_{n}=2l\pi {* z}_{n}\). As a result, this equation can be further simplified to \({I}_{{hkl}}={({\sum }_{j}^{N}{f}_{j}\cos (2l\pi {* z}_{n}))}^{2}\). The value of d was found from STEM images to be around 2.4 Å. The correlations between the calculated (0 0 0 2)/(0 0 0 l) peak intensity ratio and the c-lattice constant are shown in Extended Data Fig. 3b. This qualitative analysis helps to understand the non-monotonic variation of peak intensity ratios with c lattice constants among h-MXenes. For example, it explains why the difference between the intensities of (0 0 0 2) and (0 0 0 4) peaks gradually decreases when the c lattice constant increases for alkylimido-terminated Ti3C2 MXenes. It also explains why the (0 0 0 6) peak of Ti3C2(oca)2/3 becomes less intense when the c lattice parameter decreases from 52.93 Å to 49.45 Å in Fig. 5d.
Extended Data Fig. 5 Tilt angle of the alkyl chains of the surface termination groups relative to the surface normal.
a, Summary of the metal to surface group elemental ratios for Ti3C2(NH), alkylamine- and diamine-modified h-MXenes. *Elemental analysis was performed on C, H, and N; the Ti/N ratio was estimated by attributing the rest of the mass to Ti. We anticipate that the actual Ti/N ratio would be lower if any impurities existed. b, Change of c lattice parameters versus number of CH2 groups in the alkylamine molecules. c, Schematic of Ti3C2 sheets sandwiched by two layers of alkylimido surface termination groups. Each crystal unit cell contains two layers of Ti3C2 sheets and four layers of surface termination groups. If Ti3C2(NH) is used as the reference to account for contributions from Ti-N bonds and the van der Waals gaps, then the slope of Fig. 3b, i,e. 4.38 Å per CH2 in the alkyl chain, corresponds to four times the thickness of a single alkyl monolayer. Therefore, each CH2 unit appears to increase the thickness of the alkyl chain layer by ~1.10 Å. As the theoretical length of a fully extended alkyl chain with all-trans conformations is ~1.27 Å per CH2 group23, the tilt angle θ of organic surface group can be calculated to be arccos(1.10 Å /1.27 Å)=30°.
Extended Data Fig. 6 SEM and LAADF-STEM images of h-MXenes.
SEM and LAADF-STEM images of a, alkylamine-, b, diamine- and aromatic-amine-modified h-MXenes (scale bar in STEM images is 2 nm). The systematically smaller d-spacings measured from STEM images in comparison with powder X-ray diffraction patterns is attributed to electron beam-induced damage and strain in bended h-MXene stacks used for imaging, as confirmed by cryo-STEM imaging of microtomed h-MXene samples which showed much better agreement of interlayer distances with powder XRD data (Extended Data Fig. 5a–c). c, SEM images of PEG-amine-modified h-MXenes. d, SEM images of Ti3C2(bua)2/3 MXenes prepared with n-BuLi as deprotonating agent. e, STEM-EELS elemental analysis (line scan) of Ti3C2(dda)2/3 MXene.
Extended Data Fig. 7 STEM images of h-MXenes.
a, LAADF-STEM images of Ti3C2(pra)2/3 MXene measured at room temperature (left) and at liquid nitrogen temperature (right) with average center-to-center distances (CD) of 13.86 Å and 18.61 Å, respectively. (XRD: 17.85 Å) b, LAADF-STEM images of Ti3C2(oca)2/3 MXene measured at room temperature (left) and at liquid nitrogen temperature (right) with average CD of 17.97 Å and 22.40 Å, respectively. (XRD: 26.47 Å) c, LAADF-STEM images of Ti3C2(hda)2/3 MXene measured at room temperature (left) and at liquid nitrogen temperature (right) with average CD of 20.70 Å and 38.78 Å, respectively. (XRD: 46.67 Å) d, Comparison of center-to-center distances obtained from XRD and STEM at room and liquid nitrogen (LN) temperature, e, LAADF-STEM image of a thin h-MXene slab prepared by focused ion beam (FIB) milling of Ti3C2(pda) MXene measured at room temperature with average CD of 14.96 Å (XRD: 15.76 Å). f, Inverted ABF-STEM images of Ti3C2(tma) showing double-layered 2-thiophenemethylimido group in between Ti3C2 sheets. The visualization of S atoms is still challenging because they are relatively light as compared to Ti and less ordered than Ti3C2 sheets; beam broadening upon entering less ordered material will also reduce our ability to visualize S embedded inside an aromatic ring. The Ti/S ratio was determined to be 3/0.66 by XRF analysis. g. ABF-STEM image of d-Ti3C2(bua)2/3 viewed along [0001] direction. Fast Fourier transform (FFT, inset) showing hexagonal symmetry of the flake.
Extended Data Fig. 8 Analysis of h-MXenes before and after HBr treatment.
a, XRF and b, XPS analysis of Ti3C2(nmeda) h-MXenes before and after HBr treatment. An extra peak at ~402 eV appeared after HBr treatment, which was assigned to R-NH3+. In combination with XRF results and our analysis, we speculate that one nitrogen in diamine h-MXenes is bonded to the Ti3C2 surface while the other remains chemically accessible for protonation, or that both nitrogen atoms can be bonded to the same MXene sheet. c, Powder XRD patterns of Ti3C2(nmeda) and Ti3C2(pra)2/3 before and after HBr treatment. The (0 0 0 2) peak in the XRD pattern of Ti3C2(nmeda) shifted to smaller 2θ angles, indicating expansion of the interlayer distance after protonation. In comparison, the (0 0 0 2) peak of HBr post-treated Ti3C2(pra)2/3 does not shift. The (0 0 0 2) peak of Ti3C2(pra)2/3 did not shift to larger 2θ angles after HBr post-treatment, which provides additional evidence that terminating groups are strongly covalently bonded to Ti3C2 surface. If the organo-amines were present as intercalated ammonium cations, the HBr treatment would cause replacement of ammonium ions for protons.
Extended Data Fig. 9 Delamination of h-MXenes.
a, Schematic process of the preparation of delaminated h-MXenes. b, Powder XRD patterns of different delaminated h-MXenes. c, SEM images of delaminated Ti3C2(hda)2/3 h-MXene prepared by reacting delaminated Ti3C2Cl2 with deprotonated hexadecylamine. d, Powder XRD patterns of bulk Ti3C2(oca)2/3, and d-Ti3C2(oca)2/3 before and after oleylamine treatment. The films prepared from delaminated h-MXene solutions showed X-ray diffraction patterns with (0 0 0 l) peaks shifted to smaller 2θ angles as compared to their bulk counterparts, and the \((1 1 \overline{2} 0)\) peak vanished, as expected for re-stacked MXenes with randomly rotated individual sheets22. e, Photographs of stable colloidal solutions of delaminated h-MXenes dispersed in CHCl3 at high concentrations (top) and in dilute solutions, showing Tyndall scattering. f, TEM image of d-Ti3C2(oca/ola)2/3 MXene flakes deposited from a colloidal solution. (Inset) Fast Fourier transform (FFT) showing the crystallinity and hexagonal symmetry of the individual flake. g, AFM image of the monolayer d-Ti3C2(hda)2/3. h, Raman spectra of Ti3C2(bua)2/3, d-Ti3C2(bua)2/3, and d-Ti3C2(pra/PEG1k)2/3.
Extended Data Fig. 10 Delamination of bulk Br-terminated MXenes using deprotonated mixed amines.
Mixed amines propylamine/oleylamine or propylamine/PEG1k (9:1) were deprotonated by n-BuLi and then reacted with Ti3C2Br2 (bulk powders) at 120 °C. a, powder XRD patterns of h-MXenes with mixed termination groups. SEM image of the h-MXenes with b, pra/ola and c, pra/PEG1k mixed termination groups and (inset) photograph of the colloidal dispersion in CHCl3 (b) or NMF (c). d, Mixed amines octylamine/oleylamine (9:1 or 1:1) were deprotonated by n-BuLi and then reacted with Ti3C2Br2 (bulk powders) at 120 °C. When the molar ratio of octylamine/oleylamine is increased from 9:1 to 1:1, the XRD patterns can be deconvoluted to two sets of XRD peaks, which can be assigned to separate contributions from d-Ti3C2(oca/ola) and d-Ti3C2(ola). SEM image of the h-MXenes with e, oca/ola (9:1 in precursor) and f, oca/ola (1:1 in precursor) mixed termination groups and (inset) photograph of the colloidal dispersion in CHCl3.
Supplementary information
Supplementary Information
Supplementary materials and methods, Figs. 1-30 and Discussions 1–6.
Supplementary Data
Source_Data_for_Supplementary_Figure_S4
Supplementary Data
Source_Data_for_Supplementary_Figure_S7
Supplementary Data
Source_Data_for_Supplementary_Figure_S8
Supplementary Data
Source_Data_for_Supplementary_Figure_S12
Supplementary Data
Source_Data_for_Supplementary_Figure_S29
Supplementary Data
Source_Data_for_Computational_Models
Source data
Source Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, C., Wang, D., Lagunas, F. et al. Hybrid organic–inorganic two-dimensional metal carbide MXenes with amido- and imido-terminated surfaces. Nat. Chem. 15, 1722–1729 (2023). https://doi.org/10.1038/s41557-023-01288-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01288-w